Phenolic Compounds in Berries of Winter-Resistant Actinidia arguta Miq. and Actinidia kolomikta Maxim.: Evidence of Antioxidative Activity
Abstract
:1. Introduction
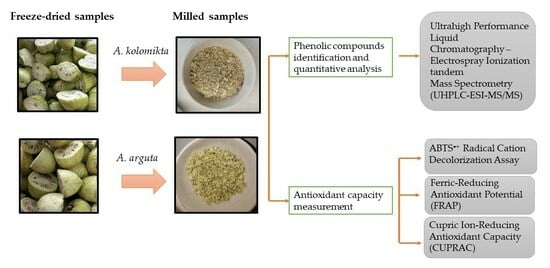
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Preparation of Berry Extracts
2.4. Determination of Bioactive Compounds Profile
2.5. Evaluation of Phenolic Compounds in Berry Samples Using the UHPLC-ESI-MS/MS Technique
2.6. Determination of Antioxidant Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Determination of Phenolic Compounds
3.2. Quantitative Composition of Phenolic Compounds
3.3. Determination of Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mesías, F.J.; Martín, A.; Hernández, A. Consumers’ growing appetite for natural foods: Perceptions towards the use of natural preservatives in fresh fruit. Food Res. Int. 2021, 150, 110749. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.; Grygorczyk, A. Consumer eating habits and perceptions of fresh produce quality. In Postharvest Handling; Elsevier: Amsterdam, The Netherlands, 2022; pp. 487–515. [Google Scholar]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Kupska, M.; Wasilewski, T.; Jędrkiewicz, R.; Gromadzka, J.; Namieśnik, J. Determination of terpene profiles in potential superfruits. Int. J. Food Prop. 2016, 19, 2726–2738. [Google Scholar] [CrossRef]
- Chacha, J.S.; Ofoedu, C.E.; Suleiman, R.A.; Jumbe, T.J.; Kulwa, K.B.M. Chapter 7—Underutilized fruits: Challenges and constraints for domestication. In Future Foods; Global Trends, Opportunities, and Sustainability Challenges; Elsevier Inc.: Amsterdam, The Netherlands, 2022; pp. 133–150. [Google Scholar]
- Wang, S.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, L. Comparative analysis of the microbial community composition between Tibetan kefir grains and milks. Food Res. Int. 2019, 116, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Liu, H.; Zhao, T.; Meng, C.; Liu, Z.; Liu, X. Bioactive compounds and in vitro antioxidant activities of peel, flesh and seed powder of kiwi fruit. Int. J. Food Sci. Tech. 2018, 53, 2239–2245. [Google Scholar] [CrossRef]
- Huang, H. Kiwifruit: The Genus ACTINIDIA. In Kiwifruit; Elsevier Science & Technology: Alpharetta, GA, USA, 2016. [Google Scholar]
- Wojdyło, A.; Nowicka, P.; Oszmiański, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D. Characteristics and pro-health properties of mini kiwi (Actinidia arguta). Hortic. Environ. Biotechnol. 2019, 60, 217–225. [Google Scholar] [CrossRef]
- Sawicki, T.; Błaszczak, W.; Latocha, P. In vitro anticholinergic and antiglycaemic properties of frost-hardy Actinidia fruit extracts and their polyphenol profile, L-ascorbic acid content and antioxidant capacity. Food Res. Int. 2023, 173, 113324. [Google Scholar] [CrossRef]
