Effects of Electron Beam Radiation on the Phenolic Composition and Bioactive Properties of Olive Pomace Extracts
Abstract
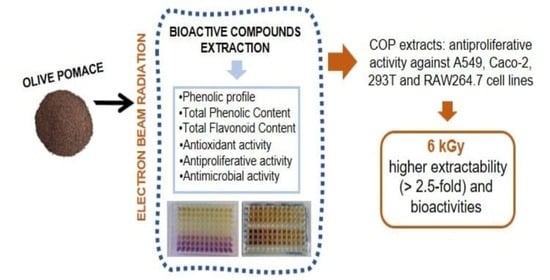
:1. Introduction
2. Materials and Methods
2.1. Olive Pomace Samples
2.2. Irradiation Experiments
2.3. Phenolic Compounds Extraction
2.4. Evaluation of Bioactive Properties
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.4.3. Antioxidant Activity
2.4.4. Antimicrobial Activity
Antibacterial Activity
Antifungal Activity
2.4.5. Cytotoxicity Assay—WST-1 Proliferation Test
2.5. Analysis of Phenolic Compounds
2.6. Statistical Analysis
3. Results and Discussion
3.1. Bioactive Properties of Olive Pomace Extracts
3.1.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
3.1.2. Antioxidant Activity
3.1.3. Antimicrobial Activity
3.1.4. Antiproliferative Activity
3.2. Phenolic Profile Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madureira, J.; Dias, M.I.; Pinela, J.; Calhelha, R.C.; Barros, L.; Santos-Buelga, C.; Margaça, F.M.A.; Ferreira, I.C.F.R.; Cabo Verde, S. The Use of Gamma Radiation for Extractability Improvement of Bioactive Compounds in Olive Oil Wastes. Sci. Total Environ. 2020, 727, 138706. [Google Scholar] [CrossRef] [PubMed]
- Malapert, A.; Reboul, E.; Loonis, M.; Dangles, O.; Tomao, V. Direct and Rapid Profiling of Biophenols in Olive Pomace by UHPLC-DAD-MS. Food Anal. Methods 2018, 11, 1001–1010. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Guillén, R.; Jiménez, A. Extraction of Interesting Organic Compounds from Olive Oil Waste. Grasas Y Aceites 2006, 57, 95–106. [Google Scholar] [CrossRef]
- Böhmer-Maas, B.W.; Otero, D.M.; Zambiazi, R.C.; Aranha, B.C. Optimization of the Extraction of Phenolic Compounds from Olive Pomace Using Response Surface Methodology. Rev. Ceres 2020, 67, 181–190. [Google Scholar] [CrossRef]
- Vitali Čepo, D.; Albahari, P.; Zovko Končić, M.; Radić, K.; Jurmanović, S.; Jug, M. Solvent Extraction and Chromatographic Determination of Polyphenols in Olive Pomace. Food Health Dis. 2017, 6, 7–14. [Google Scholar]
- Zuorro, A. Modelling of Polyphenol Recovery from Olive Pomace by Response Surface Methodology. Int. Rev. Model. Simul. 2014, 7, 1023–1028. [Google Scholar] [CrossRef]
- Suárez, M.; Romero, M.-P.; Motilva, M.-J. Development of a Phenol-Enriched Olive Oil with Phenolic Compounds from Olive Cake. J. Agric. Food Chem. 2010, 58, 10396–10403. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Heredia, A.; Guillén, R.; Jiménez, A. Production in Large Quantities of Highly Purified Hydroxytyrosol from Liquid−Solid Waste of Two-Phase Olive Oil Processing or “Alperujo”. J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef]
- Sygouni, V.; Pantziaros, A.G.; Iakovides, I.C.; Sfetsa, E.; Bogdou, P.I.; Christoforou, E.A.; Paraskeva, C.A. Treatment of Two-Phase Olive Mill Wastewater and Recovery of Phenolic Compounds Using Membrane Technology. Membranes 2019, 9, 27. [Google Scholar] [CrossRef]
