Polyphenolic Composition and Hypotensive Effects of Parastrephia quadrangularis (Meyen) Cabrera in Rat
Abstract
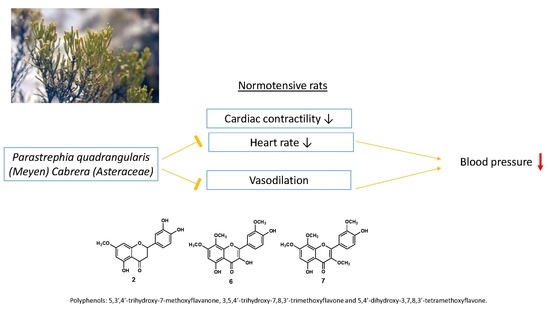
:1. Introduction
2. Material and Methods
2.1. Drugs
2.2. Plant Material
2.3. Extract Preparation
2.4. Extraction and Isolation of Secondary Compounds
2.5. UHPLC-DAD-MS Instrument
2.6. LC and MS Parameters
2.7. Animals
2.8. Langendorff
2.9. Isolation of Aortic Rings
2.10. Vascular Reactivity Experiments
2.11. Cytosolic Calcium Signal on Vascular Smooth Muscle Cells
2.12. Determination of Antioxidant Activity
2.13. Statistical Analysis
3. Results
3.1. The Hydroalcoholic Extract from P. quadrangularis Causes a Hypotensive Effect in Rats
3.2. The Hydroalcoholic Extract from P. quadrangularis (Pq) Induces an Endothelial-Dependent Vasodilator Effect in Rat Aorta
3.3. Role of Potassium Channels on Vasodilation of P. quadrangularis (Pq)
3.4. P. quadrangularis (Pq) Reduced the Contractile Response to KCl and PE
3.5. Isolation and Structural Elucidation of Compounds
3.6. Vasodilation of the Pure Compounds from P. quadrangularis (Pq)
3.7. Determination of the Antioxidant Content of P. quadrangularis (Pq)
3.8. Metabolomic Analysis of P. quadrangularis (Pq) using UHPLC-MS
3.8.1. Simple Organic Acids
3.8.2. Phenolic Acids
3.8.3. Coumarins
3.8.4. Flavonoids
3.8.5. Tremetones
3.8.6. Oxylipins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Villagrán, C.; Castro, V.; Sánchez, G.; Romo, M.; Latorre, C.; Hinojosa, L.F. La tradición surandina del desierto: Etnobotánica del área del Salar de Atacama (Provincia de El Loa, Región de Antofagasta, Chile). Estud. Atacameños 1998, 16, 7–105. [Google Scholar] [CrossRef]
- Teillier, S. High Andean flora and vegetation from Collaguasi Salar de Coposa area, Andes of northern Chile. Rev. Chil. Hist. Nat. 1998, 71, 313–329. [Google Scholar]
- Giberti, G.C. Herbal Folk Medicine in Northwestern Argentina: Compositae. J. Ethnopharmacol. 1983, 7, 321–341. [Google Scholar] [CrossRef]
- Hilgert, N.I. Plants used in home medicine in the Zenta River basin, Northwest Argentina. J. Ethnopharmacol. 2001, 76, 11–34. [Google Scholar] [CrossRef]
- Alberto, M.R.; Zampini, I.C.; Isla, M.I. Inhibition of cyclooxygenase activity by standardized hydroalcoholic extracts of four Asteraceae species from the Argentine Puna. Braz. J. Med. Biol. Res. 2009, 42, 787–790. [Google Scholar] [CrossRef]
- D’Almeida, R.E.; Isla, M.I.; Vildoza, E.D.L.; Quispe, C.; Schmeda-Hirschmann, G.; Alberto, M.R. Inhibition of arachidonic acid metabolism by the Andean crude drug Parastrephia lucida (Meyen) Cabrera. J. Ethnopharmacol. 2013, 150, 1080–1086. [Google Scholar] [CrossRef]
- Torres-Carro, R.; Isla, M.I.; Thomas-Valdes, S.; Jimenez-Aspee, F.; Schmeda-Hirschmann, G.; Alberto, M.R. Inhibition of of pro-inflammatory enzymes by medicinal plants from the Argentinean highlands (Puna). J. Ethnopharmacol. 2017, 205, 57–68. [Google Scholar] [CrossRef]
