In-Vitro Antioxidant Properties of Lipophilic Antioxidant Compounds from 3 Brown Seaweed
Abstract
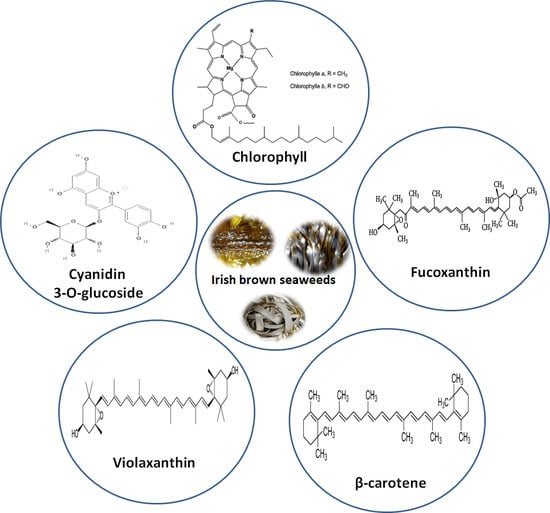
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Solvents and Standards
2.2. Seaweed Materials and Extraction Procedure
2.3. Phytochemical Constituent Analysis
2.4. Antioxidant Capacity Analysis
2.4.1. DPPH Radical Scavenging Capacity Assay
2.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.3. Metal Ion-Chelating Ability Assay
2.5. Characterization of Lipophilic Compounds using Liquid Chromatography Mass Spectrometry (LC–MS)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Content in Lipophilic Extracts
3.2. Antioxidant Capacity of Lipophilic Extracts
3.3. Characterization of Lipophilic Antioxidant Compounds by LC-ESI-MS/MS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Prior, R.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Lee, H.-H.; Lin, C.-T.; Yang, L.-L. Neuroprotection and free radical scavenging effects of Osmanthus fragrans. J. Biomed. Sci. 2007, 14, 819–827. [Google Scholar] [CrossRef]
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.U.; Łuczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, E. Natural and synthetic antioxidants: An updated overview. Free Radic. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef]
- Shahidi, F. Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Technol. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Garcia-Blairsy Reina, G.; Herrero, M.; Senorans, F.J.; Ibanez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Ganesan, P.; Kumar, C.S.; Bhaskar, N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour. Technol. 2008, 99, 2717–2723. [Google Scholar] [CrossRef]
- Herrero, M.; Jaime, L.; Martín-Álvarez, P.J.; Cifuentes, A.; Ibáñez, E. Optimization of the Extraction of Antioxidants from Dunaliella salina Microalga by Pressurized Liquids. J. Agric. Food Chem. 2006, 54, 5597–5603. [Google Scholar] [CrossRef]
- Huang, H.-L.; Wang, B.-G. Antioxidant Capacity and Lipophilic Content of Seaweeds Collected from the Qingdao Coastline. J. Agric. Food Chem. 2004, 52, 4993–4997. [Google Scholar] [CrossRef]
- Maeda, H.; Tsukui, T.; Sashima, T.; Hosokawa, M.; Miyashita, K. Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pac. J. Clin. Nutr. 2008, 17, 196–199. [Google Scholar]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Int. 2017, 99, 995–1001. [Google Scholar] [CrossRef]
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Photo protection of Haematococcus pluvialis algae by astaxanthin: Unique properties of astaxanthin deduced by EPR, optical and electrochemical studies. Antioxidants 2017, 6, 80. [Google Scholar] [CrossRef]
- Zaragozá, M.C.; López, D.; Sáiz, M.P.; Poquet, M.; Pérez, J.; Puig-Parellada, P.; Marmol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and antioxidant activity in vitro and in vivo of two Fucus vesiculosus extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Rajauria, G.; Abu-Ghannam, N. Isolation and partial characterization of bioactive fucoxanthin from Himanthalia elongata brown seaweed: A TLC-based approach. Int. J. Anal. Chem. 2013, 2013, 802573. [Google Scholar] [CrossRef]