- Peralbo-Molina, Á.; Priego-Capote, F.; Luque De Castro, M.D. Tentative Identification of Phenolic Compounds in Olive Pomace Extracts Using Liquid Chromatography-Tandem Mass Spectrometry with a Quadrupole- Quadrupole-Time-of-Flight Mass Detector. J. Agric. Food Chem. 2012, 60, 11542–11550. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound Increases the Aqueous Extraction of Phenolic Compounds with High Antioxidant Activity from Olive Pomace. LWT Food Sci. Technol. 2018, 89, 284–290. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Deng, Y.; Wang, X.; Cheng, J. Enhanced Extraction of Hydroxytyrosol, Maslinic Acid and Oleanolic Acid from Olive Pomace: Process Parameters, Kinetics and Thermodynamics, and Greenness Assessment. Food Chem. 2019, 276, 662–674. [Google Scholar] [CrossRef]
- Pavez, I.C.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an Extract Rich in Phenolic Compounds from Olive Pomace by Pressurized Liquid Extraction. Molecules 2019, 24, 3108. [Google Scholar] [CrossRef]
- Caballero, A.S.; Romero-García, J.M.; Castro, E.; Cardona, C.A. Supercritical Fluid Extraction for Enhancing Polyphenolic Compounds Production from Olive Waste Extracts. J. Chem. Technol. Biotechnol. 2020, 95, 356–362. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive Pomace as a Valuable Source of Bioactive Compounds: A Study Regarding Its Lipid- and Water-Soluble Components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial Effects of the Olive Oil Phenolic Components Oleuropein and Hydroxytyrosol: Focus on Protection against Cardiovascular and Metabolic Diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef]
- De las Hazas, M.C.L.; Rubio, L.; Macia, A.; Motilva, M.J. Hydroxytyrosol: Emerging Trends in Potential Therapeutic Applications. Curr. Pharm. Des. 2018, 24, 2157–2179. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Shamshoum, H.; Vlavcheski, F.; Tsiani, E. Anticancer Effects of Oleuropein. BioFactors 2017, 43, 517–528. [Google Scholar] [CrossRef]
- Warleta, F.; Quesada, C.S.; Campos, M.; Allouche, Y.; Beltrán, G.; Gaforio, J.J. Hydroxytyrosol Protects against Oxidative DNA Damage in Human Breast Cells. Nutrients 2011, 3, 839–857. [Google Scholar] [CrossRef]
- Buchalla, R.; Schüttler, C.; Bögl, K.W. Radiation Sterilization of Medical Devices. Effects of Ionizing Radiation on Ultra-High Molecular-Weight Polyethylene. Radiat. Phys. Chem. 1995, 46, 579–585. [Google Scholar] [CrossRef]
- Casimiro, M.H.; Ferreira, L.M.; Leal, J.P.; Pereira, C.C.L.; Monteiro, B. Ionizing Radiation for Preparation and Functionalization of Membranes and Their Biomedical and Environmental Applications. Membranes 2019, 9, 163. [Google Scholar] [CrossRef]
- Nunes, I.; Mesquita, N.; Cabo Verde, S.; Carolino, M.M.; Portugal, A.; Botelho, M.L. Bioburden Assessment and Gamma Radiation Inactivation Patterns in Parchment Documents. Radiat. Phys. Chem. 2013, 88, 82–89. [Google Scholar] [CrossRef]