- Sayago, J.E.; Ordonez, R.M.; Kovacevich, L.N.; Torres, S.; Isla, M.I. Antifungal activity of extracts of extremophile plants from the Argentine Puna to control citrus postharvest pathogens and green mold. Postharvest Biol. Technol. 2012, 67, 19–24. [Google Scholar] [CrossRef]
- Zampini, I.C.; Cuello, S.; Alberto, M.R.; Ordoñez, R.M.; Almeida, R.D.; Solorzano, E.; Isla, M.I. Antimicrobial activity of selected plant species from “the Argentine Puna” against sensitive and multi-resistant bacteria. J. Ethnopharmacol. 2009, 124, 499–505. [Google Scholar] [CrossRef]
- Ruiz, M.D.P.; Ordóñez, R.M.; Isla, M.I.; Sayago, J.E. Activity and mode of action of Parastrephia lepidophylla ethanolic extracts on phytopathogenic fungus strains of lemon fruit from Argentine Northwest. Postharvest Biol. Technol. 2016, 114, 62–68. [Google Scholar] [CrossRef]
- Rodrigo, G.C.; Almanza, G.R.; Akesson, B.; Duan, R.D. Antiproliferative activity of extracts of some Bolivian medicinal plants. J. Med. Plants Res. 2010, 4, 2204–2210. [Google Scholar]
- Gajardo, S.; Aguilar, M.; Stowhas, T.; Salas, F.; Lopez, J.; Quispe, C.; Buc-Calderon, P.; Benites, J. Determination of sun protection factor and antioxidant properties of six Chilean Altiplano plants. Bol. Latinoam. Caribe Plantas Med. Aromat. 2016, 15, 352–363. [Google Scholar]
- Echiburu-Chau, C.; Pastén, L.; Parra, C.; Bórquez, J.; Mocan, A.; Simirgiotis, M.J. High resolution UHPLC-MS characterization and isolation of main compounds from the antioxidant medicinal plant Parastrephia lucida (Meyen). Saudi Pharm. J. 2017, 25, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Benites, J.; Gutierrez, E.; López, J.; Rojas, M.; Rojo, L.; Do Céu Costa, M.; Vinardelld, M.P.; Calderon, P.B. Evaluation of analgesic activities of tremetone derivatives isolated from the chilean altiplano medicine Parastrephia lepidophylla. Nat. Prod. Commun. 2012, 7, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.E.; Benites, J.; López, J.; Rojas, M.; Díaz, P.; Ordóñez, J.; Pastene, E. Antioxidant capacity and polyphenolic content of twelve traditionally used herbal medicinal infusions from the South American Andes. Bol. Latinoam. Caribe Plantas Med. Aromat. 2009, 8, 498–508. [Google Scholar]
- Di Ciaccio, L.S.; Spotorno, V.G.; Estevez, M.M.C.; Rios, D.J.L.; Fortunato, R.H.; Salvat, A.E. Antifungal activity of Parastrephia quadrangularis (Meyen) Cabrera extracts against Fusarium verticillioides. Lett. Appl. Microbiol. 2018, 66, 244–251. [Google Scholar] [CrossRef]
- Loyola, L.A.; Jorge, N.S.; Glauco, M.B. 5,7-dihydroxy-3,8,3′,4′-tetramethoxyflavone from Parastrephia quadrangularis. Phytochemistry 1985, 24, 1871–1872. [Google Scholar] [CrossRef]
- Rao, V.S.; Paiva, L.A.; Souza, M.F.; Campos, A.R.; Ribeiro, R.A.; Brito, G.A.; Teixeira, M.J.; Silveira, E.R. Ternatin, an anti-inflammatory flavonoid, inhibits thioglycolate-elicited rat peritoneal neutrophil accumulation and LPS-activated nitric oxide production in murine macrophages. Planta Medica 2003, 69, 851–853. [Google Scholar] [CrossRef]
- Taguchi, N.; Kunisada, T.; Aoki, H.; Yuriguchi, M. External Pharmaceutical Preparation Containing Yerba Santa Extract and Specific Flavanoid for Promoting Healing of a Wound. JP2017171636A, 28 September 2017. [Google Scholar]
