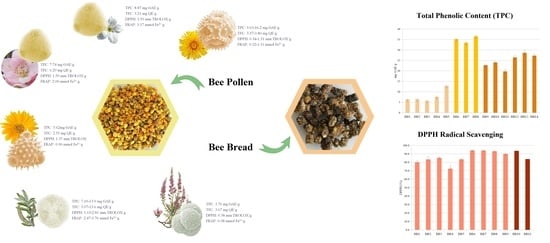
Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits
Abstract
:1. Introduction
2. Bee Collected Pollen (BP) Composition and Main Bioactivities
3. Bee Bread (BB) Composition and Main Bioactivities
4. Anti-Cancer Research with BP and BB and Its Bioactive Compounds
4.1. In Vitro Studies of BP and BB Correlated to the Bioactive Compounds
4.2. In Vivo Studies in Animal Models
Hepatoprotective Effects in Animal Experiments
4.3. Enzyme-Level Changes Induced by Bee Pollen
5. Potential of BP and BB in Complementary Therapies That Can Be Associated to Antineoplasic Treatments (Anxiety, Antinociceptive and Anti-Inflammatory Activities)
6. Clinical Trials
Chronic Prostatitis
7. Therapeutic Strategies for BP and BB and Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coşkun, T. Fonksiyonel Besinlerin Sağlığımız Üzerine Etkileri, Hacettepe Üniversitesi. Çocuk Sağlığı ve Hastalıkları Dergisi 2005, 48, 69–84. [Google Scholar]
- Giannetti, V.; Testani, E.; Recchia, L. Food consumption and innovation: Functional foods. J. Comm. Sci. Technol. Qual. 2009, 48, 213–225. [Google Scholar]
- Kıyak, S.N.; Dağlı, Y.; Zeren, Ü.; Arıburnu, M.; Gülbandılar, A.; Dönmez, M.; Okur, M. A Functional Food: “Şifalı Top”. Turk. J. Agric. Food Sci. Technol. 2014, 2, 277–279. [Google Scholar]
- Itatani, Y.; Kawada, K.; Sakai, Y. Treatment of Elderly Patients with Colorectal Cancer. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Falandry, C. Toxicity of Cancer Therapies in Older Patients. Curr. Oncol. Rep. 2018, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Fang, C.; Deng, D.; Xia, L. Research progress on common adverse events caused by targeted therapy for colorectal cancer. Oncol. Lett. 2018, 16, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.G.; Bento, C.; Zorrinho, I.; Leite, A.P. Chapter 9. Interactions between drugs and herbal teas. In Food-Drug Interact; Ramos, F., Vitoria, I., Caramona, M., Eds.; Nova Biomedical & Health, Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2018; pp. 207–225. ISBN 978-1-53613-553-4. [Google Scholar]
- Bobiș, O.; Mărghitaș, I.A.; Dezmirean, D.; Morar, O.; Bonta, V.; Chirilă, F. Quality Parameters and Nutritional Value of Different Commercial Bee Products. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2010, 67, 1–2. [Google Scholar]
- Mărgăoan, R.; Mărghitaș, L.A.; Dezmirean, D.S.; Dulf, F.V.; Bunea, A.; Socaci, S.A.; Bobiș, O. Predominant and secondary pollen botanical origins influence the carotenoid and fatty acid profile in fresh honeybee-collected pollen. J. Agric. Food Chem. 2014, 62, 6306–6316. [Google Scholar] [CrossRef] [PubMed]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee pollen: Chemical composition and therapeutic application. Evid. Based. Complement. Alternat. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.; Ellis, J.; O’Malley, M.; Nalen, C.Z. The Benefits of Pollen to Honey Bees; University of Florida: Gainesville, FL, USA, 2010; Available online: http://edis.ifas.ufl.edu/in868 (accessed on 1 January 2018).
- Alataş, İ.; Yalçın, L.İ.; ve Öztürk, A.İ. Arıcılıkta polen üretiminin koloni gelişimine ve bal verimine etkileri. Ege Tarımsal Araştırma Enstitüsü Dergisi 1997, 7, 30–42. [Google Scholar]
- Bogdanov, S. Pollen: Production, Nutrition and Health: A Review. Bee Prod. Sci. 2015, 2, 1–30. Available online: http://www.bee-hexagon.net/ (accessed on 15 August 2019).
- Nagai, T.; Nagashima, T.; Myoda, T.; Inoue, R. Preparation and functional properties of extracts from bee bread. Mol. Nutr. Food Res. 2004, 48, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Derochette, S.; Franck, T.; Mouithys-Mickalad, A.; Deby-Dupont, G.; Neven, P.; Serteyn, D. Intra- and extracellular antioxidant capacities of the new water-soluble form of curcumin (NDS27) on stimulated neutrophils and HL-60 cells. Chem. Biol. Interact. 2013, 201, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Rugendorff, E.W.; Weidner, W.; Ebeling, L.; Buck, A.C. Results of treatment with pollen extract (Cernilton®N) in chronic prostatitis and prostatodynia. BJU Int. 1993, 71, 433–438. [Google Scholar] [CrossRef]
- Shoskes, D.A. Phytotherapy in chronic prostatitis. Urology 2002, 60, 35–37. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Manickam, K. Herbal and complementary medicine in chronic prostatitis. Word. J. Urol. 2003, 21, 109–113. [Google Scholar] [CrossRef]
- Elist, J. Effects of pollen extract preparation Prostat/Poltit on lower urinary tract symptoms in patients with chronic nonbacterial prostatitis/chronic pelvic pain syndrome: A randomized, double-blind, placebo-controlled study. Urology 2006, 67, 60–63. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.H.; Schneider, M.; Ludwig, J.; Schnitker, E.; Brahler, W. Weidner. A pollen extract (Cernilton®) in patients with inflammatory chronic prostatitis-chronic pelvic pain syndrome: A multicentre, randomised, prospective, double-blind, placebo-controlled phase 3 study. Eur. Urol. 2009, 56, 544–551. [Google Scholar] [CrossRef]
- Li, Q.; Sun, M.; Wan, Z.; Liang, J.; Betti, M.; Hrynets, Y.; Wang, K. Bee Pollen Extracts Modulate Serum Metabolism in Lipopolysaccharide-induced Acute Lung Injury Mice with Anti-inflammatory Effects. J. Agric. Food Chem. 2019, 67, 7855–7868. [Google Scholar] [CrossRef]
- Al-Salem, H.S.; Bhat, R.S.; Al-Ayadhi, L.; El-Ansary, A. Therapeutic potency of bee pollen against biochemical autistic features induced through acute and sub-acute neurotoxicity. BMC Complement. Alternat. Med. 2016, 16, 120. [Google Scholar] [CrossRef]
- Velásquez, P.; Rodríguez, P.K.; Retamal, M.; Giordano, A.; Valenzuela, L.M.; Montenegro, G. Relation between composition, antioxidant and antibacterial activities and botanical origin of multi-floral bee pollen. J. Appl. Bot. Food Qual. 2017, 90, 306–314. [Google Scholar]
- Aličić, D.; Šubarić, D.; Jašić, M.; Pašalić, H.; Ačkar, Đ. Antioxidant properties of pollen. Hrana U Zdravlju I Bolesti 2014, 3, 6–12. [Google Scholar]
- Turhan, S.; Saricaoglu, F.T.; Mortas, M.; Yazici, F.; Genccelep, H. Evaluation of Color, Lipid Oxidation and Microbial Quality in Meatballs Formulated with Bee Pollen During Frozen Storage. J. Food Process. Preserv. 2017, 41, 1–8. [Google Scholar] [CrossRef]
- Basim, E.; Basim, H.; Ozcan, M. Antibacterial activities of Turkish pollen and propolis extracts against plant bacterial pathogens. J. Food Eng. 2006, 77, 992–996. [Google Scholar] [CrossRef]
- Gibriel, A.Y.; Abdeldaiem, M.H.; Ali, H.G.M. Use of the Ethanolic Extract of Bee Pollen (Bee Bread) and Gamma Irradiation for Keeping the Quality of Silver Carp (Hypophthalmichthys Molitrix) Fish Patties. Arab. J. Nucl. Sci. Appl. 2016, 49, 140–150. [Google Scholar]
- Yang, X.; Guo, D.; Zhang, J.; Wu, M. Characterization and anti-tumor activity of pollen polysaccharide. Int. Immunopharmacol. 2007, 7, 401–408. [Google Scholar] [CrossRef]
- Wang, B.; Diao, Q.; Zhang, Z.; Liu, Y.; Gao, Q.; Zhou, Y.; Li, S. Antitumor activity of bee pollen polysaccharides from Rosa rugosa. Mol. Med. Rep. 2013, 7, 1555–1558. [Google Scholar] [CrossRef]
- Medeiros, K.C.P.; Figueiredo, C.A.V.; Figueredo, T.B.; Freire, K.R.L.; Santos, F.A.R. Anti-allergic effect of bee pollen phenolic extract and myricetin in ovalbumin-sensitized mice. J. Ethnopharmacol. 2008, 119, 41–46. [Google Scholar] [CrossRef]
- Moreira, L.; Dias, L.G.; Pereira, J.A.; Estevinho, L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 2008, 46, 3482–3485. [Google Scholar] [CrossRef]
- Jannesar, M.; Shoushtari, M.S.; Ahmad, M.; Pourpak, Z. Bee Pollen Flavonoids as a Therapeutic Agent in Allergic and Immunological Disorders. Iran. J. Allergy Asthma Immunol. 2017, 16, 171–182. [Google Scholar]
- Graikou, K.; Kapeta, S.; Aligiannis, N.; Sotiroudis, G.; Chondrogianni, N.; Gonos, E.; Chinou, I. Chemical analysis of Greek pollen-Antioxidant, antimicrobial and proteasome activation properties. Chem. Cent. J. 2011, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Chantarudee, A.; Phuwapraisirisan, P.; Kimura, K.; Okuyama, M.; Mori, H.; Kimura, A.; Chanchao, C. Chemical constituents and free radical scavenging activity of corn pollen collected from Apis mellifera hives compared to floral corn pollen at Nan, Thailand. BMC Complement. Alternat. Med. 2012, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Freire, K.R.; Lins, A.; Dórea, M.C.; Santos, F.A.; Camara, C.A.; Silva, T. Palynological origin, phenolic content, and antioxidant properties of honeybee-collected pollen from Bahia, Brazil. Molecules 2012, 17, 1652–1664. [Google Scholar] [CrossRef] [PubMed]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R. Polyphenols from bee pollen: Structure, absorption, metabolism and biological activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [PubMed]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, F.; Jamali, M.; Peng, Z. Antioxidant enzyme activities and lipid oxidation in rape (Brassica campestris L.) bee pollen added to salami during processing. Molecules 2016, 21, 1439. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Kaygusuz, H.; Döker, S.; Kolaylı, S.; Erim, F.B. Characterization of Turkish honeybee pollens by principal component analysis based on their individual organic acids, sugars, minerals, and antioxidant activities. LWT 2017, 84, 402–408. [Google Scholar] [CrossRef]