- Liu, S.; Lin, J.; Wang, C.; Chen, H.; Yang, D. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chem. 2009, 114, 577–581. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Effect of hydrothermal processing on colour, antioxidant and free radical scavenging capacities of edible Irish brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 2485–2493. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Rajauria, G.; Abu-Ghannam, N.; Gupta, S. Effect of Different Solvents on Polyphenolic Content, Antioxidant Capacity and Antibacterial Activity of Irish York Cabbage. J. Food Biochem. 2012, 36, 344–358. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol. 2008, 20, 367–373. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Chandini, S.K.; Ganesan, P.; Bhaskar, N. In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem. 2008, 107, 707–713. [Google Scholar] [CrossRef]
- Hosokawa, M.; Okada, T.; Mikami, N.; Konishi, I.; Miyashita, K. Bio-functions of marine carotenoids. Food Sci. Biotechnol. 2009, 18, 1–11. [Google Scholar]
- Mhadhebi, L.; Mhadhebi, A.; Robert, J.; Bouraoui, A. Antioxidant, anti-inflammatory and antiproliferative effects of aqueous extracts of three mediterranean brown seaweeds of the genus cystoseira. Iran. J. Pharm. Res. IJPR 2014, 13, 207–220. [Google Scholar]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Abdul, F.; Singh, I.M.P. Effect of ternary solvent system on the permeability of lisinopril across rat skin in vitro. Int. J. Drug Dev. Res. 2009, 1, 67–74. [Google Scholar]
- Sahreen, S.; Khan, M.R.; Khan, R.A. Evaluation of antioxidant activities of various solvent extracts of Carissa opaca fruits. Food Chem. 2010, 122, 1205–1211. [Google Scholar] [CrossRef]
- Sun, T.; Ho, C.T. Antioxidant activities of buckwheat extracts. Food Chem. 2005, 90, 743–749. [Google Scholar] [CrossRef]
- Duan, X.-J.; Zhang, W.-W.; Li, X.-M.; Wang, B.-G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006, 95, 37–43. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant activity of fresh and processed edible seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Gordon, M.H.; Kourimská, L. Effect of antioxidants on losses of tocopherols during deep-fat frying. Food Chem. 1995, 52, 175–177. [Google Scholar] [CrossRef]
- Maoka, T.; Fujiwara, Y.; Hashimoto, K.; Akimoto, N. Rapid Identification of Carotenoids in a Combination of Liquid Chromatography/UV-Visible Absorption Spectrometry by Photodiode-Array Detector and Atomospheric Pressure Chemical Ionization Mass Spectrometry (LC/PAD/APCI-MS). J. Oleo Sci. 2002, 51, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Schütz, K.; Persike, M.; Carle, R.; Schieber, A. Characterization and quantification of anthocyanins in selected artichoke (Cynara scolymus L.) cultivars by HPLC–DAD–ESI–MS n. Anal. Bioanal. Chem. 2006, 384, 1511–1517. [Google Scholar] [CrossRef]
- Heriyanto, J.A.; Shioi, Y.; Limantara, L.; Brotosudarmo, T. Analysis of pigment composition of brown seaweeds collected from Panjang Island, Central Java, Indonesia. Philipp. J. Sci. 2017, 146, 323–330. [Google Scholar]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the colour and the chemical structure of carotenoid pigments. Food Chem. 2007, 101, 1145–1150. [Google Scholar] [CrossRef]
- Schoefs, B. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci. Technol. 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Gouvêa, A.C.; Araujo, M.C.; Schulz, D.F.; Pacheco, S.; Godoy, R.L.; Cabral, L.M. Anthocyanins standards (cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside) isolation from freeze-dried açaí (Euterpe oleraceae Mart.) by HPLC. Food Sci. Technol. 2012, 32, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Rivera, S.M.; Christou, P.; Canela-Garayoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2014, 33, 353–372. [Google Scholar] [CrossRef] [Green Version]
