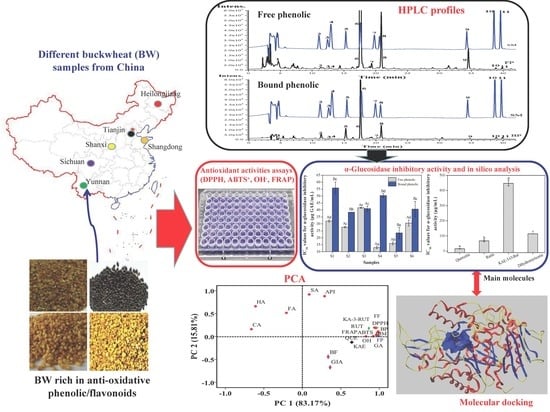
Free and Bound Phenolics of Buckwheat Varieties: HPLC Characterization, Antioxidant Activity, and Inhibitory Potency towards α-Glucosidase with Molecular Docking Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of FP and BP Fractions
2.3. Determination of Phenolic and Flavonoid Contents
2.4. Quantitative Analysis by HPLC
2.5. Antioxidant Activity Assays
2.5.1. DPPH Radical Scavenging Activity Assay
2.5.2. ABTS+ Radical Scavenging Activity Assay
2.5.3. Hydroxyl (OH•) Radical Scavenging Activity Assay
2.5.4. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6. Determination of α-Glucosidase Inhibitory Activity
2.7. Molecular Docking Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Contents (TPC) and Total Flavonoid Contents (TFC)
3.2. Quantitative HPLC Analysis of Phenolic Compositions
3.3. Antioxidant Activities
3.4. Inhibitory Activity against α-Glucosidase
3.5. Molecular Docking Analysis
3.6. Correlations between the Investigated Bio-Activities and Phenolic Compositions
3.7. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BW | buckwheat |
| DW | dried weight |
| TPC | total phenolic content |
| TFC | total flavonoid content |
| FP | free phenolic |
| BP | bound phenolic |
| FF | free flavonoid |
| BF | bound flavonoid |
| GAE | gallic acid equivalents |
| RE | rutin equivalents |
| TE | trolox equivalents |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ABTS | 2, 2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| FRAP | ferric reducing/antioxidant power |
| TPTZ | 2,4,6-tris(2-pyridyl)-s-triazine |
References
- Rosety, I.; Elosegui, S.; Pery, M.T.; Fornieles, G.; Rosety, J.M.; Díaz, A.J.; Rodríguezpareja, A.; Brenes, F.; Rosetyrodríguez, M.; Ordonez, F.J. Association between abdominal obesity and seminal oxidative damage in adults with metabolic syndrome. Rev. Med. Chile 2014, 142, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of mesenchymal stem cells isolated from metabolic syndrome and Type 2 diabetic patients as result of oxidative stress and autophagy may limit their potential therapeutic use. Stem. Cell. Rev. Rep. 2018, 14, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Nakagawa, K.; Kato, S.; Miyazawa, T.; Kimura, F.; Miyazawa, T. The combination of maternal and offspring high-fat diets causes marked oxidative stress and development of metabolic syndrome in mouse offspring. Life Sci. 2016, 151, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Nanjan, M.J.; Mohammed, M.; Kumar, B.R.P.; Chandrasekar, M.J.N. Thiazolidinediones as antidiabetic agents: A critical review. Bioorg. Chem. 2018, 77, 548–567. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Alu’Datt, M.H.; Ereifej, K.; Abu-Zaiton, A.; Alrababah, M.; Almajwal, A.; Rababah, T.; Yang, W. Anti-oxidant, anti-diabetic, and anti-hypertensive effects of extracted phenolics and hydrolyzed peptides from barley protein fractions. Int. J. Food Prop. 2012, 15, 781–795. [Google Scholar] [CrossRef]