- Campos, M.G.; Bogdanov, S.; de Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardisation of analytical methods. J. Apicult. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Human, H. Chemical composition of the ‘low quality’ pollen of sunflower (Helianthus annuus, Asteraceae). Apidologie 2013, 44, 144–152. [Google Scholar] [CrossRef]
- Phang, M.; Garg, M.L.; Sinclair, A.J. Inhibition of platelet aggregation by omega-3 polyunsaturated fatty acids is gender specific-Redefining platelet response to fish oils. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 35–40. [Google Scholar] [CrossRef]
- Gross, B.W.; Gillio, M.; Rinehart, C.D.; Lynch, C.A.; Rogers, F.B. Omega-3 fatty acid supplementation and warfarin: A lethal combination in traumatic brain injury. J. Trauma Nursing 2017, 24, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Fuenmayor, B.; Zuluaga, D.; Díaz, M.; Quicazán de, C.M.; Cosio, M.; Mannino, S. Evaluation of the physicochemical and functional properties of Colombian bee pollen. Revista MVZ Córdoba 2014, 19, 4003–4014. [Google Scholar] [CrossRef]
- Harmanescu, M.; Popovici, D.; Gergen, I. Mineral micronutrients composition of bee’s pollen. J Agroaliment. Process. Technol. 2007, 13, 175–182. [Google Scholar]
- Campos, M.G.; Webby, R.F.; Markham, K.R.; Mitchell Kevin, A.; Cunha, A.P. Age-induced diminution of free radical scavenging capacity in bee-pollens and the contribution of constituent flavonoids. J. Agric. Food Chem. 2003, 51, 742–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mărghitaş, L.A.; Stanciu, O.G.; Dezmirean, D.S.; Bobiş, O.; Popescu, O.; Bogdanov, S.; Campos, M.G. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009, 115, 878–883. [Google Scholar] [CrossRef]
- Lopes, J.; Stanciu, O.G.; Campos, M.G.; Almaraz-Abarca, N.; Muradian, L.B.; Marghitas, L.A. Bee pollen antioxidant activity—A review: Achievements and further challenges. J. Pharmacognosy 2011, 2, 25–38. [Google Scholar]
- Campos, M.G.; Mitchel, K.; Cunha, A.; Markham, K. An approach to the characterisation of Bee Pollens via their Flavonoid/Phenolic Profiles. Phytochem. Anal. 1997, 8, 181–185. [Google Scholar] [CrossRef]
- Campos, M.G.; Webby, R.F.; Markham, K.R. The Unique Occurrence of the Flavone Aglycone Tricetin in Myrtaceae Pollen. Z. Naturforsch. 2002, 57c, 944–946. [Google Scholar] [CrossRef] [Green Version]
- Campos, M.G.; Almaraz-Abarca, N.; Pires, M.; Gomes, N.; Arruda, V.; Barth, O.; Freitas, A.; Amâncio, D.; Almeida-Muradian, L. Zea mays L. pollen: An approach to its quality control. J. Agric. Sci. Technol. J. Agric. Sci. Technol. B 2015, 5, 513–522. [Google Scholar] [CrossRef]
- Negri, G.; Barreto, L.M.R.C.; Sper, F.L.; Carvalho, C.D.; Campos, M.D.G.R. Phytochemical analysis and botanical origin of Apis mellifera bee pollen from the municipality of Canavieiras, Bahia State, Brazil. Braz. J. Food Technol. 2018, 21, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Campos, M.G.; Markham, K.R. Structure Information from HPLC and on-line Measured Absorption Spectra – Flavone, Flavonols and Phenolic Acids; Coimbra Imprensa da Universidade: Coimbra, Portugal, 2007; ISBN 978-989-8074-05-8. [Google Scholar]
- Diplock, A.T.; Charleux, J.L.; Crozier-Willi, G.; Kok, F.J.; Rice-Evans, C.; Roberfroid, M. Functional food science and defence against reactive oxidative species. Br. J. Nutr. 1998, 80, 77–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, Y.; Tsuruma, K.; Shimazawa, M.; Mishima, S.; Hara, H. Comparison of bee products based on assays of antioxidant capacities. BMC Complement. Alternat. Med. 2009, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, H.; Sakamoto, T.; Acraki, Y.; Hara, H. Anti-inflammatory effect of bee pollen ethanol extract from Cistus sp. of Spanish on carrageenan-induced rat hind paw edema. BMC Complement. Alternat. Med. 2010, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Kolesarova, A.; Bakova, Z.; Capcarova, M.; Galik, B.; Juracek, M.; Simko, M. Consumption of bee pollen affects rat ovarian functions. J. Anim. Physiol. Anim. Nutr. 2013, 97, 1059–1065. [Google Scholar] [CrossRef]
- Avşar, C.; Özler, H.; Berber, I.; Civek, S. Phenolic composition, antimicrobial and antioxidant activity of Castanea sativa Mill. pollen grains from Black Sea region of Turkey. Int. Food Res. J. 2016, 23, 1711–1716. [Google Scholar]
- Campos, M.; Cunha, A.; Markham, K. Inhibition of Virulence of Pseudomonas Aeruginosa Cultures, by Flavonoids Isolated from Bee-Pollen: Possible Structure-Activity Relationships. In Polyphenol Communications 98, Proceedings of the XIX International Conference on Polyphenols, Lille, France, 1–4 September 1998; Groupe Polyphenols: Bordeaux, France, 1998. [Google Scholar]
- Fatrcová-Šramková, K.; Nôžková, J.; Kačániová, M.; Máriássyová, M.; Rovná, K.; Stričík, M. Antioxidant and antimicrobial properties of monofloral bee pollen. J. Environ. Sci. Health Part B 2013, 48, 133–138. [Google Scholar] [CrossRef]
- Bakour, M.; Fernandes, Â.; Barros, L.; Sokovic, M.; Ferreira, I.C. Bee bread as a functional product: Chemical composition and bioactive properties. LWT 2019, 109, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Leja, M.; Mareczek, A.; Wyżgolik, G.; Klepacz-Baniak, J.; Czekońska, K. Antioxidative properties of bee pollen in selected plant species. Food Chem. 2007, 100, 237–240. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Polyphenolic profile and antioxidant properties of bee-collected pollen from sunflower (Helianthus annuus L.) plant. LWT 2019, 112, 1–26. [Google Scholar] [CrossRef]
- Wu, Y.D.; Lou, Y.J. A steroid fraction of chloroform extract from bee pollen of Brassica campestris induces apoptosis in human prostate cancer PC-3 cells. Phytother. Res. 2007, 21, 1087–1091. [Google Scholar] [CrossRef]
- Pinto, B.; Caciagli, F.; Riccio, E.; Reali, D.; Šarić, A.; Balog, T.; Scarpato, R. Antiestrogenic and antigenotoxic activity of bee pollen from Cystus incanus and Salix alba as evaluated by the yeast estrogen screen and the micronucleus assay in human lymphocytes. Eur. J. Med. Chem. 2010, 45, 4122–4128. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M. Antinociceptive and antiinflammatory activities of pine (Pinus densiflora) pollen extract. Phytotherapy Res. 2007, 21, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, R.; Ishiyama, K.; Yamaguchi, M. Inhibitory effects of bee pollen Cistus ladaniferus extract on bone resorption in femoral tissues and osteoclast-like cell formation in bone marrow cells in vitro. J. Health Sci. 2006, 52, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Hamamoto, R.; Ishiyama, K.; Hashimoto, K.; Yamaguchi, M. Characterization of the active component in bee pollen Cistus ladaniferus extract in stimulating bone calcification and in inhibiting bone resorption in vitro. J. Health Sci. 2006, 52, 607–612. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hamamoto, R.; Uchiyama, S.; Ishiyama, K.; Hashimoto, K. Anabolic effects of bee pollen Cistus ladaniferus extract on bone components in the femoral-diaphyseal and-metaphyseal tissues of rats in vitro and in vivo. J. Health Sci. 2006, 52, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Hamamoto, R.; Uchiyama, S.; Ishiyama, K.; Hashimoto, K. Preventive effects of bee pollen Cistus ladaniferus extract on bone loss in streptozotocin-diabetic rats in vivo. J. Health Sci. 2007, 53, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Eraslan, G.; Kanbur, M.; Silici, S.; Liman, B.C.; Altınordulu, Ş.; Sarıca, Z.S. Evaluation of protective effect of bee pollen against propoxur toxicity in rat. Ecotoxicol. Environ. Saf. 2009, 72, 931–937. [Google Scholar] [CrossRef]
- Yıldız, O.; Can, Z.; Saral, Ö.; Yuluğ, E.; Öztürk, F.; Aliyazıcıoğlu, R.; Kolaylı, S. Hepatoprotective potential of chestnut bee pollen on carbon tetrachloride-induced hepatic damages in rats. Evid. Based. Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Mărgăoan, R.; Mărghitaș, L.A.; Dezmirean, D.; Bobis, O.; Tomos, L.; Mihai, C.; Bonta, V. Honeybee-collected pollen from Transylvania: Palynological origin, phenolic content and antioxidant activity. Bull. Uasvm Anim. Sci. Biotechnol. 2013, 70, 311–315. [Google Scholar]
- Panthong, S.; Ruangnoo, S.; Thongdeeying, P.; Sriwanthana, B.; Itharat, A. Immunomodulatory activity of Dioscorea membranacea Pierre rhizomes and of its main active constituent Dioscorealide B. BMC Complement. Alternat. Med. 2014, 14, 403. [Google Scholar] [CrossRef] [Green Version]
