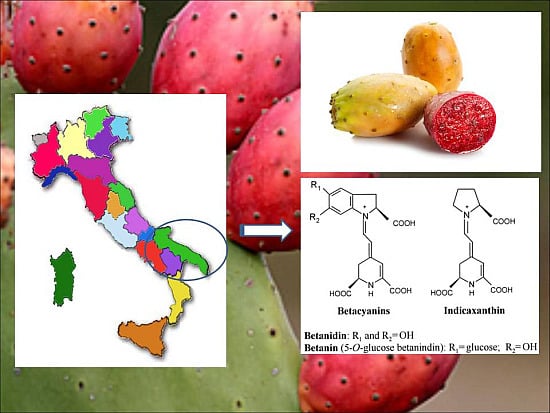
Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes
Abstract
:1. Introduction

2. Experimental Section
2.1. Sample Material, Standards and Chemical Reagents
2.2. Physico-Chemical Parameters
2.3. Fruit Extract Preparation
2.4. Phenols, Vitamin C and Antioxidant Capacity: Assessment of O. Ficus-Indica (L.) Fruit Extracts
2.5. LC/MS Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Parameters
| Variety | pH | DW (%) | TSS (°Brix) | Sucrose/Glucose/Fructose (g/100 g FW) |
|---|---|---|---|---|
| Purple | 5.89 ± 0.24 | 16.70 ± 1.20 | 12.67 ± 0.15 | ND/1.88 ± 0.04/0.78 ± 0.01 |
| Orange | 6.02 ± 0.01 | 16.10 ± 0.48 | 12.10 ± 0.14 | ND/2.14 ± 0.10/1.04 ± 0.12 |
| Significance | n.s. | n.s. | * | n.s. |
3.2. Antioxidant Capacity of Hydrophilic Cactus Pear Extract
| Betacyanin 1 | Vitamin C | Total Phenols | TEAC | ORAC | |
|---|---|---|---|---|---|
| Variety | mg/100 g FW | mg/100 g FW | mg GAE/100 g FW | mmol/100 g FW | mmol/100 g FW |
| Purple | 39.3 ± 5.2 | 36.6 ± 1.5 | 89.2 ± 3.6 | 0.61 ± 0.02 | 1.28 ± 0.02 |
| Orange | 3.6 ± 0.9 | 30.2 ± 0.3 | 69.8 ± 1.7 | 0.37 ± 0.02 | 0.98 ± 0.01 |
| Significance | *** | * | ** | *** | n.s. |
3.3. Quantification of Betanin in Cactus Pear Fruit Extracts

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stintzing, F.C.; Schieber, A.; Carle, R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur. Food Res. Technol. 2003, 216, 303–311. [Google Scholar]
- Barbera, G. History, economics and agro-ecological importance. In Agro-Ecology, Cultivation and Uses of Cactus Pear; Barbera, G., Inglese, P., Pimienta, E., Eds.; FAO Plant Production and Protection: Rome, Italy, 1995; Volume 132, pp. 1–11. [Google Scholar]
- Mondragón-Jacobo, C. Cactus pear breeding and domestication. Plant Breed. Rev. 2001, 20, 135–166. [Google Scholar]
- Caruso, M.; Currò, S.; Las Casas, G.; La Malfa, S.; Gentile, A. Microsatellite markers help to assess genetic diversity among Opuntia fiicus indica cultivated genotypes and their relation with related species. Plant Syst. Evol. 2010, 290, 85–97. [Google Scholar] [CrossRef]
- Piga, A.; del Caro, A.; Pinna, I.; Agabbio, M. Changes in ascorbic acid, polyphenol content and antioxidant activity in minimally processed cactus pear fruits. LWT—Food Sci. Technol. 2003, 36, 257–262. [Google Scholar] [CrossRef]
- Cefola, M.; Renna, M.; Pace, B. Marketability of ready-to-eat cactus pear as affected by temperature and modified atmosphere. J. Food Sci. Technol. 2014, 51, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Schieber, A.; Carle, R. Phytochemical and nutritional significance of cactus pear. Eur. Food Res. Technol. 2001, 212, 396–407. [Google Scholar] [CrossRef]
- Magloire, J.F.; Konarski, P.; Zou, D.; Stintzing, F.C.; Zou, C. Nutritional and medicinal uses of cactus pear (Opuntia sp.) cladodes and fruits. Front. Biosci. 2006, 11, 2574–2589. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Mondello, M.R.; Giuffrida, D.; Dugo, G.; Miceli, N.; Pergolizzi, S.; Taviano, M.F. Chemical characterization and biological effects of Sicilian Opuntia ficus-indica (L.) Mill. fruit juice: Antioxidant and antiulcerogenic activity. J. Agric. Food Chem. 2003, 51, 4903–4908. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Butera, D.; Pintaudi, A.M.; Allegra, M.; Livrea, M.A. Supplementation with cactus pear (Opuntia ficus-indica) fruit decreases oxidative stress in healthy humans: A comparative study with vitamic C. Am. J. Clin. Nutr. 2004, 80, 391–395. [Google Scholar] [PubMed]
- Zou, D.-M.; Brewer, M.; Garcia, F.; Feugang, J.M.; Wang, J.; Zang, R.; Liu, H.; Zou, C. Cactus pear: A natural product in cancer chemoprevention. Nutr. J. 2005, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kaur, A.; Sharma, R. Pharmacological actions of Opuntia ficus indica: A review. J. Appl. Pharm. Sci. 2012, 2, 15–18. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; García-Melo, F.; Morales-González, J.A.; Vázquez-Alvarado, P.; Muñoz-Juárez, S.; Zuñiga-Pérez, C.; Sumaya-Martínez, M.T.; Madrigal-Buiaidar, E.; Hernández-Ceruelos, A. Antioxidant and anticlastogenic capacity of prickly pear juice. Nutrients 2013, 5, 4145–4158. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin—A food colorant with biological activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- El-Mostafa, K.; El Karrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hameed, E.-S.S.; Nagaty, M.A.; Salman, M.S.; Bazaid, S.A. Phytochemicals, nutritionals and antioxidant properties of two prickly pear cactus cultivars (Opuntia ficus-indica Mill.) growing in Taif, KSA. Food Chem. 2014, 160, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cejudo-Bastante, M.J.; Chaalal, M.; Louaileche, H.; Parrado, J.; Heredia, F.J. Betalain profile, phenolic content and color characterization of different parts and varieties of Opuntia ficus-indica. J. Agric. Food Chem. 2014, 62, 8491–4499. [Google Scholar] [CrossRef] [PubMed]
- Matias, A.; Nunes, S.L.; Poejo, J.; Mecha, E.; Serra, A.T.; Amorim Madeira, P.J.; Bronze, M.R.; Duarte, C.M.M. Antioxidant and anti-inflammatory activity of a flavonoid-rich concentrate recovered from Opuntia ficus-indica juice. Food Funct. 2014, 5, 3269–3280. [Google Scholar] [CrossRef] [PubMed]
- ISTAT Data Bank. 2013. Available online: http://agri.istat.it/sag_is_pdwout/excel/Dw302013.xls (accessed on 5 February 2015).