- Borrely, S.I.; Morais, A.V.; Rosa, J.M.; Badaró-Pedroso, C.; Pereira, C.; Higa, M.C. Decoloration and Detoxification of Effluents by Ionizing Radiation. Radiat. Phys. Chem. 2016, 124, 1–5. [Google Scholar] [CrossRef]
- Hina, H.; Nafees, M.; Ahmad, T. Treatment of Industrial Wastewater with Gamma Irradiation for Removal of Organic Load in Terms of Biological and Chemical Oxygen Demand. Heliyon 2021, 7, e05972. [Google Scholar] [CrossRef]
- Barkaoui, S.; Madureira, J.; Santos, P.M.P.; Margaça, F.M.A.; Miloud, N.B.; Mankai, M.; Boudhrioua, N.M.; Cabo Verde, S. Effect of Ionizing Radiation and Refrigeration on the Antioxidants of Strawberries. Food Bioproc Tech. 2020, 13, 1516–1527. [Google Scholar] [CrossRef]
- Cabo Verde, S.; Trigo, M.J.; Sousa, M.B.; Ferreira, A.; Ramos, A.C.; Nunes, I.; Junqueira, C.; Melo, R.; Santos, P.M.P.; Botelho, M.L. Effects of Gamma Radiation on Raspberries: Safety and Quality Issues. J. Toxicol. Environ. Health A 2013, 76, 291–303. [Google Scholar] [CrossRef]
- Guerreiro, D.; Madureira, J.; Silva, T.; Melo, R.; Santos, P.M.P.; Ferreira, A.; Trigo, M.J.; Falcão, A.N.; Margaça, F.M.A.; Cabo Verde, S. Post-Harvest Treatment of Cherry Tomatoes by Gamma Radiation: Microbial and Physicochemical Parameters Evaluation. Innov. Food Sci. Emerg. Technol. 2016, 36, 1–9. [Google Scholar] [CrossRef]
- Antonio, A.L.; Fernandes, Â.; Barreira, J.C.M.; Bento, A.; Botelho, M.L.; Ferreira, I.C.F.R. Influence of Gamma Irradiation in the Antioxidant Potential of Chestnuts (Castanea Sativa Mill.) Fruits and Skins. Food Chem. Toxicol. 2011, 49, 1918–1923. [Google Scholar] [CrossRef]
- Madureira, J.; Pimenta, A.I.; Popescu, L.; Besleaga, A.; Dias, M.I.; Santos, P.M.P.; Melo, R.; Ferreira, I.C.F.R.; Cabo Verde, S.; Margaça, F.M.A. Effects of Gamma Radiation on Cork Wastewater: Antioxidant Activity and Toxicity. Chemosphere 2017, 169, 139–145. [Google Scholar] [CrossRef]
- Pereira, E.; Pimenta, A.I.; Calhelha, R.C.; António, A.L.; Barros, L.; Santos-Buelga, C.; Cabo Verde, S.; Ferreira, I.C.F.R. Infusions of Gamma Irradiated Aloysia Citrodora L. and Mentha x Piperita L.: Effects on Phenolic Composition, Cytotoxicity, Antibacterial and Virucidal Activities. Ind. Crops Prod. 2017, 97, 582–590. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef]
- Miller, A. Dosimetry for Electron Beam Application; Risø National Laboratory: Roskilde, Denmark, 1984; Volume 16. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzym. 1998, 299, 152–178. [Google Scholar] [CrossRef]