- Razali, F.N.; Sinniah, S.K.; Hussin, H.; Zainal Abidin, N.; Shuib, A.S. Tumor suppression effect of Solanum nigrum polysaccharide fraction on breast cancer via immunomodulation. Int. J. Biol. Macromol. 2016, 92, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Yang, L.; Jiang, J.G.; Zheng, C.Y.; Zhu, W. Immune enhancement effects and extraction optimization of polysaccharides from Citrus aurantium L. var. amara Engl. Food Funct. 2017, 8, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Urcan, A.C.; Criste, A.D.; Dezmirean, D.S.; Mărgăoan, R.; Caeiro, A.; Campos, M.G. Similarity of Data from Bee Bread with the Same Taxa Collected in India and Romania. Molecules 2018, 23, 2491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, M.; Karaoğlu, Ö.; Eroğlu, N.; Silici, S. Fatty Acid and Proximate Composition of Bee Bread. Gıda Teknolojisi Biyoteknolojisi 2016, 54, 497–504. [Google Scholar]
- Čeksteryté, V.; Kurtinaitienė, B.; Venskutonis, P.R.; Pukalskas, A.; Kazernavičiūtė, R.; Balžekas, J. Evaluation of antioxidant activity and flavonoid composition in differently preserved bee products. Czech J. Food Sci. 2016, 34, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Markiewicz-Żukowska, R.; Naliwajko, S.K.; Bartosiuk, E.; Moskwa, J.; Isidorov, V.; Soroczyńska, J.; Borawska, M.H. Chemical composition and antioxidant activity of chi, and its influence on the glioblastoma cell line (U87MG). J. Apicul. Sci. 2013, 57, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Silva, G.R.; Natividade, T.B.; Camara, C.A.; Silva, E.M.S.; Assis Ribeiro dos Santos, F.; Silva, T.M.S. Identification of sugar, amino acids and minerals from the pollen of Janaira Stingless Bees (Melipona subnitida). Food Nutr. Sci. 2014, 5, 1015–1021. [Google Scholar] [CrossRef] [Green Version]
- Barene, I.; Daberte, I.; Siksna, S. Investigation of Bee Bread and Development of Its Dosage Forms. Medicinos Teorija It Practika 2015, 21, 16–22. [Google Scholar] [CrossRef]
- Ivanišová, E.; Kačániová, M.; Frančáková, H.; Petrová, J.; Hutková, J.; Brovarskyi, V.; Musilová, J. Bee bread-perspective source of bioactive compounds for future. Potravinarstvo Slovak J. Food Sci. 2015, 9, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Zuluaga, C.; Serrato, J.C.; Quicazan, M. Chemical, nutritional and bioactive characterization of colombian bee bread. Chem. Eng. Trans. 2015, 43, 175–180. [Google Scholar] [CrossRef]
- Hudz, N.; Korzeniowska, K.; Wieczorek, P.P.; Schubertová, Z.; Brindza, J.; Ivanišová, E. Approaches to the Identification and Assay of Flavonoids in Bee Bread Extracts by Spectrophotometric Method. Agrobiodivers. Improv. Nutr. Health Life Qual. 2017, 1, 168–173. [Google Scholar]
- Morris, M.E.; Zhang, S. Flavonoid–drug interactions: Effects of flavonoids on ABC transporters. Life Sci. 2006, 78, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, O.; Marghitas, L.A.; Dezmirean, D. Examination of antioxidant capacity of beebread extracts by different complementary assays. Bull. Uasvm Anim. Sci. Biotechnol. 2007, 64. [Google Scholar] [CrossRef]
- Stanciu, O.; Marghitas, L.A.; Dezmirean, D. A comparison of methods used to define the antioxidant capacity of bee pollen and beebread from Romania. In Proceedings of the 43rd Croatian and 3rd International Symposium on Agriculture, Opatija, Croatia, 18–21 February 2008; Volume 751, p. 754. [Google Scholar]
- Cocan, O.; Marghitas, L.A.; Dezmirean, D. Total polyphenols, flavonoids and radical scavenging activity of beepollen and beebread collected from Transylvania area. Bull. Uasvm Anim. Sci. Biotechnol. 2006, 62. [Google Scholar] [CrossRef]
- Baltrušaitytė, V.; Venskutonis, P.R.; Čeksterytė, V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem. 2007, 101, 502–514. [Google Scholar] [CrossRef]
- Čeksterytė, V.; Račys, J.; Kaškonienė, V.; Venskutonis, P.R. Fatty acid composition in beebread. Biologija 2008, 54, 253–257. [Google Scholar] [CrossRef]
- Yin, Y.; Sui, C.; Meng, F.; Ma, P.; Jiang, Y. The omega-3 polyunsaturated fatty acid docosahexaenoic acid inhibits proliferation and progression of non-small cell lung cancer cells through the reactive oxygen species-mediated inactivation of the PI3K/Akt pathway. Lipids Health Dis. 2017, 16, 87. [Google Scholar] [CrossRef] [Green Version]
- Ceksteryte, V.; Jansen, H.J.M.E. Composition and content of fatty acids in beebread of various floral origin, collected in Lithuania and prepared for storage in different ways. Chemine Technol. 2012, 2, 57–61. [Google Scholar]
- Tavdidishvili, D.; Khutsidze, T.; Pkhakadze, M.; Vanidze, M.; Kalandia, A. Flavonoids in Georgian bee bread and bee pollen. J. Chem. Chem. Eng. 2014, 8, 676–681. [Google Scholar]
- Campos, M.G.; Cupido, M.; Tavares, R.; Consul, R. Clinical Outcomes from Tamoxifen Drug-herb Interactions. Int. J. Clin. Pharmacol. Pharmacother. 2018, 3, 140. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Kato, A.; Ando, K.; Tamura, G.; Arima, K. Effects of some fatty acid esters on the viability and transplantability of Ehrlich ascites tumor cells. Cancer Res. 1971, 31, 501–504. [Google Scholar] [PubMed]
- Ostlund, R.E., Jr.; Racette, S.B.; Stenson, W.F. Inhibition of cholesterol absorption by phytosterol-replete wheat germ compared with phytosterol-depleted wheat germ. Am. J. Clin. Nutr. 2003, 77, 1385–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, P.G.; Awad, A.B. Phytosterols as anticancer compounds. Mol. Nutr. Food Res. 2007, 51, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Habib, F.K.; Ross, M.; Burger, U.; Lewenstein, A.; Rose, K.; Jaton, J.C. Isolation and characterization of a cyclic hydroxamic acid from a pollen extract, which inhibits cancerous cell growth in vitro. J. Med. Chem. 1995, 38, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Vanderplanck, M.; Moerman, R.; Rasmont, P.; Lognay, G.; Wathelet, B.; Wattiez, R.; Michez, D. How does pollen chemistry impact development and feeding behaviour of polylectic bees? PLoS ONE 2014, 9, e86209. [Google Scholar] [CrossRef]
- Habib, F.K.; Ross, M.; Buck, A.C.; Ebeling, L.; Lewenstein, A. In vitro evaluation of the Pollen Extract Cernitin T-60, in the Regulation of Prostate Cell Growth. Br. J. Urol. 1990, 66, 393–397. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Zăhan, M.; Mărghitaş, L.A.; Dezmirean, D.S.; Erler, S.; Bobiş, O. Antiproliferative activity and apoptotic effects of Filipendula ulmaria pollen against C26 mice colon tumour cells. J. Apicul. Sci. 2016, 60, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Sobral, F.; Calhelha, R.; Barros, L.; Dueñas, M.; Tomás, A.; Santos-Buelga, C.; Vilas-Boas, M.; Ferreira, I. Flavonoid composition and antitumor activity of bee bread collected in northeast Portugal. Molecules 2017, 22, 248. [Google Scholar] [CrossRef] [Green Version]
- Borawska, M.H.; Markiewicz-Żukowska, R.; Naliwajko, S.K.; Moskwa, J.; Bartosiuk, E.; Socha, K.; Mariak, Z. The interaction of bee products with temozolomide in human diffuse astrocytoma, glioblastoma multiforme and astroglia cell lines. Nutr. Cancer 2014, 66, 1247–1256. [Google Scholar] [CrossRef]
- Uçar, M.; Değer, O.; Gerigelmez, A.Y.; Cengiz, S.; Barlak, Y.; Ovalı, E. Effect of Turkish pollen and propolis extracts on caspase-3 activity in myeloid cancer cell lines. Trop. J. Pharm. Res. 2016, 15, 2445–2449. [Google Scholar] [CrossRef] [Green Version]
- Cooper, M.E.; Vranes, D.; Youssef, S.; Stacker, S.A.; Cox, A.J.; Rizkalla, B.; Gilbert, R.E. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes 1999, 48, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Oxygen sensing and metabolic homeostasis. Mol. Cell. Endocrinol. 2014, 397, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Izuta, H.; Shimazawa, M.; Tsuruma, K.; Araki, Y.; Mishima, S.; Hara, H. Bee products prevent VEGF-induced angiogenesis in human umbilical vein endothelial cells. BMC Complement. Altern. Med. 2009, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasouli, H.; Norooznezhad, A.H.; Rashidi, T.; Hoseinkhani, Z.; Mahnam, A.; Tarlan, M.; Mansouri, K. Comparative in vitro/theoretical studies on the anti-angiogenic activity of date pollen hydro-alcoholic extract: Highlighting the important roles of its hot polyphenols. BioImpacts BI 2018, 8, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanem, K.Z.; Ramadan, M.M.; Ghanem, H.Z.; Fadel, M. Improving the production of unsaturated fatty acid esters and flavonoids from date palm pollen and their effects as anti-breast-cancer and antiviral agents: An in-vitro study. J. Arab. Soc. Med. Res. 2015, 10, 47–55. [Google Scholar]
- Omar, W.A.W.; Azhar, N.A.; Fadzilah, N.H.; Kamal, N.N.S.N.M. Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines. Asian Pac. J. Trop. Biomed. 2016, 6, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Youn, C.K.; Kim, J.; Park, J.H.; Do, N.Y.; Cho, S.I. Role of autophagy in cisplatin-induced ototoxicity. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1814–1819. [Google Scholar] [CrossRef]
- Athira, K.V.; Madhana, R.M.; Lahkar, M. Flavonoids, the emerging dietary supplement against cisplatin-induced nephrotoxicity. Chem. Biol. Interact. 2016, 248, 18–20. [Google Scholar]