- Nam, T.G.; Lim, T.G.; Lee, B.H.; Lim, S.; Kang, H.; Eom, S.H.; Yoo, M.; Jang, H.W.; Kim, D.O. Comparison of anti-Inflammatory effects of flavonoid-Rich common and tartary buckwheat sprout extracts in lipopolysaccharide-stimulated RAW 264.7 and peritoneal macrophages. Oxid. Med. Cell. Longev. 2017, 2017, 9658030. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef]
- Sedej, I.; Sakač, M.; Mandić, A.; Mišan, A.; Tumbas, V.; Čanadanović-Brunet, J. Buckwheat (Fagopyrum esculentum Moench) grain and fractions: Antioxidant compounds and activities. J. Food Sci. 2012, 77, 954–959. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Zheng, S.; Lu, K.; Zhao, G.; Jian, M. The composition, antioxidant and anti-proliferative capacities of phenolic compounds extracted from tartary buckwheat bran (Fagopyrum tartaricum (L.) Gaerth). J. Funct. Foods 2016, 22, 145–155. [Google Scholar] [CrossRef]
- Chandramohan, G.; Alnumair, K.S.; Veeramani, C.; Alsaif, M.A.; Almajwal, A.M. Protective effect of kaempferol, a flavonoid compound, on oxidative mitochondrial damage in streptozotocin-induced diabetic rats. Progr. Nutr. 2015, 17, 3. [Google Scholar]
- Lee, K.H.; Choib, E.M. Myricetin, a naturally occurring flavonoid, prevents 2-deoxy-ribose induced dysfunction and oxidative damage in osteoblastic MC3T3-E1 cells. Eur. J. Pharmacol. 2008, 591, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Q.; Zhang, Q.H. Advances in the development of functional foods from buckwheat. Crit. Rev. Food Technol. 2001, 41, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.R.; Li, X.H.; Yeonbok, K.; Soocheon, C.; Sunju, K.; Sangun, P. Phenolic compoundsin different organs of tartary buckwheat (Fagopyrum tataricum Gaertn.) cultivars. Aust. J. Crop. Sci. 2013, 7, 1861–1865. [Google Scholar]
- Qin, P.Y.; Wang, Q.A.; Shan, F.; Hou, Z.H.; Ren, G.X. Nutritional composition and flavonoids content of flour from different buckwheat cultivars. Int. J. Food Sci. Technol. 2010, 45, 951–958. [Google Scholar] [CrossRef]
- Lu, Y.; Lv, J.; Hao, J.; Niu, Y.; Whent, M.; Costa, J.; Yu, L. Genotype, environment, and their interactions on the phytochemical compositions and radical scavenging properties of soft winter wheat bran. LWT Food Sci. Technol. 2015, 60, 277–283. [Google Scholar] [CrossRef]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef]
- Vollmannová, A.; Margitanová, E.; Tóth, T.; Timoracká, M.; Urminská, D.; Bojn Anská, T.; Čičová, I. Cultivar influence on total polyphenol and rutin contents and total antioxidant capacity in buckwheat, amaranth, and quinoa seeds. Czech J. Food Sci. 2013, 31, 589–595. [Google Scholar] [CrossRef]
- Dvořáková, M.; Guido, L.F.; Dostálek, P.; Skulilová, Z.; Moreira, M.M.; Barros, A.A. Antioxidant properties of free, soluble ester and insoluble-bound phenolic compounds in different barley varieties and corresponding malts. J. Inst. Brew. 2012, 114, 27–33. [Google Scholar] [CrossRef]