- Go, L.; Tian, H.; Lu, P.-J.; Wang, J.-P.; Wang, Y.-F. Chemical constituents of Sapium sebiferum leaves. Zhongguo Zhongyao Zazhi 2015, 40, 1518–1522. [Google Scholar] [CrossRef]
- Brito, I.; Bórquez, J.; Simirgiotis, M.; Cárdenas, A. Crystal structure of 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4H-chromen-4-one, C19H18O8. Z. Krist. New Cryst. Struct. 2018, 233, 61. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Schmeda-Hirschmann, G.; Avendaño, M.; Sepúlveda, B.; Winterhalter, P. Fast high resolution Orbitrap MS fingerprinting of the resin of Heliotropium taltalense Phil. from the Atacama Desert. Ind. Crops Prod. 2016, 85, 159–166. [Google Scholar] [CrossRef]
- Garneau, F.-X.; Collin, G.J.; Jean, F.-I.; Gagnon, H.; Lopez Arze, J.B. Essential oils from Bolivia. XII. Asteraceae: Ophryosporus piquerioides (DC) Benth. ex Baker. J. Essent. Oil Res. 2013, 25, 388–393. [Google Scholar] [CrossRef]
- Cifuentes, F.; Paredes, A.; Palacios, J.; Muñoz, F.; Carvajal, L.; Nwokocha, C.R.; Morales, G. Hypotensive and antihypertensive effects of a hydroalcoholic extract from Senecio nutans Sch. Bip. (Compositae) in mice: Chronotropic and negative inotropic effect, a nifedipine-like action. J. Ethnopharmacol. 2016, 179, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.; Palacios, J.; Quispe, C.; Nwokocha, C.R.; Morales, G.; Kuzmicic, J.; Cifuentes, F. Hydroalcoholic extract and pure compounds from Senecio nutans Sch. Bip (Compositae) induce vasodilation in rat aorta through endothelium-dependent and independent mechanisms. J. Ethnopharmacol. 2016, 192, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, F.; Bravo, J.; Norambuena, M.; Stegen, S.; Ayavire, A.; Palacios, J. Chronic exposure to arsenic in tap water reduces acetylcholine-induced relaxation in the aorta and increases oxidative stress in female rats. Int. J. Toxicol. 2009, 28, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Rameshrad, M.; Babaei, H.; Azarmi, Y.; Fouladia, D.F. Rat aorta as a pharmacological tool for in vitro and in vivo studies. Life Sci. 2016, 145, 190–204. [Google Scholar] [CrossRef]
- Larrazabal-Fuentes, M.; Palma, J.; Paredes, A.; Mercado, A.; Neira, I.; Lizama, C.; Sepulveda, B.; Bravo, J. Chemical composition, antioxidant capacity, toxicity and antibacterial activity of the essential oils from Acantholippia deserticola (Phil.) Moldenke (Rica rica) and Artemisia copa Phil. (Copa copa) extracted by microwave assisted hydrodistillation. Ind. Crop. Prod. 2019, 142, 111830. [Google Scholar] [CrossRef]
- Cifuentes, F.; Palacios, J.; Kuzmicic, J.; Carvajal, L.; Muñoz, F.; Quispe, C.; Nwokocha, C.R.; Morales, G.; Norambuena-Soto, I.; Chiong, M.; et al. Vasodilator and hypotensive effects of pure compounds and hydroalcoholic extract of Xenophyllum poposum (Phil) V.A Funk (Compositae) on rats. Phytomedicine 2018, 50, 99–108. [Google Scholar] [CrossRef]