- Dugbartey, G.J.; Peppone, L.J.; de Graaf, I.A. An integrative view of cisplatin-induced renal and cardiac toxicities: Molecular mechanisms, current treatment challenges and potential protective measures. Toxicology 2016, 371, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Naghizadeh, B.; Mansouri, S.M.T.; Mashhadian, N.V. Crocin attenuates cisplatin-induced renal oxidative stress in rats. Food. Chem. Toxicol. 2010, 48, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Bodiga, V.L.; Kudle, M.R.; Bodiga, S. Silencing of PKC-α, TRPC1 or NF-κB expression attenuates cisplatin-induced ICAM-1 expression and endothelial dysfunction. Biochem. Pharmacol. 2015, 98, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.L.; Chu, J.G.; Jian, X.M.; Dong, J.Z.; Wang, L.P.; Li, G.X.; Yang, N.B. Curcumin attenuates lipopolysaccharide/d-galactosamine-induced acute liver injury by activating Nrf2 nuclear translocation and inhibiting NF-kB activation. Biomed. Pharmacother. 2017, 91, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Abdella, E.M.; Tohamy, A.; Ahmad, R.R. Antimutagenic activity of Egyptian propolis and bee pollen water extracts against cisplatin-induced chromosomal abnormalities in bone marrow cells of mice. Iran. J. Cancer Prevent. 2009, 2, 175–181. [Google Scholar]
- Munsted, K.; Bogdanov, S. Bee products and their potential use in modern medicine. J. Api. Prod. Api. Med. Sci. 2009, 1, 57–63. [Google Scholar] [CrossRef]
- Quintanilha, J.C.F.; de Sousa, V.M.; Visacri, M.B.; Amaral, L.S.; Santos, R.M.M.; Zambrano, T.; Moriel, P. Involvement of cytochrome P450 in cisplatin treatment: Implications for toxicity. Cancer Chemother. Pharmacol. 2017, 80, 223–233. [Google Scholar] [CrossRef]
- Campos, M.G.; Machado, J.; Costa, M.L.; Lino, S.; Correia, F.; Maltez, F. Case Report: Severe Hematological, Muscle and Liver Toxicity Caused by Drugs and Artichoke Infusion Interaction in an Elderly Polymedicated Patient. Curr. Drug Saf. 2018, 13, 44–50. [Google Scholar] [CrossRef]
- Costa, M.L.; Rodrigues, J.A.; Azevedo, J.; Vasconcelos, V.; Eiras, E.; Campos, M.G. Hepatotoxicity induced by paclitaxel interaction with turmeric in association with a microcystin from a contaminated dietary supplement. Toxicon 2018, 150, 207–211. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, N.R.; Harjai, K. Feeding Bee Pollen and Bee Bread to Mice: Effect and Antioxidant Status. Int. J. Ther. Appl. 2014, 18, 26–29. [Google Scholar]
- Devi, A.; Kumar, N.R.; Kaur, J. Evaluation of the antioxidative potential of Bee Products: Pollen and Bee Bread against Staphylococcus aureus Infected Balb/c mice. J. Chem. Pharm. Sci. 2016, 974, 2115. [Google Scholar]
- Bhandari, P.S.; Strant, M. Bee bread—Various usages of bee bread and clinical experiences. In Proceedings of the No Bees No Life Apıtherapy Symposium, Lukovica and Maribor, Slovenia, 23–24 October 2015; p. 26. [Google Scholar]
- Wojcicki, J.; Hinek, A.; Samochowiec, L. The protective effect of pollen extracts against allyl alcohol damage of the liver. Arch. Immunol. Ther. Exp. 1985, 33, 841–849. [Google Scholar]
- Wojcicki, J.; Samochowiec, L.; Bartłkomowicz, B.; Hinek, A.; Jaworska, M.; Gawrońska-Szklarz, B. Effect of pollen extract on the development of experimental atherosclerosis in rabbits. Atherosclerosis 1986, 62, 39–45. [Google Scholar] [CrossRef]
- Almaraz-Abarca, N.; Campos, M.G.; Avila-Reyes, J.A.; Naranjo-Jimenez, N.; Corral, J.H.; Gonzalez-Valdez, L.S. Antioxidant activity of polyphenolic extract of monofloral honeybee-collected pollen from mesquite (Prosopis juliflora, Leguminosae). J. Food Compos. Anal. 2007, 20, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Šarić, A.; Balog, T.; Sobočanec, S.; Kušić, B.; Šverko, V.; Rusak, G.; Marotti, T. Antioxidant effects of flavonoid from Croatian Cystus incanus L. rich bee pollen. Food Chem. Toxicol. 2009, 47, 547–554. [Google Scholar] [CrossRef]
- Cheng, N.; Ren, N.; Gao, H.; Lei, X.; Zheng, J.; Cao, W. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem. Toxicol. 2013, 55, 234–240. [Google Scholar] [CrossRef]
- Tohamy, A.A.; Abdella, E.M.; Ahmed, R.R.; Ahmed, Y.K. Assessment of anti-mutagenic, anti-histopathologic and antioxidant capacities of Egyptian bee pollen and propolis extracts. Cytotechnology 2014, 66, 283–297. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Shen, Z.; Geng, Q.; Wu, Z.; Shi, P.; Miao, X. Protective effect of Schisandra chinensis bee pollen extract on liver and kidney injury induced by cisplatin in rats. Biomed. Pharmacother. 2017, 95, 1765–1776. [Google Scholar] [CrossRef]
- Hassan, W.A.; El-kashlan, A.M.; Mohamed, N.A. Egyptian date palm pollen ameliorates testicular dysfunction induced by cadmium chloride in adult male rats. J. Am. Sci. 2012, 8, 659–669. [Google Scholar]
- Saral, Ö.; Yildiz, O.; Aliyazicioğlu, R.; Yuluğ, E.; Canpolat, S.; Öztürk, F.; Kolayli, S. Apitherapy products enhance the recovery of CCL4-induced hepatic damages in rats. Turk. J. Med. Sci. 2016, 46, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Khalil, F.A.; El-Sheikh, N.M. The effects of dietary Egyptian propolis and bee pollen supplementation against toxicity if sodium fluoride in rats. J. Am. Sci. 2010, 11, 310–316. [Google Scholar]
- Asakawa, K.; Nandachi, N.; Satoh, S.; Honma, M.; Namikata, S.; Ishii, M.; Kishimoto, T. Effects of cernitin pollen-extract (Cernilton®) on inflammatory cytokines in sex-hormone-induced nonbacterial prostatitis rats. Hinyokika kiyo. Acta Urol. Japon. 2001, 47, 459–465. [Google Scholar]
- Karampour, N.S.; Hemmati, A.A.; Malmir, A. The anxiolytic effect of bee pollen hydroalcoholic extract in mice. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 301–305. [Google Scholar] [CrossRef]
- Coleta, M.; Batista, M.T.; Campos, M.G.; Carvalho, R.; Cotrim, M.D.; Lima, T.C.; Cunha, A.P. Neuropharmacological evaluation of the putative anxiolytic effect of Passiflora edulis Sims., its sub-fractions and flavonoid constituents. Phytother. Res. 2006, 20, 1067–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleta, M.; Campos, M.G.; Cotrim, M.D.; Lima, T.C.M.D.; Cunha, A.P. Assessment of luteolin (3′,4′,5,7-tetrahydroxyflavone) neuropharmacologicalactivity. Behav. Brain Res. 2008, 189, 75–82. [Google Scholar] [CrossRef] [Green Version]
- El-Ansary, A.; Al-Salem, H.S.; Asma, A.; Al-Dbass, A. Glutamate excitotoxicity induced by orally administered propionic acid, a short chain fatty acid can be ameliorated by bee pollen. Lipids Health Dis. 2017, 16, 96. [Google Scholar] [CrossRef] [Green Version]
- Shehu, M.B. The Effect of Beebread on Wound Healing in Malnourished Rabbits. Ph.D. Thesis, Universiti Sains, Gelugor, Penang, Malaysia, 2014; p. 58. [Google Scholar]
- Bakour, M.; Al-Waili, N.S.; El Menyiy, N.; Imtara, H.; Figuira, A.C.; Al-Waili, T.; Lyoussi, B. Antioxidant activity and protective effect of bee bread (honey and pollen) in aluminum-induced anemia, elevation of inflammatory makers and hepato-renal toxicity. J. Food Sci. Technol. 2017, 54, 4205–4212. [Google Scholar] [CrossRef]
- Hani, B.; Dalila, B.; Saliha, D.; Harzallah, D.; Ghadbane, M.; Khennouf, S. Microbiological sanitary aspects of pollen. Adv. Environ. Biol. 2012, 6, 1415–1420. [Google Scholar]
- Stanciu, O.G.; Marghitas, L.A.; Dezmirean, D. Macro-and oligo-mineral elements from honeybee-collected pollen and beebread harvested from Transylvania (Romania). Bull. Uasvm Anim. Sci. Biotechnol. 2009, 66, 1–2. [Google Scholar]
- De-Melo, A.A.M.; de Almeida-Muradian, L.B. Health Benefits and Uses in Medicine of Bee Pollen. In Bee Products-Chemical and Biological Properties; Springer: Cham, Switzerland, 2017; pp. 261–276. [Google Scholar]
- Collins, M.M.; Stafford, R.S.; O’leary, M.P.; Barry, M.J. How common is prostatitis? A national survey of physician visits. J. Urol. 1998, 159, 1224–1228. [Google Scholar] [CrossRef]
- Barbalias, G.A. Clinical and therapeutical guidelines for chronic prostatitis. Eur. Urol. 2000, 37, 116–117. [Google Scholar] [CrossRef]
- Schaeffer, A.J. Classification (traditional and National Institutes of Health) and demographics of prostatitis. Urology 2002, 60, 5–6. [Google Scholar] [CrossRef]
- Aitken, R.J.; Buckingham, D.; Harkiss, D. Use of a xanthine oxidase free radical generating system to investigate the cytotoxic effects of reactive oxygen species on human spermatozoa. Reproduction 1993, 97, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamirande, E.D.; Gagnon, C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int. J. Androl. 1993, 16, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Kong, X.; Qian, Y.; Xu, D.; Liu, H.; Zhu, Y.; Qi, J. Therapeutic efficacy of Cernilton in benign prostatic hyperplasia patients with histological prostatitis after transurethral resection of the prostate. Int. J. Clin. Exp. Med. 2015, 8, 11268. [Google Scholar] [PubMed]