- Barkaoui, S.; Mankai, M.; Miloud, N.B.; Kraïem, M.; Madureira, J.; Cabo Verde, S.; Boudhrioua, N. Effect of Gamma Radiation Coupled to Refrigeration on Antioxidant Capacity, Sensory Properties and Shelf Life of Strawberries. LWT Food Sci. Technol. 2021, 150, 112088. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic Profile and Antioxidant Activity of Coleostephus myconis (L.) Rchb.f.: An Underexploited and Highly Disseminated Species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Ammar, S.; Contreras, M.d.M.; Gargouri, B.; Segura-Carretero, A.; Bouaziz, M. RP-HPLC-DAD-ESI-QTOF-MS Based Metabolic Profiling of the Potential Olea Europaea by-Product “Wood” and Its Comparison with Leaf Counterpart. Phytochem. Anal. 2017, 28, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, A.; Pati, S.; Minervini, F.; D’Antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, Isoverbascoside, and Their Derivatives Recovered from Olive Mill Wastewater as Possible Food Antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef]
- Kabbash, E.M.; Abdel-Shakour, Z.T.; El-Ahmady, S.H.; Wink, M.; Ayoub, I.M. Comparative Metabolic Profiling of Olive Leaf Extracts from Twelve Different Cultivars Collected in Both Fruiting and Flowering Seasons. Sci. Rep. 2023, 13, 1–13. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Chemical Screening of Olive Biophenol Extracts by Hyphenated Liquid Chromatography. Anal. Chim. Acta 2007, 603, 176–189. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; Contreras, M.D.M.; Carvalheiro, F.; Duarte, L.C.; Roseiro, L.B.; Romero, I.; Castro, E. Recovery of Bioactive Compounds from Industrial Exhausted Olive Pomace through Ultrasound-Assisted Extraction. Biology 2021, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.R.; Anwar, M.M.; Sallam, E.M. Improving Quality and Shelf-Life of Minced Beef Using Irradiated Olive Leaf Extract. J. Food Process Preserv. 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Harrison, K.; Were, L.M. Effect of Gamma Irradiation on Total Phenolic Content Yield and Antioxidant Capacity of Almond Skin Extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- Hussain, P.R.; Suradkar, P.; Javaid, S.; Akram, H.; Parvez, S. Influence of Postharvest Gamma Irradiation Treatment on the Content of Bioactive Compounds and Antioxidant Activity of Fenugreek (Trigonella foenum-graceum L.) and Spinach (Spinacia oleracea L.) Leaves. Innov. Food Sci. Emerg. Technol. 2016, 33, 268–281. [Google Scholar] [CrossRef]
- Hussain, P.R.; Wani, A.M.; Meena, R.S.; Dar, M.A. Gamma Irradiation Induced Enhancement of Phenylalanine Ammonia-Lyase (PAL) and Antioxidant Activity in Peach (Prunus persica Bausch, Cv. Elberta). Radiat. Phys. Chem. 2010, 79, 982–989. [Google Scholar] [CrossRef]
- Brenes, M.; Medina, E.; Romero, C.; De Castro, A. Antimicrobial Activity of Olive Oil. Agro Food Ind. Hi Tech 2007, 18, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Brenes, M.; Romero, C.; García, A.; De Castro, A. Main Antimicrobial Compounds in Table Olives. J. Agric. Food Chem. 2007, 55, 9817–9823. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Ferreira, I.C.F.R.; Calhelha, R.; Andrade, P.B.; Valentão, P.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolics and Antimicrobial Activity of Traditional Stoned Table Olives “Alcaparra”. Bioorg. Med. Chem. 2006, 14, 8533–8538. [Google Scholar] [CrossRef]