- Zuo, L.; Wang, Z.; Fan, Z.; Tian, S.; Liu, J. Evaluation of Antioxidant and antiproliferative properties of three Actinidia (Actinidia kolomikta, Actinidia arguta, Actinidia chinensis) extracts in vitro. Int. J. Mol. Sci. 2012, 13, 5506–5518. [Google Scholar] [CrossRef]
- Horák, M.; Šnurkovič, P.; Ondrášek, I.; Balík, J.; Srilaong, V. Comparison of some physico-chemical parameters of kiwiberry (Actinidia arguta) cultivars from a cold climate. Folia Hortic. 2019, 31, 375–383. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, H.; Zhao, B.; Lei, D.; Zhou, X.; Yao, W.; Fan, J.; Zhang, Y.; Chen, Q.; Wang, Y.; et al. Comparative changes of health-promoting phytochemicals and sugar metabolism of two hardy kiwifruit (Actinidia arguta) cultivars during fruit development and maturity. Front. Plant Sci. 2022, 13, 1087452. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Dong, K.; Yin, X.; Hao, C.; Zhang, W.; Irfan, M.; Chen, L.; Wang, Y. Metabolomic and transcriptomic exploration of the uric acid-reducing flavonoids biosynthetic pathways in the fruit of Actinidia arguta Sieb. Zucc. Front. Plant Sci. 2022, 13, 1025317. [Google Scholar] [CrossRef] [PubMed]
- Waswa, E.N.; Ding, S.; Wambua, F.M.; Mkala, E.M.; Mutinda, E.S.; Odago, W.O.; Amenu, S.G.; Muthui, S.W.; Linda, E.L.; Katumo, D.M.; et al. The genus Actinidia lindl. (Actinidiaceae): A comprehensive review on its ethnobotany, phytochemistry, and pharmacological properties. J. Ethnopharmacol. 2024, 319, 117222. [Google Scholar] [CrossRef] [PubMed]
- D’Amelia, V.; Aversano, R.; Chiaiese, P.; Carputo, D. The antioxidant properties of plant flavonoids: Their exploitation by molecular plant breeding. Phytochem. Rev. 2018, 17, 611–625. [Google Scholar] [CrossRef]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological applications and health-promoting properties of flavonols: An updated view. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef] [PubMed]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Česonienė, L. Assessment of phenological and microphenological timing of Actinidia kolomikta. Hortic. Veg. Grow. 2004, 23, 82–91. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol. 1999, 299, 152–178. [Google Scholar]
- Urbonavičiūtė, A.; Jakštas, V.; Kornyšova, O.; Janulis, V.; Maruška, A. Capillary electrophoretic analysis of flavonoids in single-styled hawthorn (Crataegus monogyna Jacq.) ethanolic extracts. J. Chromatogr. A 2006, 1112, 339–344. [Google Scholar] [CrossRef]
- Heil, M.; Baumann, B.; Andary, C.; Linsenmair, E.K.; McKey, D. Extraction and quantification of “condensed tannins” as a measure of plant anti-herbivore defence? Revisiting an old problem. Naturwissenschaften 2002, 89, 519–524. [Google Scholar] [CrossRef]