- Münstedt, K.; Voss, B.; Kullmer, U.; Schneider, U.; Hübner, J. Bee pollen and honey for the alleviation of hot flushes and other menopausal symptoms in breast cancer patients. Mol. Clin. Oncol. 2015, 3, 869–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buck, A.C.; Rees, R.W.; Ebeling, L. Treatment of chronic prostatitis and prostatodynia with pollen extract. Br. J. Urol. 1989, 64, 496–499. [Google Scholar] [CrossRef]
- Buck, A.C.; Cox, R.; Rees, R.W.M.; Ebeling, L.; John, A. Treatment of Outflow Tract Obstruction due to Benign Prostatic Hyperplasia with the Pollen Extract Cernilton®: A Double-blind, Placebo-controlled Study. Br. J. Urol. 1990, 66, 398–404. [Google Scholar] [CrossRef]
- Chen, H.J.; Wang, Z.P.; Chen, Y.R.; Qin, D.S.; Fu, S.J.; Ma, B.L. Effects of pollen extract EA-10, P5 on chronic prostatitis or infertility with chronic prostatitis. Acta Pharmacol. Sin. 2002, 23, 1035. [Google Scholar]
- Monden, K.; Tsugawa, M.; Ninomiya, Y. A Japanese version of the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI, Okayama version) and the clinical evaluation of cernitin pollen extract for chronic non-bacterial prostatitis. Nippon Hinyokika Gakkai Zasshi 2002, 93, 539–547. [Google Scholar]
- Li, N.C.; Na, Y.Q.; Guo, H.Q. Clinical study with Prostat (Poltit) for treatment for chronic nonbacterial prostatitis. Chin. J. Urol. 2003, 9, 28. [Google Scholar]
- Cai, T.; Wagenlehner, F.M.; Luciani, L.G.; Tiscione, D.; Malossini, G.; Verze, P.; Bartoletti, R. Pollen extract in association with vitamins provides early pain relief in patients affected by chronic prostatitis/chronic pelvic pain syndrome. Exp. Ther. Med. 2014, 8, 1032–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togo, Y.; Ichioka, D.; Miyazaki, J.; Maeda, Y.; Kameyama, K.; Yasuda, M.; Hiyama, Y.; Takahashi, S.; Nagae, H.; Hirota, S.; et al. Japanese Research Group for Urinary Tract Infection (JRGU) Oral administration of cernitin pollen extract (Cernilton® for 30 days might be useful to avoid unnecessary biopsy in prostate biopsy candidates: A preliminary study. Int. J. Urol. 2018, 25, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadava, D.; Kane, S.E. The effect of brassinolide, a plant steroid hormone, on drug resistant small-cell lung carcinoma cells. Biochem. Biophys. Res. Commun. 2017, 493, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, F.; Turriziani, M.; Tortorelli, G.; Avvisati, G.; Torino, F.; De Vecchis, L. Triazene compounds: Mechanism of action and related DNA repair systems. Pharmacol. Res. 2007, 56, 275–287. [Google Scholar] [CrossRef]
- Aoki, T.; Mizutani, T.; Ishikawa, M.; Sugiyama, K.; Hashimoto, N. A first feasibility study of temozolomide for Japanese patients with recurrent anaplastic astrocytoma and glioblastoma multiforme. Int. J. Clin. Oncol. 2003, 8, 301–304. [Google Scholar] [CrossRef]
- Kim, J.T.; Kim, J.S.; Ko, K.W.; Kong, D.S.; Kang, C.M.; Kim, M.H.; Son, M.J.; Song, H.S.; Shin, H.J.; Lee, D.S.; et al. Metronomic treatment of temozolomide inhibits tumor cell growth through reduction of angiogenesis and augmentation of apoptosis in orthotopic models of gliomas. Oncol. Rep. 2006, 16, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, M.; Macchione, N.; Ferrante, C.; Chiavaroli, A.; Recinella, L.; Carradori, S.; Zengin, G.; Cesa, S.; Leporini, L.; Leone, S.; et al. Graminex pollen: Phenolic pattern, colorimetric analysis and protective effects in immortalized prostate cells (PC3) and rat prostate challenged with LPS. Molecules 2018, 23, 1145. [Google Scholar] [CrossRef] [Green Version]


| Properties | Bee Pollen Type |
|---|---|
| Antibiotic | Castanea spp., Eucalyptus spp., Taraxacum spp., Trifolium spp., Zea mays L. |
| Anti-atherogenic | Aesculus hippocastanum L., Castanea sativa Mill., Prunus spp., Salix spp. |
| Anti-anemia | Acacia spp., Citrus spp., Crataegus spp., Papaver spp., Tilia spp. |
| Antitussives | Papaver spp. |
| Diuretic | Centaurea cyanus L., Prunus spp., Taraxacum spp. |
| Digestive | Acacia spp., Lavandula spp., Rosmarinus officinalis L. |
| Cardioprotective | Crataegus spp. |
| Hepatoprotective | Aesculus hippocastanum L., Castanea sativa Mill., Cystus incanus L., Prosopis juliflora (Sw.) DC., Schisandra chinensis (Turcz.) Baill., Taraxacum spp. |
| Kidney function | Brassica napus L., Phoenix dactylifera L., Schisandra chinensis Turcz.) Baill., Trifolium alexandrinum L., Zea mays L. |
| Immunomodulating | Eucalyptus spp., Malus spp. |
| Ulcer healing | Brassica napus L. |
| Functional Properties | BP and BB Type | Extract Type or Concentration | Bioactivity | References |
|---|---|---|---|---|
| Anti microbial | Castanea sativa Mill. | 10 g of Castanea sativa Mill pollen (A1–A5, E1–E4) extracted by 100 mL of methanol from nine different populations | Inhibition zone diameter: A1 (9–21 mm) against Candida albicans ATCC 14053, Bacillus cereus 7064, Enterococcus faecalis ATCC 51299, Methicillin Resistant Staphylococcus aureus (MRSA), Micrococcus luteus, Staphylococcus aureus ATCC 6538; A2 (9–18 mm) against Candida krusei ATCC 6258, Escherichia coli ATCC 11293, MRSA, M. luteus, S. aureus; A3 (9–20 mm) against C. krusei, E. coli, MRSA, M. luteus, S. aureus; A4 (9–21 mm) against C. parapsilosis, B. cereus, E. faecalis, MRSA, M. luteus, S. aureus; A5 (9–21 mm) against E. coli, MRSA, M. luteus, S. aureus; E1 (9–23 mm) against C. krusei, Candida parapsilosis ATCC 22019, B. cereus, MRSA, M. luteus, S. aureus, ancomycin Resistant Enterococcus (VRE); E2 (9–22 mm) against C. krusei, C. albicans, B. cereus, E. coli, MRSA, M. luteus, S. aureus; E3 (12–21 mm) against C. krusei, MRSA, M. luteus, S. aureus; E4 (10–21 mm) C. krusei, C. albicans, C. parapsilosis, B. cereus, MRSA, M. luteus, S. aureus | [58] |
| Ranunculus sardous Crantz., Ulex europaeus L. | N/S | Marked antibiotic activity against Pseudomonas aeruginosa due to herbacetin derivates | [59] | |
| Brassica napus subsp. napus L. | 10 g of pollen extracted in 99.9% and 70% (v/v) methanol (MEh and MEl) and 96% and 70% (v/v) ethanol (Eh and El) | MEh and MEl: 2.33–3.00 mm against Listeria monocytogenes CCM 4699; 1.33–2.66 mm against Pseudomonas aeruginosa CCM 1960; 2.33–3.67 mm against Staphylococcus aureus CCM 3953; 2.00–3.83 mm against Salmonella enterica CCM 4420; 1.67–3.00 mm against Escherichia coli CCM 3988; Eh and El: 2.33–3.67 mm against L. monocytogenes; 1.67–3.67 mm against P. aeruginosa; 2.33–3.00 mm against S. aureus; 2.00–3.00 mm against S. enterica; 2.33–3.67 mm against E. coli; | [60] | |
| Helianthus annuus L. | 10 g of pollen extracted in 99.9% and 70% (v/v) methanol (MEh and MEl) and 96% and 70% (v/v) ethanol (Eh and El) | MEh and MEl: 2.33–3.67 mm against L. monocytogenes; 2.00–2.67 mm against P. aeruginosa; 1.67–2.00 mm against S. aureus; 2.67–3.33 mm against S. enterica; 1.33–2.67 mm against E. coli; Eh and El: 2.33–2.67 mm against L. monocytogenes; 1.00–2.67 mm against P. aeruginosa; 1.00–2.67 mm against S. aureus; 2.17–3.67 mm against S. enterica; 1.67–2.67 mm against E. coli; | [60] | |
| Papaver somniferum L. | 10 g of pollen extracted in 99.9% and 70% (v/v) methanol (MEh and MEl) and 96% and 70% (v/v) ethanol (Eh and El) | MEh and MEl: 0.67–2.67 mm against L. monocytogenes; 1.00–1.67 mm against P. aeruginosa; 1.67–2.50 mm against S. aureus; 1.67–2.67 mm against S. enterica; 2.00–2.67 mm against E. coli; Eh and El: 2.00–2.33 mm against L. monocytogenes; 1.00–1.67 mm against P. aeruginosa; 1.67–3.67 mm against S. aureus; 1.67–2.17 mm against S. enterica; 1.83–3.00 mm against E. coli; | [60] | |
| BB—predominant Bupleurum spinosum Gouan.; Anethum graveolens L. | Hydro methanolic BB extract of 20 mg/mL in water | MIC: 0.04 mg/mL against B. cereus; 0.25 mg/mL against E. coli; 0.175 mg/mL against S. aureus, L. monicytogenes, Enterobacter cloacae, Salmonella typhimurium; MBC: 0.08 mg/mL against B. cereus; 0.35 mg/mL against S. aureus, L. monicytogenes, E. coli, E. cloacae, S. typhimurium | [61] | |
| BB—predominant Bupleurum spinosum Gouan.; Anethum graveolens L. | Hydro methanolic BB extract of 20 mg/mL in water | MIC: 0.35 mg/mL against Aspergillus ochraceus; 0.50 mg/mL against Aspergillus fumigatus, 0.70 mg/mL against Penicillium funiculosum; 1.00 mg/mL against Aspergillus niger, Penicillium ochrochloron, Penicillium verrucosum var. cyclopium; MBC: 0.70 mg/mL against A. ochraceus; 1.00 mg/mL against A. fumigates, P. funiculosum; 1.40 mg/mL against A. niger, P. ochrochloron, P.v. cyclopium | [61] | |
| Antioxidant | Selected monofloral species | 2 g of BP extracted in 15 mL methanol | DPPH value ranging between: 0.135–2.814 mmol Trolox g–1, in Pinus spp. and Salix spp.; TEAC value ranging between: 0.546–6.838 mmol Trolox g–1, in Pinus spp. and Salix spp. 0.255–5.355 mmol Fe(II) g–1, in Knautia arvensis (L.) Coulter and Matricaria chamomilla L. | [47] |
| BB—predominant Bupleurum spinosum Gouan.; Anethum graveolens L. | 1 g of BB stirred with 30 mL methanol/water (80:20 v/v) mixture and prepared at a final concentration of 20 mg/mL in water | Total antioxidant capacity (mg AA/g extract) 143 ± 22 DPPH assay (EC50, mg/mL) 0.98 ± 0.06 ABTS assay (EC50, mg/mL) 0.50 ± 0.04 Reducing power (EC50, mg/mL) 0.19 ± 0.03 | [61] | |