- Markin, D.; Duek, L.; Berdicevsky, I. In Vitro Antimicrobial Activity of Olive Leaves. Mycoses 2003, 46, 132–136. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]
- Yakhlef, W.; Arhab, R.; Romero, C.; Brenes, M.; de Castro, A.; Medina, E. Phenolic Composition and Antimicrobial Activity of Algerian Olive Products and By-Products. LWT Food Sci. Technol. 2018, 93, 323–328. [Google Scholar] [CrossRef]
- Anter, J.; Tasset, I.; Demyda-Peyrás, S.; Ranchal, I.; Moreno-Millán, M.; Romero-Jimenez, M.; Muntané, J.; Luque de Castro, M.D.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Evaluation of Potential Antigenotoxic, Cytotoxic and Proapoptotic Effects of the Olive Oil by-Product “Alperujo”, Hydroxytyrosol, Tyrosol and Verbascoside. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 772, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Carrasco-Pancorbo, A.; Simal-Gándara, J.; Giampieri, F.; et al. Characterization of Phenolic Extracts from Brava Extra Virgin Olive Oils and Their Cytotoxic Effects on MCF-7 Breast Cancer Cells. Food Chem. Toxicol. 2018, 119, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez-Román, D.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Pérez-Sánchez, A.; Herrero, M.; Ibañez, E.; Micol, V.; Zarrouk, M.; Segura-Carretero, A.; et al. Use of Advanced Techniques for the Extraction of Phenolic Compounds from Tunisian Olive Leaves: Phenolic Composition and Cytotoxicity against Human Breast Cancer Cells. Food Chem. Toxicol. 2012, 50, 1817–1825. [Google Scholar] [CrossRef]
- Madureira, J.; Albuquerque, B.; Dias, M.I.; Pinela, J.; Calhelha, R.C.; Santos-Buelga, C.; Margaça, F.M.A.; Ferreira, I.C.F.R.; Cabo Verde, S.; Barros, L. Ultrasound-Assisted Extraction of Hydroxytyrosol and Tyrosol from Olive Pomace Treated by Gamma Radiation: Process Optimization and Bioactivity Assessment. Food Funct. 2023, 14, 3038–3050. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Romero, M.-P.; Ramo, T.; Macià, A.; Motilva, M.-J. Methods for Preparing Phenolic Extracts from Olive Cake for Potential Application as Food Antioxidants. J. Agric. Food Chem. 2009, 57, 1463–1472. [Google Scholar] [CrossRef]
- Sánchez De Medina, V.; Priego-Capote, F.; Jiménez-Ot, C.; Luque De Castro, M.D. Quality and Stability of Edible Oils Enriched with Hydrophilic Antioxidants from the Olive Tree: The Role of Enrichment Extracts and Lipid Composition. J. Agric. Food Chem. 2011, 59, 11432–11441. [Google Scholar] [CrossRef]



| Total Phenolic Content (mg GAE/g Extract) | Total Flavonoid Content (mg CAE/g Extract) | FRAP (mmol FES/g Extract) | DPPH (IC50, µg/mL) | |
|---|---|---|---|---|
| COP samples | ||||
| 0 kGy | 71 ± 1 b | 131 ± 1 b | 1.46 ± 0.01 b,c | 480 ± 9 b,c |
| 6 kGy | 95 ± 3 a | 143 ± 1 a | 1.78 ± 0.04 a | 545 ± 13 a |