- De Quiros, A.R.-B.; Frecha-Ferreiro, S.; Vidal-Pérez, A.M.; López-Hernández, J. Antioxidant compounds in edible brown seaweeds. Eur. Food Res. Technol. 2010, 231, 495–498. [Google Scholar] [CrossRef]
- Enzell, C.; Francis, G.; Liaaen-Jensen, S. Mass spectrometric studies of carotenoids. I. Occurrence and intensity ratios of M–92 and M–106 peaks. Acta Chem. Scand. 1968, 22, 1054–1055. [Google Scholar] [CrossRef]
- Milenković, S.M.; Zvezdanović, J.B.; Anđelković, T.D.; Marković, D.Z. The identification of chlorophyll and its derivatives in the pigment mixtures: HPLC-chromatography, visible and mass spectroscopy studies. Adv. Technol. 2012, 1, 16–24. [Google Scholar]
- Lim, C.K. High-Performance Liquid Chromatography and Mass Spectrometry of Porphyrins, Chlorophylls and Bilins; World Scientific Publishing Co.: Singapore, 2009; Volume 2, p. 177. [Google Scholar]
- Zvezdanović, J.B.; Petrović, S.M.; Marković, D.Z.; Andjelković, T.D.; Andjelković, D.H. Electrospray ionization mass spectrometry combined with ultra high performance liquid chromatography in the analysis of in vitro formation of chlorophyll complexes with copper and zinc. J. Serb. Chem. Soc. 2014, 79, 689–706. [Google Scholar] [CrossRef]
- Hultin, H. Oxidation of lipids in seafoods. In Seafoods: Chemistry, Processing Technology and Quality; Springer: Dordrecht, The Netherlands, 1994; pp. 49–74. [Google Scholar]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Santoso, J.; Yoshie, Y.; Suzuki, T. Polyphenolic compounds from seaweeds: Distribution and their antioxidative effect. Dev. Food Sci. 2004, 42, 169–177. [Google Scholar]
- Takeshi, S.; Yumiko, Y.-S.; Joko, S. Mineral components and anti-oxidant activities of tropical seaweeds. J. Ocean Univ. China 2005, 4, 205–208. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Identification and characterization of phenolic antioxidant compounds from brown Irish seaweed Himanthalia elongata using LC-DAD–ESI-MS/MS. Innov. Food Sci. Emerg. Technol. 2016, 37, 261–268. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]

 : H. elongata;
: H. elongata;  : L. saccharina;
: L. saccharina;  : L. digitata]. Data are expressed as mean ± SD (n = 6). Ferric Reducing Antioxidant Power (FRAP) values are expressed as mg Trolox equivalent (TE)/g extract (dry weight). Letters (a–g) on each bar are significantly different (p < 0.05) for various solvents, for each individual species. Letters (p–r) on bars at a specific solvent (Mix 4) are significantly different (p < 0.05) among the three species. Mix 1: n-hexane and diethyl ether; Mix 2: n-hexane and chloroform; Mix 3: diethyl ether and chloroform; Mix 4; n-hexane, diethyl ether and chloroform. All the solvents were mixed either 1:1 or 1:1:1 ratio (v/v).
: L. digitata]. Data are expressed as mean ± SD (n = 6). Ferric Reducing Antioxidant Power (FRAP) values are expressed as mg Trolox equivalent (TE)/g extract (dry weight). Letters (a–g) on each bar are significantly different (p < 0.05) for various solvents, for each individual species. Letters (p–r) on bars at a specific solvent (Mix 4) are significantly different (p < 0.05) among the three species. Mix 1: n-hexane and diethyl ether; Mix 2: n-hexane and chloroform; Mix 3: diethyl ether and chloroform; Mix 4; n-hexane, diethyl ether and chloroform. All the solvents were mixed either 1:1 or 1:1:1 ratio (v/v).