| Selected monofloral species | 0.25 mL BP in 80% methanol | Total antioxidant activity (%): 6.8–86.4 in Zea mays L. and Sinapis alba L. DPPH value (%): 8.6–91.3 in Lamium purpureum L. and Aesculus hippocastanum L. HRSA (%): 10.5–98.0 in Aesculus hippocastanum L. and Pyrus communis L. | [62] | |
| Helianthus annus L. | 0.5 g of pollen extracted with 10 mL of 80% methanol and 50% ethanol | Methanolic extract: TPC: 816 mg/kg GAE of DW; TFC: 843 mg/kg QE DW; ABTS radical scavenging activity: 95.5%; Ethanolic extract: TPC: 2907 mg/kg GAE of DW; TFC: 865 mg/kg QE DW; ABTS radical scavenging activity: 75% | [63] | |
| Anti-carcinogenic | Brassica rapa L. | 1.95 g pollen fraction (chloroform extract) with 12.5, 25, 50 and 100 μg/mL of pollen extract administered for 24 h | Citotoxicity in MCF-7, Hela, BEL-7402, BCG-823, KB, A549 and HO8910 cells with 100 μg/mL extract ↑ caspase-3 enzyme activity; ↓ expression of anti-apoptic proteins Bcl-2 | [64] |
| Cistus x incanus L., Salix alba L. | 1 g bee pollen mixed with 9 mL 70% ethanol with final concentrations of: 1 mg/mL, 10 mg/mL and 100 mg/mL at 24 h until harvest (72 h) | C. incanus extract 2 induced toxicity at 355.6 mg/mL S. alba extract 2 induced toxicity at 660 mg/mL, (91.82–7.46%, Inhibition of 17-β estradiol activity | [65] | |
| Anti-inflammatory | Pinus densiflora Siebold & Zucc. | Three times extracted pollen with 70% ethanol, with orally administered dose of 100 and 200 mg/kg | Significant effect in formalin test of mice with pollen (100 and 200 mg/kg) at the first (0–5 min) and second phase (15–30 min); Dose of 200 mg/kg delayed the response of mice to hot plate thermal stimulation; ↓ inflammation induced by carrageenan, formalin and arachidonic acid, due to flavonoid content in Pinus pollen | [66] |
| Cistus spp. | 200 g of BP extracted with water or 95% ethanol; with orally administered dose of BP (300 mg/kg), Water BP (300 mg/kg), EtOH BP (100 and 300 mg/kg) | ↓ inhibition of carrageenan-induced edema at 300 mg/kg water PB; ↑ inhibition of carrageenan-induced edema at 100 and 300 mg/kg (48.4% and 43.5%) ↑ anti-inflammatory effect of ethanol extract, strong inhibition of carrageenan-induced paw edema ↑ inhibition of COX-1 and COX-2 in water BP (IC50: 150 μg/mL and 10.3 μg/mL) ↑ inhibitionof COX-1 and COX-2 in ethanol BP (IC50: > 150 μg/mL) | [56] | |
| Anti-osteoporosis | Cistuscreticus L. | 5 g of BP in 20 mL distilled water, with concentrations of 10, 100, 1000 µg/mL | ↑ calcium content (mg/g dry bone) on VD3-induced decrease, in the femoral-diaphyseal and metaphyseal tissues by BP dose increase ↑ calcium content (mg/g dry bone) on PGE2-induced decrease, in the femoral-diaphyseal and metaphyseal tissues by BP dose increase ↓ calcium content (mg/g dry bone) on PTH-induced decrease, in the femoral-diaphyseal and metaphyseal tissues by BP dose increase ↓ glucose consumed (mg/g dry bone) on PTH-stimulated glucose consumption in the femoral-diaphyseal and metaphyseal tissues by BP dose increase ↓ lactic acid production (mg/g dry bone) on PTH-stimulated lactic acid production in the femoral-diaphyseal and ↑ lactic acid production (mg/g dry bone) in the metaphyseal tissues by BP dose increase ↓ TRACP (nmol/min/mg protein) on PTH-induced increase in TRACP activity in the femoral-diaphyseal and metaphyseal tissues by BP dose increase | [67] |
| Cistuscreticus L. | 5 g of BP in 20 mL distilled water, with concentrations of 10, 50 and 100 µg/mL BP extracts fractioned to less than MW 1000 (A), from MW 1000 to MW 10,000 (B) and greater than MW 10,000 (C) | ↑ calcium content (mg/g dry bone) in rat femoral-diaphyseal tissues in the presence of 50 μg/mL BP extract (< MW 1000) and moderately higher in the presence of 100 μg/mL in all BP fractioned extracts ↑ calcium content (mg/g dry bone) in rat femoral-diaphyseal and -methaphyseal tissues by dose increase of 25 and 50 μg/mL BP extract (< MW 1000) ↑ osteoclast-like MNCs (number/culture) on PTH-induced osteoclastic cell formation by dose decrease (10 and 50 µg/mL) and higer fractioned extracts | [68] | |
| Cistuscreticus L. | 5 g of BP in 20 mL distilled water (oral administration) 20 g of BP in 99.5% ethanol (30 mL) for use on tissues of rats; Concentrations: 1, 5 or 10 mg/mL 100 g body weight orally administered to rats for 7 days; 10, 100 and 1000 µg/mL water and ethanol extracts | ↑ calcium content (mg/g dry bone) in the femoral-diaphyseal and metaphyseal tissues by oral administration of BP water extracts (5 and 10 mg/mL/100 g body weight) ↑ calcium content (mg/g dry bone) in the femoral-diaphyseal and metaphyseal tissues in the presence of water-solubilized extract (100 or 1000 µg/mL) and ethanol extract (1000 µg/mL) by dose increase ↑ calcium content (mg/g dry bone) in the femoral-diaphyseal tissues with water-solubilized extract (100 µg/mL) ↑ alkaline phosphatase (µmol/min/mg protein) activity and DNA (mg/g wet bone) content in the presence of water-solubilized extract (100 or 1000 µg/mL) | [69] | |
| Brassica napus L., Camellia sinensis (L.) Kuntze., Fagopyrum esculentum Moench. | 5 g of BP in 20 mL distilled water (oral administration) 20 g of BP in 99.5% ethanol (30 mL) for use on tissues of rats; Concentrations: 1, 5 or 10 mg/mL 100 g body weight orally administered to rats for 7 days; 10, 100 and 1000 µg/mL water and ethanol extracts | ↑ calcium content (mg/g dry bone) in the femoral-diaphyseal or metaphyseal tissues in the presence of water-solubilized extract (100 µg/mL), best results in C. sinensis (L.) Kuntze (> 240 mg/g dry bone calcium content) | [69] | |
| Cistuscreticus L. | 5 g of BP in 20 mL distilled water, with final concentrations of 5, 10 or 20 mg/mL 100 g body weight orally administered to rats for 14 days | ↑ calcium content in the femoral-diaphyseal (5, 10 mg/100 g) or metaphyseal (5, 10 or 20 mg/100 g) tissues in the presence of water-solubilized extract (5, 10 or 20 mg/100 g) in STZ-diabetic rats ↓ serum glucose (mg/dL) concentration by BP dose increase ↓ triglyceride concentration ↑ alkaline phosphatase (µmol/min/mg protein) activity and DNA (mg/g wet bone) content in the presence of water-solubilized extract (5, 10 or 20 mg/100 g) by dose increase ↓ serum calcium (mg/dL) concentration in STZ-diabetic rats by BP dose increase ↑ inorganic phosphorus (mg/dL) concentration in STZ-diabetic rats by BP dose increase | [70] | |
| Hepatoprotective | Brasssica napus L. | 30 g of BP in 100 mL distilled water, with orally administered concentrations of: G1 (control): 1mL of distillated water + 1 mL SO; G2: 100 mg/kg/bw/day of WSBP +1 mL SO; G3: 20 mg/kg/bw/day propoxur in 1 mL distilled water and 1 mL SO; G4: 20 mg/kg/bw/day propoxur in 1 mL volume of soy oil and with 100mg/kg/bw/day of WSBP orally administered for 14 days | ↓ plasma and tissue (liver, kidney, brain, heart) MDA (nmol/mL-nmol/mg-prot) levels in the WSBP-treated group (G2) and propoxur + WSBP (G4) compared to propoxour treated ones (G3); ↑ erythrocyte and tissue (liver, kidney, brain, heart) SOD (U/mg Hb-U/mg-prot) activities in WSBP-treated group (G2) and Propoxur + WSBP (G4) compared to propoxour treated ones (G3); ↑ erythrocyte and tissue (liver, kidney, brain, heart) GSH-Px (U/mgHb-U/mg-prot) activities in WSBP-treated group (G2) and propoxur + WSBP (G4) compared to propoxour treated ones (G3); ↑ serum T-protein and albumin (mg/dL) levels in WSBP-treated group (G2) and propoxur + WSBP (G4) compared to propoxour treated ones (G3); ↓ creatin (mg/dL) levels WSBP-treated group (G2) and propoxur + WSBP (G4) compared to propoxour treated ones (G3) | [71] |
| Castanea sativa L. | 1 g of BP with 10 mL methanol, with orally administered concentrations: G1 (control): 0.9% NaCl (i.p.); G2 (control): 0.8 mL/kg OO (i.p.); G3 (control) 0.8 mL/kg Ethanol (i.p.); G4 (CCI4) 0.8 mL/kg CCI4 in OO; G5 (Silibinin) 0.8 mL/kg CCI4 in OO + Silibinin (50 mg/kg/day) gavage; G6 (low BP) 0,8 mL/kg CCI4 in OO (i.p.) + BP (200 mg/kg/day) gavage; G7 (high BP) 0,8 mL/kg CCI4 in OO (i.p.) + BP (400 mg/kg/day) gavage | Protective effect of hepatocytes from oxidative stress Healing of liver damage induced by CCI4 (carbon tetrachloride) toxicity ↓ weight loss % in G6 (low pollen) and G7 (high pollen) compared to G4 (CCI4) G5 (silibinin) and G1-3; ↓ plasma AST, ALT (U/L) and MDA (nmol/mL plasma) levels in G5 (silibinin), G6 (low pollen) and G7 (high pollen) compared to G4 (CCI4) and ↑ levels compared to control G1-3; ↑ liver MDA (nmol/g tissue) in G6 (low pollen) and G7 (high pollen) compared to G5 (silibinin) and control G1-3; ↓ SOD (U/g liver) in G6 (low pollen) and G7 (high pollen) compared to G5 (silibinin) and control G1-3 ↑ AI in G6 (low pollen) compared to G5 (silibinin)and control G1-3 G6 (low pollen) decreased fatty degeneration and regeneration in hepatocytes G7 (high pollen) decreased fatty degeneration | [72] |
| BP or BB | Cell Lines | Treatment Schemes | Obtained Results | References |