- Didier, F.; Catherine, F.; Odile, T.; Jean-Louis, L. Caffeoyl derivatives: Major antioxidant compounds of some wild herbs of the Asteraceae family. Food Sci. Nutr. 2011, 2, 181–192. [Google Scholar]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Žvikas, V.; Janulis, V.; Gómez-Serranillos, M.P. Antioxidant activity, neuroprotective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Česonienė, L.; Štreimikytė, P.; Liaudanskas, M.; Žvikas, V.; Viškelis, P.; Viškelis, J.; Daubaras, R. Berries and Leaves of Actinidia kolomikta (Rupr. & Maxim.) Maxim.: A Source of Phenolic Compounds. Plants 2022, 11, 147. [Google Scholar] [PubMed]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa ‘Hayward’ and Actinidia eriantha ‘Bidan’. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and proanthocyanidins: Chemical structures, food sources, bioactivities, and product development. Food Rev. Int. 2023, 39, 4581–4609. [Google Scholar] [CrossRef]
- Macedo, C.; Costa, P.C.; Rodrigues, F. Bioactive compounds from Actinidia arguta fruit as a new strategy to fight glioblastoma. Food Res. Int. 2023, 175, 113770. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Xie, D.; Sharma, S.B. Proanthocyanidins–a final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P. Anticholinergic effects of Actinidia arguta fruits and their polyphenol content determined by liquid chromatography-photodiode array detector-quadrupole/time of flight-mass spectrometry (LC-MS-PDA-Q/TOF). Food Chem. 2019, 271, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Kwok, K. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Kurakane, S.; Yamada, N.; Sato, H.; Igarashi, K. Anti-diabetic effects of Actinidia arguta polyphenols on rats and KK-Ay mice. Food Sci. Technol. Res. 2011, 17, 93–102. [Google Scholar] [CrossRef]
- Ozden, E.M.; Bingol, Z.; Mutlu, M.; Karagecili, H.; Köksal, E.; Goren, A.C.; Alwasel, S.H.; Gulcin, İ. Antioxidant, antiglaucoma, anticholinergic, and antidiabetic effects of kiwifruit (Actinidia deliciosa) oil: Metabolite profile analysis using LC-HR/MS, GC/MS and GC-FID. Life 2023, 13, 1939. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Ali, F.; Siddique, Y.H. Bioavailability and pharmaco-therapeutic potential of luteolin in overcoming Alzheimer’s disease. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2019, 18, 352–365. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Teng, K.; Zang, H. Actinidia arguta (Sieb. et Zucc.) Planch. ex Miq.: A Review of phytochemistry and pharmacology. Molecules 2023, 28, 7820. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Guo, Y.; How, K.; Acosta, A.; Documet, D.; Liang, C.; Arul, D.; Wood, S.; Moon, K.; Oliver, L.S. Five questions on how biochemistry can combat climate change. BBA Adv. 2023, 4, 100111. [Google Scholar] [CrossRef] [PubMed]