| 11 kGy | 86 ± 2 a | 132 ± 2 b | 1.51 ± 0.02 b | 500 ± 10 b |
| EOP samples | ||||
| 0 kGy | 67.8± 0.8 b,c | 115 ± 1 c,d | 1.40 ± 0.02 c,d | 561 ± 9 a |
| 6 kGy | 60 ± 2 c | 112 ± 1 d | 1.41 ± 0.02 b,c,d | 440 ± 9 c |
| 11 kGy | 70 ± 3 b | 118 ± 1 c | 1.35 ± 0.02 d | 462 ± 5 b,c |
| B. cereus | S. aureus | L. monocytogenes | E. faecalis | E. coli | S. Typhymurium | P. fluorescens | A. section Nigri | A. fumigatus | C. albicans | |
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/mL) | ||||||||||
| COP samples | ||||||||||
| 0 kGy | 20 | 20 | 20 | >60 | 60 | 60 | 60 | >100 | >100 | >100 |
| 6 kGy | 20 | 20 | 20 | >60 | 60 | 60 | 60 | >100 | >100 | >100 |
| 11 kGy | 20 | 20 | 20 | >60 | 60 | 60 | 60 | >100 | >100 | >100 |
| EOP samples | ||||||||||
| 0 kGy | 20 | >60 | 20 | >60 | 60 | 60 | 60 | >100 | >100 | >100 |
| 6 kGy | 20 | >60 | 20 | >60 | 60 | 60 | 60 | >100 | >100 | >100 |
| 11 kGy | 20 | >60 | 20 | >60 | 60 | 60 | 60 | >100 | >100 | >100 |
| MBC (mg/mL) | MFC (mg/mL) | |||||||||
| COP samples | ||||||||||
| 0 kGy | 20 | 40 | 60 | >60 | 60 | 100 | 60 | >100 | >100 | >100 |
| 6 kGy | 20 | 40 | 60 | >60 | 60 | 100 | 60 | >100 | >100 | >100 |
| 11 kGy | 20 | 40 | 60 | >60 | 60 | 100 | 60 | >100 | >100 | >100 |
| EOP samples | ||||||||||
| 0 kGy | 20 | >60 | >60 | >60 | >100 | >100 | >100 | >100 | >100 | >100 |
| 6 kGy | 20 | >60 | >60 | >60 | >100 | >100 | >100 | >100 | >100 | >100 |
| 11 kGy | 20 | >60 | >60 | >60 | >100 | >100 | >100 | >100 | >100 | >100 |
| Peak | Rt (min) | λmax (nm) | Molecular Ion [M-H] (m/z) | MS 2 (m/z) | MS 3 (m/z) | Tentative Identification |
|---|---|---|---|---|---|---|
| 1 | 4.26 | 277 | 315 | Hydroxytyrosol-1-β-glucoside | ||
| 2 | 5.38 | 281 | 153 | 123(100) | Hydroxytyrosol | |
| 3 | 7.86 | 220, 277 | 137 | Tyrosol | ||
| 4 | 11.93 | 278, 324 | 753 | 639(100) | 621(100), 529(57), 487(62), 459(10) | β-Hydroxyverbascoside isomer 1 |
| 5 | 12.36 | 282, 321 | 753 | 639(100) | 621(100), 529(6) | β-Hydroxyverbascoside isomer 2 |
| 6 | 16.99 | 292, 329 | 737 | 623(100) | 461(100) | Verbascoside |
| 7 | 18.96 | 234, 283, 326 | 707 | 593(100) | 447(52), 285(100) | Luteolin-7-O-rutinoside |
| 8 | 19.32 | 234, 284, 329 | 815 | 701(100) | 377(100), 307(41), 275(29) | Oleuropein-O-hexoside |
| 9 | 19.7 | 236, 285, 324 | 737 | 623(100) | 461(100) | Isoverbascoside |
| 10 | 22.5 | 239, 281, 323 | 779 | 665(100) | 623(100), 503(32), 461(49), 443(24) | Acetylverbascoside derivative |
| Compound | Quantification (mg/g Extract) | |||||
|---|---|---|---|---|---|---|
| COP | EOP | |||||
| 0 kGy | 6 kGy | 11 kGy | 0 kGy | 6 kGy | 11 kGy | |
| Hydroxytyrosol-1-β-glucoside 1 | 3.7 ± 0.1 b | 9 ± 1 a | 9.4 ± 0.4 a | 3.6 ± 0.4 b | 3.2 ± 0.4 b | 2.7 ± 0.3 b |
| Hydroxytyrosol 1 | 10.1 ± 0.2 b,c | 26 ± 2 a | 27.1 ± 0.4 a | 11.06 ± 0.43 b | 9 ± 1 b,c | 7.9 ± 0.5 c |
| Tyrosol 2 | 2.43 ± 0.05 b | 6.9 ± 0.3 a | 7.7 ± 0.3 a | 2.7 ± 0.2 b | 2.2 ± 0.1 b | 1.95 ± 0.09 b |