|---|---|---|---|---|
| Cernitin T-60 (water-soluble pollen extract with > 90% pollen w/w) | Human prostate cell line DU-145 | The inhibitory patterns for both the naturally occurring fraction designated as FV-7 in the water soluble component of the pollen extract Cernilton® and an authentic synthetic sample of DIBOA were tested at 1, 10 and 100 g/ml | ↓ Growth inhibition (1 μg/mL) of V-7 or DIBOA for day 1–6.; ↑ Inhibitory effect (10 μg/mL) of 50% at day 1 and 80% at day 5; Complete shutdown of the proliferative effects (100 μg/mL) achieved from day 1 to 6 | [100] |
| Chloroform extract from Brassica rapa L. BP (CPBC) | Human cancer cell lines (PC-3, lncap, MCF-7, Hela, BEL-7402, BCG-823, KB, A549 and HO8910) | Cell lines treated with various concentrations of CPBC (12.5–100 μg/mL) for 24 h | CPBC remarkably induced concentration-dependent cytotoxicity in PC-3 and lncap cells; 100 μg/mL CPBC could induce cytotoxicity in MCF-7, Hela, BEL7402, BCG-823, KB, A549 and HO8910 cells | [64] |
| Extracted and fractionated BP polysaccharides from Rosa × rugosa Thunb. (WRPP) | Human colon cancer HT-29 and HCT116 cells | Cells treated with varying concentrations (0, 0.5, 2, 5 mg/mL) of various BP polysaccharides for 72 h | Fractions and sub-fractions of WRPP showed a concentration-dependent proliferation-inhibitory effect on HT-29 and HCT116 cells | [29] |
| BB ethanolic extracts (ebbs) obtained from three different samples of BB from Poland | Glioblastoma cell line (U87MG) | BB extract—effects of EBB1, EBB2, EBB3 (10, 20, 30, 50, 100 µg/mL) on the viability of glioblastoma cell line (U87MG) were studied after 24 h, 48 h and 72 h of treatment. | time-dependent inhibitory effect on the viability of U87MG cells treated EBB; The main inhibitory effect of EBB was observed after 72 h; EBB treatment decreased cell viability to 49–66%. | [81] |
| Salix spp. Beebread (EBB) extract | DASC, U87MG, svgp12 | Cytotoxic effect using MTT assay: EBB (50 mg/mL), combination with TMZ (20 mm) on cells after 24 h, 48 h and 72 h of the treatment | ↓ Cell viability: EBB = 62.4 ± 4.6% on U87MG after 72 h; ↓ Cell viability: EBB + TMZ: 82.9–85.2% after 48 h and 70.7–80.0% after 72 h on U87MG; ↓ Cell viability: EBB + TMZ: 46.2 ± 3.0% after 72 h on SVGp12 | [105] |
| Date palm pollen (DPP) and volatile esters of fermented and non-fermented Phoenix dactylifera L. Pollens (FDPPS) | MCF-7 cell line | Antioxidant activities were determined using DPPH assay, the ferric reducing antioxidant power assay and ABTS assay. Anti-breast-cancer and antiviral activities were determined using the MTT assay | ↑ Antioxidant activity of the FDPP extract of 3.16, 3.42, and 2.14 times that of the DPP extract as determined by the ABTS, ferric reducing antioxidant power (FRAP) and DPPH assays; ↑ Anticancer activity of FDPP against the MCF-7 cell line (IC50: 9.52 μg/mL) compared with the DPP extract (IC50: 96.22 μg/mL); ↑ Antiviral activity of FDPP (CC50: 16.5 μg/mL) compared with DPP (CC50: 38.8 μg/mL). | [111] |
| BPE (bee pollen extract) | MCF-7 and L929 cell lines | Antioxidant activities determined with DPPH assay. Antiproliferative activity at different concentrations of BPE and cisplatin was determined using MTT assay on MCF-7 and L929 cell lines. | BPE EC50: 0.5 mg/mL; BPE IC50: 15 mg/mL on MCF-7 and 26 mg/mL in normal cell line L929; CP IC50: 20 μmol/L on MCF-7 | [112] |
| Dimethyl sulfoxide (DMSO) extracts of BP | HL-60 Myeloid Cancer Cell Lines | DMSO extracts of BP were incubated separately with HL-60 cells, and caspase-3 activity evaluated | ↑ Apoptosis DMSO extract of pollen (2 mg/mL): 52.2%; ↓ Cell viability: 62% | [106] |
| Six BB samples (BB1-BB5, BBC) | Human tumor cell lines: MCF-7, NCI-H460, Hela, HepG2; Non-Tumor Porcine Liver Cells: PLP2 | In vitro assays—cytotoxicity (ranging from > 400 to 68 µg/mL) on all cell lines | BB1 GI25: 164 µg/mL on MCF-7; 345 µg/mL on Hela; BB2 GI25: 84 µg/mL on MCF-7; BB3 GI25: 164 µg/mL on MCF-7; 253 µg/mL on NCI-H460; 225 µg/mL on Hela; 67 µg/mL on HepG2; BB4 GI25: 85 µg/mL on NCI-H460; 209 µg/mL on Hela; BB5 GI25: 68 µg/mL on NCI-H460; 276 µg/mL on Hela; BBC GI25: 366 µg/mL on Hela; None of the BB samples showed toxicity for normal cells | [104] |
| Animal Models | The Type of BP, Collection Site and Application Method | Applied Treatment | Effects of BP Administration | References |
|---|---|---|---|---|
| Eighty male Wistar rats weighing 180–240 g were divided into eight equal groups | Cernitin T60 and Cernitin GBX of specially selected plants, Sweden, orally administered | G1—control; G2—allyl alcohol (AA); G3—AA + Cernitin T60 2.5 mg/kg/day; G4—AA + Cernitin T60 50 mg/kg/day; G5—AA + Cernitin GBX 2.5 mg/kg/day; G6—AA + Cernitin GBX 50 mg/kg/day; G7—AA + Cernitin GBX 2.5 mg/kg/day + Cernitin T60 50 mg/kg/day; G8—AA + Cernitin GBX 50 mg/kg/day + Cernitin T60 50 mg/kg/day. | ↑ liver cells apoptosis in G2 ↓ complete cell degeneration in G4 ↓ hepatotoxicity in G1 and G6 ↓ bilirubin level and liver weight; ↓activity ALT and AST in G3 and G4: ↓ serum enzymes activity in G7 and G8 | [127] |
| Forty male mongrel rabbits with initial body weight 3.0–3.8 kg fed with a standard basic diet, randomly divided into four equal groups | Cernitin T60 and Cernitin GBX—from six plant species: Rye grass, Maize, Timothy grass, Pine, Alder flower and Orchard grass; orally administered | G1—control, G2—HFD, G3– HFD + pollen extracts (Cernitin T60—50 mg/kg/24 h + Cernitin GBX—10 mg/kg/24 h) orally, G4—HFD + clofibrate (Pharmaceutical Works ‘Polfa’/25 mg/kg/24 h) orally. HFD = (g/kg/24 h) cholesterol (0.5), hydrogenated coconut oil (l.0), cholic acid (0.1). The experiment lasted 12 weeks | The intima of the aorta of rabbits of G1 (controls) was unchanged. G2, G4 HFD fed groups— ↑ atherosclerotic plaques, the plaque coverage averaging 83.5% compared to only 33.7% in the pollen extract-treated animals. ↑ weight of livers G2 and G4 | [128] |
| S180-bearing mice | Brassica rapa L. pollen polysaccharide (LBPP), Wuhan, China; orally administered | G1—normal saline injections. G2—control; G3—polysaccharide LBPP (50 mg/kg body weight); G4—thpolysaccharide LBPP (100 mg/kg body weight); G5—polysaccharide LBPP (200 mg/kg body weight); G6—cyclophosphamid (Cy, 20 mg/kg body weight | ↓ growth of S180 (51.26% inhibition rate) with RPP ↓ toxicity RPP + Cy displayed synergism and reduced its toxicity on immune organs. ↑ antitumor activity of RPP ↓ toxicity of RPP on liver, kidney, spleen and thymus | [28] |
| Eighty male CF1 mice (19–21 g) divided into 8 groups | Bee pollen from mesquite (Prosopis juliflora (Sw.) DC.) collected in April in Mexico, extracts of two flavonol concentration (9.794 μg/mL and 21.751 μg/mL), 200 μL orally | G1—provided with cooking oil, G2—200 mL of mesquite BP extract; G3—200 mL of extract of mesquite BP; G4—200 mL of vitamin E (400 UI); G5 intoxicated with bromobenzene—200 mL, 94.211 mg/mL in cooking oil; G6-8 intoxicated with bromobenzene—200 mL, 94.211 mg/mL in cooking oil after the administration of vitamin E (400 UI) | ↑ antioxidant activity in vivo on the liver of bromobenzene-intoxicated mice; ↓ MDA in the in vitro biological systems, flavonols (0.07 mg/mL) in mesquite pollen extract; ↓ MDA in the in vivo system, flavonols (21.751 mg/mL); ↓ Liver LPO (the highest dose) | [129] |
| Female CBA/Hr mice aged 4 months. Experimental and control group consisted of 10 mice each | Cystus incanus L. BP from location in Central Croatia’s Dalmatia coast and offshore islands; orally administered to mice | Mice were fed 14 days before testing either with commercial food pellets (control group) or with commercial food pellets mixed with bee pollen (100 mg/kg bw) | ↓ TBARS in the liver, but without effect in brain; ↑ AOE in the liver, brain and lysate of erythrocytes; ↓ hepatic LPO; ↑ Apoptosis Hspa9a, Tnfsf6 (liver); ↓ Casp 1 and Cc121c; ↑ SOD, GSH-Px and CAT activity in the lysate of erythrocytes (100 mg/kg bw) | [130] |
| Male Kunming mice divided into five groups of 12 animals each | Schisandra chinensis (Turcz.) Baill. pollen extract (SCPE) from Xi’an, China; administered daily orally for 42 days | In vivo study: SCPE (10, 20 and 40 g/kg) administered to CCl4-induced acute liver damage in mice | SCPE—total phenolic content (53.74 ± 1.21 mg GAE/g), total flavonoid content (38.29 ± 0.91 mg Rutin/g); ↓ ALT, AST in acute liver damage induced by CCl4, ↓ MDA formation in liver, ↑ SOD and GSH-Px | [131] |
| Forty-nine 12 weeks old Sprague-Dawley rats divided in seven groups | BP collected during flowering season in Turkey (Western Black Sea region) with dominant component Castanea sativa L. (> 45%), 200 mg/kg/day orally, 400 mg/kg/day orally, 7 days | G1—control, 0.9% NaCl (i.p.); G2—control, 0.8 mL/kg olive oil (i.p.); G3—control, 0.8 mL/kg Ethanol (i.p.); G4—CCI4, 0.8 mL/kg CCI4 in olive oil; G5—Silibinin, 0.8 mL/kg CCI4 in olive oil + Silibinin (50 mg/kg/day) gavage; G6—low pollen, 0,8 mL/kg CCI4 in olive oil (i.p.) + Pollen (200 mg/kg/day) gavage; G7—high pollen, 0,8 mL/kg CCI4 in olive oil (i.p.) + Pollen (400 mg/kg/day) gavage | ↑ AI in G4 compared to G5–G7. Toxicity: ↑ plasma ALT and AST ↑ MDA in liver, RBC and plasma; ↓ SOD in plasma, RBC and liver Pollen administered groups: ↓ Plasma ALT: high dose ↓ Plasma AST ↓ MDA in the plasma, RBC and liver | [72] |