- Camarena-Tello, J.C.; Martínez-Flores, H.E.; Garnica-Romo, M.G.; Padilla-Ramírez, J.S.; Saavedra-Molina, A.; Alvarez-Cortes, O.; Bartolomé-Camacho, M.C.; Rodiles-López, J.O. Quantification of phenolic compounds and in vitro radical scavenging abilities with leaf extracts from two varieties of Psidium guajava L. Antioxidants 2018, 7, 34. [Google Scholar] [CrossRef]
- Tuyen, P.T.; Xuan, T.D.; Khang, D.T.; Ahmad, A.; Quan, N.V.; Tu, A.T.; Anh, H.; Minh, T.N. Phenolic compositions and antioxidant properties in bark, flower, inner skin, kernel and leaf extracts of Castanea crenata Sieb. et Zucc. Antioxidants 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, Y.; Wu, Y.N.; Xia, F.G.; Wu, Z.Q. Quickly verifying the antioxidant contribution of the individual composition in natural antioxidants by HPLC-free radical scavenging detection. LWT Food Sci. Technol. 2018, 96, 461–468. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Liu, Y.; Zhang, Q.; Li, Y.; Wu, Z. Release of phenolics compounds from Rubus idaeus L. dried fruits and seeds during simulated in vitro digestion and their bio-activities. J. Funct. Foods 2018, 46, 57–65. [Google Scholar] [CrossRef]
- Husain, S.R.; Cillard, J.; Cillard, P. Hydroxyl radical scavenging activity of flavonoids. Phytochem 1987, 26, 2489–2491. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Zhang, J.; Zhang, W.; Hu, X.; Li, W.; Liu, S. Extraction methods for the releasing of bound phenolics from Rubus idaeus L. leaves and seeds. Ind. Crop. Prod. 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Luo, Y.; Huang, K.; Wu, Z. Quickly screening for potential α-glucosidase inhibitors from guava leaves tea by bioaffinity ultrafiltration coupled with HPLC-ESI-TOF/MS Method. J. Agric. Food Chem. 2018, 66, 1576–1582. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Gao, F.; Zhao, Y.; Cai, S.; Pang, M. The free, esterified, and insoluble-bound phenolic profiles of Rhus chinensis mill. fruits and their pancreatic lipase inhibitory activities with molecular docking analysis. J. Funct. Foods 2018, 40, 729–735. [Google Scholar] [CrossRef]
- Wu, L.F.; Liu, Y.F.; Qin, Y.; Wang, L.; Wu, Z.Q. HPLC-ESI-qTOF-MS/MS characterization, antioxidant activities and inhibitory ability of digestive enzymes with molecular docking analysis of various parts of raspberry (Rubus ideaus L.). Antioxidants 2019, 8, 274. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, Y.; Ma, Y.; Cheng, G.; Cai, S. Phenolic composition, antioxidant properties, and inhibition toward digestive enzymes with molecular docking analysis of different fractions from Prinsepia utilis royle fruits. Molecules 2018, 23, 3373. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, C.; Yao, Y.; Xu, B. Alteration of phenolic profiles and antioxidant capacities of common buckwheat and tartary buckwheat produced in China upon thermal processing. J. Sci. Food Agric. 2019, 99, 5565–5576. [Google Scholar] [CrossRef]