- Wang, Y.; Qing, W.-X.; Li, L.-X.; Zhao, D.-B.; Liu, X.-H. Isolation and identification of 5,3′,4′-trihydroxy-7-methoxyflavanone from Artemisia sphaerocephala Kraschen. Chin. J. Struct. Chem. 2014, 33, 199–203. [Google Scholar]
- Shin, H.J.; Nam, J.-W.; Yoon, U.J.; Han, A.-R.; Seo, E.-K. Identification of three new flavonoids from the peels of Citrus unshiu. Helv. Chim. Acta 2012, 95, 240–245. [Google Scholar] [CrossRef]
- Shimokawa, K.; Mashima, I.; Asai, A.; Ohno, T.; Yamada, K.; Kita, M.; Uemura, D. Biological activity, structural features, and synthetic studies of (−)-ternatin, a potent fat-accumulation inhibitor of 3T3-L1 adipocytes. Chem. Asian J. 2008, 3, 438–446. [Google Scholar] [CrossRef]
- Chang, F.-R.; Huang, S.-T.; Liaw, C.-C.; Yen, M.-H.; Hwang, T.-L.; Chen, C.-Y.; Hou, M.-F.; Yuan, S.-S.; Cheng, Y.-B.; Wu, Y.-C. Diterpenes from Grangea maderaspatana. Phytochemistry 2016, 131, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B.X. Fast Detection of Phenolic Compounds in Extracts of Easter Pears (Pyrus communis) from the Atacama Desert by Ultrahigh-Performance Liquid Chromatography and Mass Spectrometry (UHPLC-Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Banerjee, D.; Dutta, N.L. Euphorbetin: A new bicoumarin from Euphorbia lathyris L. Tetrahedron Lett. 1972, 13, 601–604. [Google Scholar] [CrossRef]
- Liang, S.; Feng, Y.; Tian, J.-M.; Lu, M.; Xiong, Z.; Zhang, W.-D. Coumarins from Daphne feddei and their potential anti-inflammatory activities. J. Asian Nat. Prod. Res. 2011, 13, 1074–1080. [Google Scholar] [CrossRef]
- Kumari, G.N.K.; Rao, L.J.M.; Rao, N.S.P. Myricetin methyl ethers from Solanum pubescens. Phytochemistry 1984, 23, 2701–2702. [Google Scholar] [CrossRef]
- Brito, I.; Simirgiotis, M.; Muñoz, R.; Benites, J.; Pasten, L.; Bórquez, J.; Cárdenas, A. Crystal structure of 11-(p-coumaroyloxy)-tremetone, C22H20O5. Z. Krist. New Cryst. Struct. 2017, 232, 13–14. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Rodríguez-Pérez, C.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comprehensive, untargeted, and qualitative RP-HPLC-ESI-QTOF/MS2 metabolite profiling of green asparagus (Asparagus officinalis). J. Food Compos. Anal. 2016, 46, 78–87. [Google Scholar] [CrossRef]
- Simirgiotis, M.; Ramirez, J.; Schmeda-Hirschmann, G.; Kennelly, E. Bioactive coumarins and HPLC-PDA-ESI-ToF-MS metabolic profiling of edible queule fruits (Gomortega keule), an endangered endemic Chilean species. Food Res. Int. 2013, 54, 532–543. [Google Scholar] [CrossRef]
- Ardiles, A.; Barrientos, R.; Simirgiotis, M.J.; Bórquez, J.; Sepúlveda, B.; Areche, C. Gastroprotective Activity of Parastrephia quadrangularis (Meyen), Cabrera from the Atacama Desert. Molecules 2018, 23, 2361. [Google Scholar] [CrossRef] [Green Version]