 : H. elongata;
: H. elongata;  : L. saccharina;
: L. saccharina;  : L. digitata]. Data are expressed as mean ± SD (n = 6). Ferric Reducing Antioxidant Power (FRAP) values are expressed as mg Trolox equivalent (TE)/g extract (dry weight). Letters (a–g) on each bar are significantly different (p < 0.05) for various solvents, for each individual species. Letters (p–r) on bars at a specific solvent (Mix 4) are significantly different (p < 0.05) among the three species. Mix 1: n-hexane and diethyl ether; Mix 2: n-hexane and chloroform; Mix 3: diethyl ether and chloroform; Mix 4; n-hexane, diethyl ether and chloroform. All the solvents were mixed either 1:1 or 1:1:1 ratio (v/v).
: L. digitata]. Data are expressed as mean ± SD (n = 6). Ferric Reducing Antioxidant Power (FRAP) values are expressed as mg Trolox equivalent (TE)/g extract (dry weight). Letters (a–g) on each bar are significantly different (p < 0.05) for various solvents, for each individual species. Letters (p–r) on bars at a specific solvent (Mix 4) are significantly different (p < 0.05) among the three species. Mix 1: n-hexane and diethyl ether; Mix 2: n-hexane and chloroform; Mix 3: diethyl ether and chloroform; Mix 4; n-hexane, diethyl ether and chloroform. All the solvents were mixed either 1:1 or 1:1:1 ratio (v/v).

| Organic | Dielectric | Yield | TPC | TFC | TCC | TChC |
|---|---|---|---|---|---|---|
| Solvents | Constant (ε) | % | mg GAE/g | mg QE/g | (μg/g) | (µg/g) |
| H. elongata | ||||||
| n-hexane | 2.0 | 0.20 ± 0.02 a,p | 14.1 ± 0.79 a | 11.3 ± 2.50 a | 1.55 ± 0.12 a | 3.23 ± 1.01 a |
| Diethyl ether | 4.3 | 0.17 ± 0.01 b | 165.2 ± 1.46 b | 92.1 ± 5.64 b | 2.18 ± 0.93 b | 2.41 ± 1.00 b |
| Chloroform | 5.0 | 0.11 ± 0.01 c | 71.2 ± 2.33 c | 37.5 ± 3.75 c | 2.81 ± 1.03 c | 6.62 ± 1.34 c |
| Mix 1 | 3.2 | 0.05 ± 0.02 e | 121.5 ± 3.67 d | 60.4 ± 4.02 d | 1.79 ± 0.22 d | 1.70 ± 0.91 d |
| Mix 2 | 3.5 | 0.16 ± 0.03 b,d | 152.3 ± 1.98 e | 55.8 ± 2.60 d | 1.93 ± 0.31 d | 1.56 ± 0.61 d |
| Mix 3 | 4.7 | 0.16 ± 0.02 b | 88.9 ± 2.96 f | 85.8 ± 3.82 b | 2.66 ± 1.04 e | 3.68 ± 1.12 e |
| Mix 4 * | 3.8 | 0.14 ± 0.01 d | 180.2 ± 1.84 g,p | 131.3 ± 4.51 e,p | 3.15 ± 0.91 f,p | 3.86 ± 1.22 e,p |
| L. saccharina | ||||||
| n-hexane | 2.0 | 0.19 ± 0.03 a,q | 9.5 ± 1.93 a | 6.9 ± 0.88 a | 1.45 ± 0.42 a | 4.66 ± 1.03 a |