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Rapid microplate high-throughput methodology for assessment of Folin-Ciocalteu reducing capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef]
- Paradiso, A.; Cecchini, C.; de Gara, L.; D’Egidio, M.G. Functional and rheological properties of dough from immature durum wheat. J. Cereal Sci. 2006, 43, 216–222. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Spirito, R.; Gerardi, C.; Durante, M.; Nicoletti, I. Nutraceutical properties in organic strawberry from South Italy. Acta Hort. 2012, 926, 683–690. [Google Scholar]
- Cai, Y.; Sun, M.; Corke, H. Identification and distribution of simple and acylated betacyanins in the Amaranthaceae. J. Agric. Food Chem. 2001, 49, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef] [PubMed]
- El Kossori, R.L.; Villaume, C.; El Boustani, E.; Sauvaire, Y.; Mejean, L. Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus-indica sp.). Plant Foods Hum. Nutr. 1998, 52, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ouelhazi, N.K.; Ghrir, R.; Diêp Le, K.H.; Lederer, F. Invertase from Opuntia ficus-indica fruits. Phytochemistry 1992, 31, 59–61. [Google Scholar] [CrossRef]
- Yeddes, N.; Cherif, J.K.; Guyot, S.; Sotin, H.; Ayadi, M.T. Comparative study of antioxidant power, polyphenols, flavonoids and betacyanins of the peel and pulp of three Tunisian Opuntia forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef]
- Yahia, E.M.; Mondragón-Jacobo, C. Nutritional components and anti-oxidant capacity of ten cultivars and lines of cactus pear fruit (Opuntia spp.). Food Res. Int. 2011, 44, 2311–2318. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, J.A.; Almela, L.; Obón, J.M.; Castellar, M.R. Determination of antioxidant constituents in cactus pear fruits. Plant Foods Hum. Nutr. 2010, 65, 253–259. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of Sicilian prickly pear (Opuntia ficus-indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [PubMed]
- Castellar, M.R.; Solano, F.; Obón, J.M. Betacyanin and other antioxidants production during growth of Opuntia stricta (Haw.) fruits. Plant Foods Hum. Nutr. 2012, 67, 337–343. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Niki, E. Antioxidant capacity: Which capacity and how to assess it? J. Berry Res. 2011, 1, 169–176. [Google Scholar]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Santiago, E.; Yahia, E.M. Identification and quantification of betalains from the fruits of 10 Mexican prickly pear cultivars by high-performance liquid chromatography and electrospray ionization mass spectrometry. J. Agric. Food Chem. 2008, 56, 5758–5764. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, M.; Wu, H.; Huang, R.; Corke, H. Characterization and quantification of betacyanin pigments from diverse Amaranthus species. J. Agric. Food Chem. 1998, 46, 2063–2070. [Google Scholar] [CrossRef]
- Patel, S. Reviewing the prospect of Opuntia pears as low cost functional foods. Rev. Environ. Sci. Biotechnol. 2013, 12, 223–234. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, C.; Negro, C.; Tommasi, N.; Gerardi, C.; Mita, G.; Miceli, A.; De Bellis, L.; Blando, F. Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes. Antioxidants 2015, 4, 269-280. https://doi.org/10.3390/antiox4020269
Albano C, Negro C, Tommasi N, Gerardi C, Mita G, Miceli A, De Bellis L, Blando F. Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes. Antioxidants. 2015; 4(2):269-280. https://doi.org/10.3390/antiox4020269
Chicago/Turabian StyleAlbano, Clara, Carmine Negro, Noemi Tommasi, Carmela Gerardi, Giovanni Mita, Antonio Miceli, Luigi De Bellis, and Federica Blando. 2015. "Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes" Antioxidants 4, no. 2: 269-280. https://doi.org/10.3390/antiox4020269
APA StyleAlbano, C., Negro, C., Tommasi, N., Gerardi, C., Mita, G., Miceli, A., De Bellis, L., & Blando, F. (2015). Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes. Antioxidants, 4(2), 269-280. https://doi.org/10.3390/antiox4020269