| β-Hydroxyverbascoside isomer 1 3 | 0.65 ± 0.01 b | 1.7 ± 0.1 a | 1.8 ± 0.1 a | 0.29 ± 0.01 c | 0.25 ± 0.02 c | 0.2 ± 0.01 c |
| β-Hydroxyverbascoside isomer 2 3 | 0.70 ± 0.01 b | 1.88 ± 0.08 a | 2.08 ± 0.09 a | 0.30 ± 0.01 c | 0.26 ± 0.02 c | 0.25 ± 0.01 c |
| Verbascoside 3 | 1.73 ± 0.06 b | 4 ± 1 a | 4.85 ± 0.09 a | 1.15 ± 0.03 b,c | 0.89 ± 0.08 c | 0.81 ± 0.05 c |
| Luteolin-7-O-rutinoside 4 | 1.198 ± 0.003 b | 1.76 ± 0.01 a | 1.99 ± 0.10 a | 1.18 ± 0.01 b | 1.12 ± 0.02 b | 1.10 ± 0.02 b |
| Oleuropein-O-hexoside 5 | 1.15 ± 0.07 c | 2.9 ± 0.2 b | 3.7 ± 0.2 a | 1.34 ± 0.06 c | 1.05 ± 0.08 c | 1.05 ± 0.08 c |
| Isoverbascoside 3 | 0.24 ± 0.01 d | 0.546 ± 0.003 b | 0.68 ± 0.03 a | 0.44 ± 0.01 b,c | 0.32 ± 0.03 c,d | 0.32 ± 0.02 d |
| Acetylverbascoside derivative 3 | 0.25 ± 0.02 b | 0.6 ± 0.1 a | 0.72 ± 0.02 a | 0.246 ± 0.001 b | 0.20 ± 0.02 b | 0.19 ± 0.02 b |
| Total phenylethanoid derivatives | 21 ± 1 b | 53 ± 7 a | 58 ± 2 a | 21 ± 2 b | 17 ± 2 b | 15 ± 2 b |
| Total flavonoids | 1.198 ± 0.005 b | 1.76 ± 0.01 a | 1.99 ± 0.17 a | 1.18 ± 0.01 b | 1.12 ± 0.03 b | 1.10 ± 0.04 b |
| Total phenolic compounds | 22 ± 1 b | 55 ± 7 a | 60 ± 2 a | 22 ± 2 b | 18 ± 2 b | 17 ± 2 b |
| This Study | Suárez et al., 2009 [57] | Sánchez de Medina et al., 2011 [58] | ||
|---|---|---|---|---|
| Crude olive pomace, 6 kGy | Solid–liquid extraction at atmospheric pressure | Solid–liquid extraction at high pressure | Microwave irradiation | |
| Hydroxytyrosol | 26 | 2.79 | 2.54 | 0.11 |
| Tyrosol | 6.9 | 0.32 | 0.09 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madureira, J.; Gonçalves, I.; Cardoso, J.; Dias, M.I.; Santos, P.M.P.; Margaça, F.M.A.; Santos-Buelga, C.; Barros, L.; Cabo Verde, S. Effects of Electron Beam Radiation on the Phenolic Composition and Bioactive Properties of Olive Pomace Extracts. Antioxidants 2024, 13, 558. https://doi.org/10.3390/antiox13050558
Madureira J, Gonçalves I, Cardoso J, Dias MI, Santos PMP, Margaça FMA, Santos-Buelga C, Barros L, Cabo Verde S. Effects of Electron Beam Radiation on the Phenolic Composition and Bioactive Properties of Olive Pomace Extracts. Antioxidants. 2024; 13(5):558. https://doi.org/10.3390/antiox13050558
Chicago/Turabian StyleMadureira, Joana, Inês Gonçalves, Jéssica Cardoso, Maria Inês Dias, Pedro M. P. Santos, Fernanda M. A. Margaça, Celestino Santos-Buelga, Lillian Barros, and Sandra Cabo Verde. 2024. "Effects of Electron Beam Radiation on the Phenolic Composition and Bioactive Properties of Olive Pomace Extracts" Antioxidants 13, no. 5: 558. https://doi.org/10.3390/antiox13050558
APA StyleMadureira, J., Gonçalves, I., Cardoso, J., Dias, M. I., Santos, P. M. P., Margaça, F. M. A., Santos-Buelga, C., Barros, L., & Cabo Verde, S. (2024). Effects of Electron Beam Radiation on the Phenolic Composition and Bioactive Properties of Olive Pomace Extracts. Antioxidants, 13(5), 558. https://doi.org/10.3390/antiox13050558