| Diethyl ether | 4.3 | 0.12 ± 0.01 b | 53.4 ± 0.96 b | 33.1 ± 2.65 b | 2.14 ± 0.83 b | 3.48 ± 0.93 b |
| Chloroform | 5.0 | 0.08 ± 0.02 c,d | 12.5 ± 0.32 c | 7.5 ± 0.00 a | 2.59 ± 0.91 c | 7.82 ± 1.54 c |
| Mix 1 | 3.2 | 0.06 ± 0.01 d | 29.1 ± 0.64 d | 17.5 ± 1.77 c | 1.86 ± 0.63 d | 2.75 ± 1.08 d |
| Mix 2 | 3.5 | 0.11 ± 0.04 b | 25.2 ± 1.61 e | 15.0 ± 3.54 c | 2.08 ± 0.39 b | 2.46 ± 1.17 e |
| Mix 3 | 4.7 | 0.09 ± 0.01 c | 39.8 ± 1.61 f | 22.5 ± 1.77 d | 2.73 ± 0.84 e | 3.48 ± 1.32 b |
| Mix 4 * | 3.8 | 0.07 ± 0.01 c,d | 73.4 ± 0.32 g,q | 56.3 ± 1.77 e,q | 2.75 ± 0.88 e,q | 3.62 ± 1.22 f,p |
| L. digitata | ||||||
| n-hexane | 2.0 | 0.17 ± 0.02 a,r | 7.7 ± 0.64 a | 4.4 ± 0.88 a | 1.20 ± 1.24 a | 6.19 ± 1.42 a |
| Diethyl ether | 4.3 | 0.14 ± 0.02 a,d | 48.9 ± 2.25 b | 29.4 ± 2.65 b | 1.35 ± 1.64 b | 5.65 ± 1.64 b |
| Chloroform | 5.0 | 0.09 ± 0.01 b,c | 15.2 ± 2.25 c | 8.1 ± 0.88 c | 1.93 ± 1.17 c | 8.86 ± 1.93 c |
| Mix 1 | 3.2 | 0.08 ± 0.02 c | 29.1 ± 2.57 d | 18.1 ± 0.88 d | 1.39 ± 1.28 b | 4.62 ± 1.76 d |
| Mix 2 | 3.5 | 0.11 ± 0.04 b | 29.5 ± 2.57 e | 18.1 ± 2.65 d | 1.44 ± 0.68 d | 2.47 ± 1.48 e |
| Mix 3 | 4.7 | 0.12 ± 0.00 b,d | 46.8 ± 2.57 f | 26.3 ± 1.77 b,e | 1.43 ± 1.29 d | 2.71 ± 1.39 f |
| Mix 4 * | 3.8 | 0.11 ± 0.02 b | 52.7 ± 1.93 g,r | 31.9 ± 2.65 e,r | 2.19 ± 1.37 e,r | 2.88 ± 1.08 f,q |
| Peak No | tR [min] | λmax [nm] | Molecular Ion Species M+ [m/z] | Fragment Ions (MS–MS) [m/z] | Identification |
|---|---|---|---|---|---|
| H. elongata | |||||
| 1 | 2.1 | 269,424 | -- | -- | Unidentified |
| 2 | 2.5 | 282,532 | 449 [M + H]+ | 287 [M + H − 162]+ | Cyanidin-3-O-glucoside |
| 3 | 3.2 | 326,425 | -- | -- | Carotenoid |
| 4 | 3.8 | 453,480 | 536.9 [M + H]+ | 444.2 [M + H − 92]+ 430.3 [M + H − 106]+ | β-carotene |
| 5 | 5.3 | 272,408,505 | 601.5 [M + H]+ | 583.5 [M + H − 18]+ 565.5 [M + H − 18 − 18]+ | Violaxanthin |
| 6 | 6.2 | 278,430,620,662 | 893.5 [M + H]+ | 615.2 [M + H − 278]+ | Chlorophyll a derivative |
| 7 | 8.5 | 276,420,446 | -- | -- | Carotenoid |
| 8 | 9.8 | 275,430,592,664 | -- | -- | Chlorophyll |
| 9 | 11.5 | 273,423,513 | -- | Carotenoid | |
| 10 | 13.5 | 327,420,472,505 | -- | -- | Carotenoid |
| 11 | 15.4 | 266,332,448 | 659.6 [M + H]+ | 641.6 [M + H − 18]+ 581.5 [M + H − 78]+ | Fucoxanthin |
| 12 | 24.1 | 278,422,508 | -- | -- | Carotenoid |
| L. saccharina | |||||