- Paulauskienė, A.; Tarasevičienė, Ž.; Žebrauskienė, A.; Pranckietienė, I. Effect of controlled atmosphere storage conditions on the chemical composition of super hardy Kiwifruit. Agronomy 2020, 10, 822. [Google Scholar] [CrossRef]
- Latocha, P. The nutritional and health benefits of Kiwiberry (Actinidia arguta)—A Review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef]
- Bader Ul Ain, H.; Tufail, T.; Javed, M.; Tufail, T.; Arshad, M.U.; Hussain, M.; Gull Khan, S.; Bashir, S.; Al Jbawi, E.; Abdulaali Saewan, S. Phytochemical profile and pro-healthy properties of berries. Int. J. Food Prop. 2022, 25, 1714–1735. [Google Scholar] [CrossRef]
- Latocha, P.; Krupa, T.; Wołosiak, R.; Worobiej, E.; Wilczak, J. Antioxidant activity and chemical difference in fruit of different Actinidia sp. Int. J. Food Sci. Nutr. 2010, 61, 381–394. [Google Scholar] [CrossRef]






| Cultivar | Origin | * Average Berry Weight, g |
|---|---|---|
| A. kolomikta | ||
| ‘Sentiabrskaja’ | Russia | 2.04 ± 0.04 a |
| ‘Aromatnaja’ | Russia | 2.31 ± 0.19 ab |
| ‘Matovaja’ | Russia | 2.39 ± 0.17 b |
| ‘VIR-2’ | Russia | 2.74 ± 0.22 c |
| ‘Milema’ | Lithuania | 3.6 ± 0.10 d |
| A. arguta | ||
| ‘Purpurova Sadova’ | Ukraine | 5.32 ± 0.24 a |
| ‘Izumrudna’ | Ukraine | 5.94 ± 0.75 ab |
| ‘Figurna’ | Ukraine | 6.23 ± 0.43 b |
| ‘Kijivskaja Hibridna’ | Ukraine | 7.18 ± 0.08 c |
| ‘Kijivskaja Krupnoplidna’ | Ukraine | 10.37 ± 0.39 d |
| Compound | Parent Ion (m/z) | Daughter Ion (m/z) | Cone Voltage, V | Collision Energy, eV |
|---|---|---|---|---|
| Neochlorogenic acid | 353 | 191 | 32 | 14 |
| Kaempferol-3-O-rutinoside (Nictoflorin) | 593 | 285 | 36 | 20 |
| Quercetin 3-O-glucoside (Isoquercitrin) | 463 | 301 | 52 | 28 |
| Luteolin-4-O-glucoside (Juncein) | 447 | 285 | 36 | 16 |
| Luteolin-7-rutinoside (Scolymoside) | 593 | 285 | 82 | 36 |
| Procyanidin B1 | 577 | 289 | 50 | 20 |
| Procyanidin C1 | 865.2 | 125 | 56 | 60 |
| (+)-Catechin | 289 | 123 | 60 | 34 |
| Chlorogenic acid | 353 | 191 | 32 | 14 |
| Phloridzin | 435 | 273 | 42 | 14 |
| Quercetin | 301 | 151 | 48 | 20 |
| Isorhamnetin-3-O-rutinoside (Narcissin) | 623 | 315 | 70 | 32 |
| Ferulic acid | 193 | 134 | 32 | 18 |
| Procyanidin B2 | 577 | 289 | 50 | 20 |
| (−)-Epicatechin | 289 | 123 | 60 | 34 |
| Caffeic acid | 179 | 107 | 36 | 22 |
| Kaempferol-3-O-glucoside (Astragalin) | 447 | 284 | 54 | 28 |
| Quercetin-3-O-rutinoside (Rutin) | 609 | 300 | 70 | 38 |
| Quercetin-3-O-galactoside (Hyperoside) | 463 | 300 | 50 | 26 |
| Phenolic Compound, µg/g DW | Actinidia kolomikta | Actinidia arguta | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Milema’ | ‘Sentiabrskaja’ | ‘VIR-2’ | ‘Matovaja’ | ‘Aromatnaja’ | ‘Izumrudna’ | ‘Kijevskaja Krupnoplidna’ | ‘Figurna’ | ‘Purpurova Sadova’ | ‘Kijevskaja Hibridna’ | |