- Nyström, L.; Lampi, A.M.; Andersson, A.A.M.; Kamaleldin, A.; Gebruers, K.; Courtin, C.M.; Delcour, J.A.; Li, L.; Ward, J.L.; Fraś, A. Phytochemicals and dietary fiber components in rye varieties in the HEATHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9758–9767. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Shen, S.R.; Lai, Y.J.; Wu, S.C. Rutin and quercetin, bioactive compounds from tartary buckwheat, prevent liver inflammatory injury. Food Funct. 2013, 4, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Xiang, J.; Li, W.; Ndolo, V.U.; Beta, T. A comparative study of the phenolic compounds and in vitro antioxidant capacity of finger millets from different growing regions in Malawi. J. Cereal Sci. 2019, 87, 143–149. [Google Scholar] [CrossRef]
- Deng, Y.T.; Lin-Shiau, S.Y.; Shyur, L.F.; Lin, J.K. Pu-erh tea polysaccharides decrease blood sugar by inhibition of α-glucosidase activity in vitro and in mice. Food Funct. 2015, 6, 1539–1546. [Google Scholar] [CrossRef]
- Laar, F.A.V.D.; Lucassen, P.L.; Akkermans, R.P.; Van De Lisdonk, E.H.; Rutten, G.E.; Van Weel, C. α-Glucosidase inhibitors for patients with Type 2 diabetes. Diabetes Care 2005, 28, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Jeong, E.Y.; Cho, K.S.; Lee, H.S. α-amylase and α-glucosidase inhibitors isolated from Triticum aestivum L. sprouts. J. Korean Soc. Appl. Biol. 2012, 55, 47–51. [Google Scholar] [CrossRef]
- Pramai, P.; Abdul Hamid, N.A.; Mediani, A.; Maulidiani, M.; Abas, F.; Jiamyangyuen, S. Metabolite profiling, antioxidant, and α-glucosidase inhibitory activities of germinated rice: Nuclear-magnetic-resonance-based metabolomics study. J. Food Drug Anal. 2018, 26, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wei, S.; Zhou, M.J.; Ren, G.X. Antioxidant and α-glucosidase inhibitory activity of colored grains in China. J. Agric. Food Chem. 2010, 58, 770–774. [Google Scholar]
- Qin, P.; Wu, L.; Yao, Y.; Ren, G. Changes in phytochemical compositions, antioxidant and α-glucosidase inhibitory activities during the processing of tartary buckwheat tea. Food Res. Int. 2013, 50, 562–567. [Google Scholar] [CrossRef]
- Zeng, L.; Ding, H.F.; Hua, X.; Zhang, G.W.; Gong, D.M. Galangin inhibits α-glucosidase activity and formation of non-enzymatic glycation products. Food Chem. 2019, 271, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Hosseinighazvini, S.M.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]




| Abbre. | Collect Location | Type | Color | Phenolic Contents (mg GAE/g DW) | Flavonoid Contents (mg RE/g DW) | ||||
|---|---|---|---|---|---|---|---|---|---|
| FP | BP | TP | FF | BF | TF | ||||
| S1 | Tianjin, China | Tartary buckwheat | Black | 7.69 ± 0.35c | 0.73 ± 0.04b | 8.42 ± 0.39d | 14.48 ± 0.26d | 1.07 ± 0.04b | 15.55 ± 0.30d |
| S2 | Sichuan, China | Common buckwheat | Green black | 5.33 ± 0.27a | 0.96 ± 0.02c | 6.29 ± 0.28b | 7.37 ± 0.21a | 1.17 ± 0.03b | 8.54 ± 0.23a |