| 1 | 1.9 | 278,424 | -- | -- | Unidentified |
| 2 | 2.5 | 326,425 | -- | -- | Carotenoid |
| 3 | 3.0 | 269,425 | -- | -- | Unidentified |
| 4 | 3.4 | 424,572 | -- | -- | Carotenoid |
| 5 | 3.8 | 430,620,662 | 893.5 [M + H]+ | 615.2 [M + H − 278]+ | Chlorophyll a derivative |
| 6 | 4.2 | 272,425,532 | -- | -- | Carotenoid |
| 7 | 5.0 | 276,413,504 | 601 [M + H]+ | 583 [M + H − 18]+ 565 [M + H − 18 − 18]+ | Violaxanthin |
| 8 | 5.9 | 422,532 | -- | -- | Carotenoid |
| 9 | 8.3 | 276,424,504 | -- | -- | Carotenoid |
| 10 | 8.9 | 431,483,665 | -- | -- | Chlorophyll |
| 11 | 10.9 | 421,572 | Carotenoid | ||
| 12 | 12.6 | 266,332,448 | 659.2 [M + H]+ | 641.2 [M + H − 18]+ 581.5 [M + H − 78]+ | Fucoxanthin |
| 13 | 19.7 | 276,429,527 | -- | -- | Carotenoid |
| L. digitata | |||||
| 1 | 1.8 | 282,532 | 449 [M + H]+ | 287 [M + H − 162]+ | Cyanidin-3-O-glucoside |
| 2 | 4.4 | 425,572 | -- | -- | Carotenoid |
| 3 | 5.6 | 273,410,505 | 601.5 [M + H]+ | 583.5 [M + H − 18]+ 565.5 [M + H − 18 − 18]+ | Violaxanthin |
| 4 | 7.4 | 320,531 | -- | -- | Unidentified |
| 5 | 8.1 | 425,483,529 | -- | -- | Carotenoid |
| 6 | 9.4 | 451,484,507,641 | 906.2 [M + H]+ | 628.2 [M + H − 278]+ | Chlorophyll b derivative |
| 7 | 10.4 | 275,430,620,662 | 893.5 [M + H]+ | 615.2 [M + H − 278]+ | Chlorophyll a derivative |
| 8 | 11.1 | 328,536 | -- | -- | Unidentified |
| 9 | 13.0 | 277,359,420 | -- | -- | Carotenoid |
| 10 | 13.7 | 425,572 | -- | -- | Carotenoid |
| 11 | 15.9 | 274,376,427,527 | -- | -- | Carotenoid |
| 12 | 18.8 | 274,399,429,528 | -- | -- | Carotenoid |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajauria, G. In-Vitro Antioxidant Properties of Lipophilic Antioxidant Compounds from 3 Brown Seaweed. Antioxidants 2019, 8, 596. https://doi.org/10.3390/antiox8120596
Rajauria G. In-Vitro Antioxidant Properties of Lipophilic Antioxidant Compounds from 3 Brown Seaweed. Antioxidants. 2019; 8(12):596. https://doi.org/10.3390/antiox8120596
Chicago/Turabian StyleRajauria, Gaurav. 2019. "In-Vitro Antioxidant Properties of Lipophilic Antioxidant Compounds from 3 Brown Seaweed" Antioxidants 8, no. 12: 596. https://doi.org/10.3390/antiox8120596
APA StyleRajauria, G. (2019). In-Vitro Antioxidant Properties of Lipophilic Antioxidant Compounds from 3 Brown Seaweed. Antioxidants, 8(12), 596. https://doi.org/10.3390/antiox8120596