| Phenolic acids | ||||||||||
| Neochlorogenic acid | 8.10 ± 3.49 g | 8.11 ± 1.80 g | 12.57 ± 3.48 e | 6.71 ± 1.85 h | 8.44 ± 2.05 f | 6.13 ± 0.19 i | 20.75 ± 0.94 b | 58.19 ± 3.36 a | 14.35 ± 1.1 d | 19.06 ± 2.02 c |
| Chlorogenic acid | 35.98 ± 2.78 h | 40.61 ± 2.89 g | 41.47 ± 5.95 f | 44.79 ± 4.02 e | 211.74 ± 6.24 a | 59.12 ± 1.55 c | 52.06 ± 5.04 d | 61.77 ± 3.56 b | 4.22 ± 0.58 j | 25.74 ± 4.06 i |
| Ferulic acid | 8.18 ± 0.54 d | 9.02 ± 0.81 b | 4.26 ± 0.39 h | 3.90 ± 1.03 i | 4.99 ± 2.20 g | 14.25 ± 1.45 a | 8.68 ± 0.33 c | 5.48 ± 1.23 f | 6.47 ± 1.16 e | 3.61 ± 0.21 j |
| Caffeic acid | 14.57 ± 2.96 d | 6.46 ± 0.29 e | 4.22 ± 0.36 j | 23.89 ± 2.72 b | 87.57 ± 9.73 a | 4.84 ± 0.13 g | 4.60 ± 0.93 i | 5.16 ± 1.25 f | 4.78 ± 1.02 h | 15.93 ± 0.81 c |
| Flavonols | ||||||||||
| Kaempferol-3-O-rutinoside | 26.42 ± 5.47 a | 6.93 ± 0.15 a | 12.00 ± 1.21 c | 17.34 ± 0.99 b | 11.13 ± 1.24 d | 5.24 ± 0.79 h | 4.65 ± 1.17 i | 2.64 ± 0.40 j | 5.89 ± 1.0 f | 5.40 ± 0.47 g |
| Isorhamnetin-3-O-rutinoside | 7.58 ± 0.56 f | 7.49 ± 1.15 f | 8.90 ± 0.75 e | 14.55 ± 0.52 a | 11.45 ± 0.78 c | 6.77 ± 0.99 g | 3.88 ± 0.75 h | 2.94 ± 0.98 i | 9.51 ± 1.45 d | 13.31 ± 1.61 b |
| Kaempferol-3-O-glucoside | 387.53 ± 10.59 b | 65.37 ± 11.14 e | 109.69 ± 19.83 d | 301.79 ± 14.27 c | 564.94 ± 28.15 a | 49.06 ± 3.80 f | 6.90 ± 0.94 j | 44.68 ± 2.61 g | 7.95 ± 1.12 i | 12.47 ± 1.25 h |
| Rutin | 7.16 ± 0.51 h | 8.00 ± 0.72 g | 14.51 ± 0.98 f | 15.11 ± 1.33 e | 32.16 ± 2.18 d | 6.33 ± 0.31 i | 81.46 ± 6.86 a | 43.12 ± 0.75 b | 37.86 ± 1.76 c | 43.47 ± 1.87 b |
| Isoquercitrin | 283.49 ± 20.4 fe | 123.14 ± 19.40 i | 236.18 ± 34.19 h | 958.42 ± 31.95 b | 1078.48 ± 27.35 a | 99.60 ± 5.74 j | 621.82 ± 7.87 c | 383.23 ± 21.43 e | 593.15 ± 9.61 d | 257.02 ± 20.54 g |
| Quercetin | 11.23 ± 1.83 b | 3.51 ± 1.83 h | 5.36 ± 2.09 d | 4.83 ± 0.91 e | 4.20 ± 0.64 f | 4.27 ± 0.76 f | 7.48 ± 1.40 c | 3.76 ± 0.77 g | 4.79 ± 1.07 e | 15.93 ± 1.07 a |
| Phloridzin | 6.50 ± 3.70 d | 3.69 ± 1.15 e | 2.69 ± 0.86 f | 11.05 ± 1.08 a | 2.71 ± 0.51 f | 2.77 ± 0.24 f | 9.49 ± 1.26 b | 9.46 ± 1.44 b | 3.61 ± 1.20 e | 9.11 ± 0.63 c |
| Hyperoside | 91.49 ± 7.59 e | 50.06 ± 6.43 h | 78.53 ± 8.78 g | 262.53 ± 6.33 b | 295.83 ± 9.97 a | 47.08 ± 1.85 i | 25.58 ± 2.19 j | 116.95 ± 8.01 d | 156.59 ± 8.60 e | 79.37 ± 10.37 i |
| Flavones | ||||||||||
| Luteolin-4-O-glucoside | 53.28 ± 4.09 b | 8.81 ± 1.74 g | 16.86 ± 4.03 d | 42.72 ± 4.03 c | 80.97 ± 6.14 a | 6.95 ± 0.48 i | 8.07 ± 1.61 h | 11.07 ± 0.99 f | 11.55 ± 0.96 f | 14.38 ± 2.27 e |