| Male albino mice divided in: six groups with 11 animals each. | Effects of water extracts of Egyptian bee pollen (WEBP) from Beni-Suef, Upper Egypt, on cisplatin (CDDP) induced hepatic, renal, testicular and genotoxicity in male albino mice; Orally administered | G1—negative control (0.9% NaCl solution by intraperitoneal injection (i.p.) twice/week for 3 weeks). G2—i.p. injection of CDDP (2.8 mg/kg b. wt.) twice/week for 3 weeks. G3—8.4 mg/kg b. wt. of propolis extract oral/day for 14 days. G4—WEBP (140 mg/kg b. wt.) oral/once/day for 14 days. G5 and G6—CDDP injected i.p. with (2.8 mg/kg b. wt. twice/week) alone for 1 week and for the next 2 weeks were given WSDP and WEBP by oral intubation and i.p. injection of CDDP | The treatment of mice with WEBP at a dose of 140 mg/kg b. wt./day, for 14 days with CDDP (2.8 mg/kg b. wt.) resulted in: ↓ Lipid peroxidation in the liver, kidney and testis ↑ CAT and GSH in the liver, kidney and testis The positive control animals taken CDDP alone showed toxic histological and genetical manifestations ↑ LPO in kidney, liver and testis ↓ CAT and GSH in the kidney, liver and testis | [132] |
| 36 adult male Sprague Dawley rats divided into six groups of six animals each | Schisandra chinensis (Turcz.) Baill. bee pollen extract (SCBPE) from Jiaozhou, Shandong, China; intragastrically administered (i.g.) in mice | G1—normal saline (10 mL/kg/day) for 12 days and i.p. with saline (10 mL/kg) at the 7th day G2—normal saline (10 mL/kg/day) for 12 days and i.p. CP (8 mg/kg) at the 7th day G3—i.g., VC (400 mg/kg/day) for 12 days days and i.p. CP (8 mg/kg) at the 7th day G4-6—SCBPE (400, 800, 1200 mg/kg/day) for 12 days and i.p. CP (8 mg/kg) at the 7th day | ↓ MDA in kidney and dose-dependent in liver ↓ iNOS in liver and dose-dependent in kidney ↑ SOD dose-dependent in liver and kidney ↑ CAT in the liver and kidney ↑ GSH in the liver and kidney | [133] |
| Molecules | Organs | Species | Agents | Change | References |
|---|---|---|---|---|---|
| Oxidative stress | |||||
| CAT | Liver, brain, heart | rats | Propoxur | ↑ | [71] |
| kidney | rats | Propoxur | ↓ | [71] | |
| Testis | rats | CdCl2 | ↑ | [134] | |
| Plasma | rats | CCl4 | ↓ | [135] | |
| Liver | rats | CCl4 | ↓ | [135] | |
| Liver, kidney, testis | mice | Cisplatin | ↑ | [132] | |
| Liver, kidney | rats | Cisplatin | ↑ | [133] | |
| GST | Liver, kidney, testis | mice | Cisplatin | ↑ | [132] |
| GSH | Liver, kidney | rats | Cisplatin | ↑ | [133] |
| Testis, prostate | rats | CdCl2 | ↑ | [134] | |
| Brain | rats | F | ↑ | [136] | |
| SOD | Liver, kidney, heart, brain | rats | Propoxur | ↑ | [71] |
| Testis | rats | CdCl2 | ↑ | [134] | |
| Liver | mice | CCl4 | ↑ | [131] | |
| Liver | rats | CCl4 | ↑ | [72] | |
| Liver | rats | CCl4 | ↓ | [135] | |
| Plasma | rats | CCl4 | ↑ | [135] | |
| Liver, kidney | rats | Cisplatin | ↑ | [133] | |
| GSH-Px | Liver, kidney, heart, brain | rats | Propoxur | ↑ | [71] |
| Liver | mice | CCl4 | ↑ | [131] | |
| MDA | Liver | mice | Bromobenzene | ↓ | [129] |
| Brain | rats | F | ↓ | [136] | |
| Liver, kidney | rats | Cisplatin | ↓ | [133] | |
| Liver, kidney, testis | mice | Cisplatin | ↓ | [132] | |
| Liver | mice | CCl4 | ↓ | [131] | |
| Liver | rats | CCl4 | ↓ | [72] | |
| Liver | rats | CCl4 | ↓ | [135] | |
| Liver, kidney, heart, brain | rats | Propoxur | ↓ | [71] | |
| iNOS | Liver, kidney | rats | Cisplatin | ↓ | [133] |
| Inflammation | |||||
| IL-6 | Prostate, testis | rats | β-estradiol | ↓ | [137] |
| TNF-α | Prostate, testis | rats | β-estradiol | ↓ | [137] |
| BP | Patient Disorders | Treatment Schemes | Obtained Results | References |
|---|---|---|---|---|
| BP extract Cernilton® (several different plants from Sweden) | 15 patients with chronic relapsing non-bacterial prostatitis or prostatodynia | Cernilton® administration varied from 1 to 18 months | ↑ lasting relief and symptom-free in seven patients ↑ improvement in six patients ↓ response in two patients | [154] |
| BP extract Cernilton® (several different plants in southern Sweden) | 53 patients with benign prostatic hyperplasia (BPH) entered in a double-blind placebo-controlled study | Patients were administered Cernilton® (n = 29) and placebo (n = 24) in a dose of two capsules for 6 months | ↑ subjective improvement with Cernilton® (69%) compared with placebo (30%) ↓ residual urine in Cernilton®-treated and in the antero-posterior (A-P) diameter of the prostate on ultrasound | [155] |
| BP extract Cernilton® R N | treatment of chronic prostatitis syndrome in 90 patients; G1—those without associated complicating factors (CFs) (n = 72) G2—those with complicating factors, i.e., urethral structures, prostatic calculi, bladder neck sclerosis (n = 18) | Cernilton® R N given in a dose of 1 tablet tid and in most cases treatment was continued for 6 months | ↑ response in the group without CFs, 56 (78%); 26 (36%) were cured of their symptoms and signs ↑ in flow rate ↓ in leucocyturia in the post-prostate massage urine (VB3) and ↓ complement C3/coeruloplasmin in the ejaculate in 30 (42%) in the patients with CFs, only one patient showed a response. | [16] |
| BP extract EA-10, P5 (375 mg/pill) | G1—68 cases of CP G2—63 cases of infertility with CP | G1: group A (n = 25): EA-10, P5 + Roxithromycin; group B (n = 21): EA-10, P5; group C (n = 22): Roxithromycin; G2: group A (n = 20): EA-10, P5 + Roxithromycin; group B (n = 21): EA-10, P5; group C (n = 22): Roxithromycin; Administration twice daily for 4 weeks | Pre-treatment group: ↑ LEPS, MDA, NO ↓ Zn content and SOD; Post-treatment: ↑ LEPS, Zn content and sperm motility and viability ↓ MDA and NO | [156] |
| Cernilton®/Cernitin pollen extract | 87 patients: G1: control (n = 18); G2: NIH chronic prostatitis category III (n = 34); G3: BPH (n = 35) | Patients received two capsule daily from 4 to 6 weeks | ↑ pain/discomfort domain score (G1: 0.39; G2: 9.79; G3: 1.66) ↑ QoL (G1: 0.39; G2: 8.21; G3: 4.17) | [157] |
| Prostat/Poltit | 115 patients with chronic nonbacterial prostatitis | Each patient was given 1 tablet of prostat (70 mg P5 + 4 mg EA10) twice a day for 8 weeks | ↓ NIH-CPSI and QoL ↓ symptom rating scores ↓ WBC counts in prostate massage fluid | [158] |
| Prostat/Poltit (74 mg highly defined extract of BP from selected Graminae spp.) | Two groups: 58 patients between 20 and 55 years old with chronic nonbacterial prostatitis or chronic pelvic pain syndrome were randomized to receive Prostat/Poltit (n = 30) or placebo (n = 28) | The dose was three tablets/day. The placebo tablets were identical in appearance to the active tablets, but contained no pollen extract. | patients taking Prostat/Poltit: ↑ clinically improvement or symptom-free in 22 patients, compared to ↑ improvement in 10 of the patients taking placebo | [19] |
| BP extract Cernilton® | 139 men randomly allocated to the pollen extract (n = 70) or placebo (n = 69). | Participants were randomised to receive oral capsules of the pollen extract (2 capsules q8 h) or placebo for 12 wk | ↑ individual domains pain, QoL and NIH-CPSI score after 12 wk of treatment with pollen extract compared to placebo. Adverse events were minor in all patients studied. | [20] |
| DEPROX 500® (1 g pollen extract (500 mg per tablet) and vitamins | 87 males (25 class IIIa and 62 class IIIb) with a mean age of 33.6 ± 5.9 years with chronic prostatitis/chronic pelvic pain syndrome | Participants were randomised to receive oral capsules of DEPROX 500® (two capsules/day; n = 41) or ibuprofen (600 mg, one tablet three times/day; n = 46) for four weeks | ↓ NIH-CPSI total score by ≥ 25% ↓ Adverse events in the DEPROX 500® treatment group compared to ibuprofen ↑ pain relief and QoL in DEPROX 500® treatment group ↑ antioxidant activity of the pollen extract and protective effect on nerves by vitamins combination | [159] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. https://doi.org/10.3390/antiox8120568
Mărgăoan R, Stranț M, Varadi A, Topal E, Yücel B, Cornea-Cipcigan M, Campos MG, Vodnar DC. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants. 2019; 8(12):568. https://doi.org/10.3390/antiox8120568
Chicago/Turabian StyleMărgăoan, Rodica, Mirela Stranț, Alina Varadi, Erkan Topal, Banu Yücel, Mihaiela Cornea-Cipcigan, Maria G. Campos, and Dan C. Vodnar. 2019. "Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits" Antioxidants 8, no. 12: 568. https://doi.org/10.3390/antiox8120568
APA StyleMărgăoan, R., Stranț, M., Varadi, A., Topal, E., Yücel, B., Cornea-Cipcigan, M., Campos, M. G., & Vodnar, D. C. (2019). Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants, 8(12), 568. https://doi.org/10.3390/antiox8120568