- Lund-Johansen, P.; Omvik, P. Acute and chronic hemodynamic effects of drugs with different actions on adrenergic receptors: A comparison between alpha blockers and different types of beta blockers with and without vasodilating effect. Cardiovasc. Drugs Ther. 1991, 5, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Schinzari, F.; Tesauro, M.; Cardillo, C. Vascular hyperpolarization in human physiology and cardiovascular risk conditions and disease. Acta Physiol. 2017, 219, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, F.; Palacios, J.; Paredes, A.; Nwokocha, C.R.; Paz, C. 8-Oxo-9-Dihydromakomakine Isolated from Aristotelia chilensis Induces Vasodilation in Rat Aorta: Role of the Extracellular Calcium Influx. Molecules 2018, 23, 3050. [Google Scholar] [CrossRef] [Green Version]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [Green Version]










| Blood Pressure | Control | 10 mg/kg Pq | 20 mg/kg Pq | 40 mg/kg Pq | 80 mg/kg Pq |
|---|---|---|---|---|---|
| MAP, mmHg | 128 ± 4 | 103 ± 2 | 94 ± 8 ** | 82 ± 6 *** | 55 ± 3 *** |
| Blood Pressure | Control | 10 mg/kg Pq | 20 mg/kg Pq | 40 mg/kg Pq |
|---|---|---|---|---|
| SBP, mmHg | 111 ± 6 | 107 ± 2 | 103 ± 2 | 99 ± 2 |
| DBP, mmHg | 75 ± 6 | 73 ± 1 | 66 ± 3 | 49 ± 1 ** |
| PP, mmHg | 36 ± 5 | 35 ± 3 | 37 ± 3 | 50 ± 2 * |
| Drugs | IC50 (µg/mL) |
|---|---|
| Control | 122 ± 1 |
| Endo-denuded | 272 ± 2 *** |
| L-NAME | 903 ± 2 *** |
| ODQ | 104 ± 2 *** |
| BaCl2 | 275 ± 4 *** |
| Glibenclamide | 122 ± 1 |
| TEA | 410 ± 2 *** |
| Drugs | EC50 |
|---|---|
| KCl (mM) | |
| Control | 21.0 ± 1 |
| Pq | 24.0 ± 4 |
| Nimodipine | n.c. |
| PE (nM) | |
| Control | 29.9 ± 1 |
| Pq | 95.0 ± 2 ** |
| Nimodipine | 89.8 ± 1 * |
| Antioxidant Assay | Pq | Trolox | Ascorbic Acid |
|---|---|---|---|
| Total phenolics a | 482 ± 19 | - | - |
| Total flavonoids b | 140 ± 4 | - | - |
| FRAP c | 760 ± 12 | - | - |
| ABTS d | 127 ± 5 * | 77 ± 2 | - |
| DPPH d | 201 ± 4 * | 61 ± 3 | - |
| NO d | 498 ± 5 * | - | 48 ± 1 |
| Molybdate d | 115 ± 4 * | - | 23 ± 1 |
| Hexacyanoferrate (III) d | 73 ± 2 * | - | 19 ± 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifuentes, F.; Palacios, J.; Nwokocha, C.R.; Bórquez, J.; Simirgiotis, M.J.; Norambuena, I.; Chiong, M.; Paredes, A. Polyphenolic Composition and Hypotensive Effects of Parastrephia quadrangularis (Meyen) Cabrera in Rat. Antioxidants 2019, 8, 591. https://doi.org/10.3390/antiox8120591
Cifuentes F, Palacios J, Nwokocha CR, Bórquez J, Simirgiotis MJ, Norambuena I, Chiong M, Paredes A. Polyphenolic Composition and Hypotensive Effects of Parastrephia quadrangularis (Meyen) Cabrera in Rat. Antioxidants. 2019; 8(12):591. https://doi.org/10.3390/antiox8120591
Chicago/Turabian StyleCifuentes, Fredi, Javier Palacios, Chukwuemeka R. Nwokocha, Jorge Bórquez, Mario J. Simirgiotis, Ignacio Norambuena, Mario Chiong, and Adrián Paredes. 2019. "Polyphenolic Composition and Hypotensive Effects of Parastrephia quadrangularis (Meyen) Cabrera in Rat" Antioxidants 8, no. 12: 591. https://doi.org/10.3390/antiox8120591
APA StyleCifuentes, F., Palacios, J., Nwokocha, C. R., Bórquez, J., Simirgiotis, M. J., Norambuena, I., Chiong, M., & Paredes, A. (2019). Polyphenolic Composition and Hypotensive Effects of Parastrephia quadrangularis (Meyen) Cabrera in Rat. Antioxidants, 8(12), 591. https://doi.org/10.3390/antiox8120591