| Luteolin-7-rutinoside | 26.75 ± 0.84 b | 6.82 ± 0.69 e | 13.48 ± 2.24 d | 19.93 ± 2.86 c | 87.40 ± 6.58 a | 5.56 ± 0.83 f | 3.97 ± 0.19 h | 2.36 ± 0.48 i | 5.58 ± 1.23 f | 5.24 ± 0.62 g |
| Flavon-3-ols | ||||||||||
| (+)-Catechin | 41.05 ± 2.20 b | 34.14 ± 5.67 c | 22.59 ± 3.15 f | 30.42 ± 4.79 d | 69.71 ± 5.63 a | 27.05 ± 3.07 e | 11.19 ± 1.84 bh | 10.41 ± 2.89 i | 14.33 ± 2.13 g | 11.10 ± 0.28 h |
| (−)-Epicatechin | 361.79 ± 43.14 c | 200.45 ± 11.24 e | 456.71 ± 14.00 a | 236.15 ± 12.65 d | 393.15 ± 34.47 b | 23.79 ± 3.16 g | 17.29 ± 3.05 j | 22.99 ± 1.45 h | 44.90 ± 5.27 f | 21.00 ± 2.55 i |
| Procyanidin B1 | 199.42 ± 32.90 a | 122.20 ± 33.23 d | 157.56 ± 27.50 b | 144.40 ± 8.39 c | 198.04 ± 9.87 a | 144.10 ± 8.03 c | 29.83 ± 7.68 f | 20.72 ± 4.14 g | 35.97 ± 2.99 e | 28.25 ± 4.10 f |
| Procyanidin C1 | 163.73 ± 5.19 c | 113.92 ± 2.98 f | 289.24 ± 27.60 a | 128.20 ± 5.75 d | 255.18 ± 7.93 b | 116.70 ± 1.56 e | 19.10 ± 1.29 dh | 12.39 ± 1.71 j | 36.90 ± 1.00 g | 18.44 ± 2.32 i |
| Procyanidin B2 | 262.22 ± 16.58 c | 208.17 ± 11.48 d | 465.27 ± 32.68 a | 207.20 ± 8.20 d | 336.95 ± 11.67 b | 157.52 ± 12.54 e | 34.03 ± 3.57 g | 29.14 ± 2.41 i | 55.19 ± 3.59 f | 32.84 ± 3.66 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Česonienė, L.; Januškevičė, V.; Saunoriūtė, S.; Liaudanskas, M.; Žvikas, V.; Krikštolaitis, R.; Viškelis, P.; Urbonavičienė, D.; Martusevičė, P.; Zych, M.; et al. Phenolic Compounds in Berries of Winter-Resistant Actinidia arguta Miq. and Actinidia kolomikta Maxim.: Evidence of Antioxidative Activity. Antioxidants 2024, 13, 372. https://doi.org/10.3390/antiox13030372
Česonienė L, Januškevičė V, Saunoriūtė S, Liaudanskas M, Žvikas V, Krikštolaitis R, Viškelis P, Urbonavičienė D, Martusevičė P, Zych M, et al. Phenolic Compounds in Berries of Winter-Resistant Actinidia arguta Miq. and Actinidia kolomikta Maxim.: Evidence of Antioxidative Activity. Antioxidants. 2024; 13(3):372. https://doi.org/10.3390/antiox13030372
Chicago/Turabian StyleČesonienė, Laima, Viktorija Januškevičė, Sandra Saunoriūtė, Mindaugas Liaudanskas, Vaidotas Žvikas, Ričardas Krikštolaitis, Pranas Viškelis, Dalia Urbonavičienė, Paulina Martusevičė, Marcin Zych, and et al. 2024. "Phenolic Compounds in Berries of Winter-Resistant Actinidia arguta Miq. and Actinidia kolomikta Maxim.: Evidence of Antioxidative Activity" Antioxidants 13, no. 3: 372. https://doi.org/10.3390/antiox13030372
APA StyleČesonienė, L., Januškevičė, V., Saunoriūtė, S., Liaudanskas, M., Žvikas, V., Krikštolaitis, R., Viškelis, P., Urbonavičienė, D., Martusevičė, P., Zych, M., Daubaras, R., Balčiūnaitienė, A., & Viškelis, J. (2024). Phenolic Compounds in Berries of Winter-Resistant Actinidia arguta Miq. and Actinidia kolomikta Maxim.: Evidence of Antioxidative Activity. Antioxidants, 13(3), 372. https://doi.org/10.3390/antiox13030372