| S3 | Yunnan, China | Common buckwheat | Black | 6.57 ± 0.25b | 0.74 ± 0.02b | 7.31 ± 0.27c | 11.88 ± 0.53c | 1.28 ± 0.09c | 13.16 ± 0.57c |
| S4 | Shanxi, China | Tartary buckwheat | Light yellow | 13.74 ± 0.24d | 0.66 ± 0.03a | 14.40 ± 0.25e | 26.60 ± 0.86e | 0.72 ± 0.01a | 27.32 ± 0.79e |
| S5 | Shangdong, China | Common buckwheat | Dark yellow | 5.18 ± 0.25a | 0.63 ± 0.03a | 5.81 ± 0.27a | 10.07 ± 0.12b | 0.85 ± 0.03a | 10.92 ± 0.12b |
| S6 | Heilongjiang, China | Tartary buckwheat | Dark green | 6.19 ± 0.01b | 0.90 ± 0.03c | 7.09 ± 0.05c | 13.53 ± 0.85d | 1.38 ± 0.04c | 14.91 ± 0.87c |
| Analytes | Status | Contents (μg/g DW) | |||||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | ||
| Gallic acid (GA) | FP | 22.96 ± 5.4c | 13.30 ± 2.60b | 9.55 ± 1.56a | 75.01 ± 1.53d | ND | ND |
| BP | 67.06 ± 0.82a | 64.76 ± 0.75a | 71.18 ± 3.12b | 60.66 ± 1.07a | 68.73 ± 0.38b | 59.79 ± 1.00a | |
| TP | 90.02 ± 4.99d | 78.05 ± 3.32c | 80.74 ± 4.90c | 135.67 ± 1.31e | 68.73 ± 0.38b | 59.79 ± 1.00a | |
| 4-Hydroxybenzoic acid (4-HA) | FP | 99.39 ± 0.71a | 97.63 ± 1.07a | 96.80 ± 0.40a | 93.26 ± 0.55a | 122.57 ± 1.23c | 102.64 ± 8.18b |
| BP | 175.29 ± 6.65d | 165.75 ± 3.58c | 78.85 ± 5.33b | 164.31 ± 8.44c | 193.72 ± 10.89e | 61.57 ± 7.28a | |
| TP | 274.68 ± 6.53d | 263.38 ± 2.55c | 175.65 ± 4.95b | 257.58 ± 8.04c | 296.58 ± 12.12e | 164.21 ± 6.72a | |
| 5-Caffeoylquinic acid (5-CA) | FP | 4.20 ± 0.06b | 6.73 ± 6.03c | 17.68 ± 2.06d | ND | 19.55 ± 0.71d | 2.20 ± 0.09a |
| BP | 33.82 ± 2.43b | 2.61 ± 0.99a | 46.96 ± 0.01c | ND | ND | ND | |
| TP | 38.02 ± 2.49d | 9.65 ± 5.43b | 64.64 ± 2.06e | ND | 19.55 ± 0.71c | 2.20 ± 0.09a | |
| Syringic acid (SA) | FP | ND | 19.39 ± 0.44a | ND | 31.99 ± 3.35b | 36.75 ± 0.51b | 34.50 ± 7.91b |
| BP | 66.97 ± 2.43d | 27.46 ± 1.97c | 6.69 ± 0.47b | 25.74 ± 2.76c | 4.28 ± 0.16a | 6.93 ± 1.21b | |
| TP | 66.97 ± 2.43d | 46.85 ± 1.96b | 6.69 ± 0.47a | 57.73 ± 4.90c | 41.03 ± 0.64b | 41.44 ± 6.90b | |
| Dihydromyricetin (DIM) | FP | ND | ND | ND | ND | ND | ND |
| BP | 123.13 ± 12.37c | 299.93 ± 1.43e | 83.25 ± 0.57b | 148.95 ± 0.42d | 57.94 ± 0.50a | 57.85 ± 0.14a | |
| TP | 123.13 ± 12.37c | 299.93 ± 1.43e | 83.25 ± 0.57b | 148.95 ± 0.42d | 57.94 ± 0.50a | 57.85 ± 0.14a | |
| Rutin (RUT) | FP | 3813.38 ± 110.33a | 1294.63 ± 38.38b | 3273.15 ± 86.34d | 6288.26 ± 144.01f | 2646.60 ± 142.33c | 3409.12 ± 66.11e |
| BP | 244.75 ± 7.45b | 236.66 ± 11.82b | 324.15 ± 21.98c | 85.02 ± 4.76a | 222.05 ± 11.55c | 416.83 ± 10.87d | |
| TP | 4058.13 ± 107.06e | 1531.29 ± 32.71a | 3597.30 ± 64.89c | 6373.28 ± 148.57f | 2868.66 ± 140.69b | 3825.95 ± 76.95d | |
| Ferulic acid (FA) | FP | ND | 9.26 ± 0.18a | 12.77 ± 0.31b | 9.69 ± 0.21a | 10.95 ± 0.37a | 12.39 ± 0.17b |
| BP | 3.88 ± 0.10a | 3.41 ± 0.13a | 3.71 ± 0.19a | 6.32 ± 0.83b | 3.15 ± 0.11a | 3.08 ± 0.09a | |
| TP | 3.88 ± 0.10a | 12.67 ± 0.29b | 16.49 ± 0.37c | 16.01 ± 0.68c | 14.10 ± 0.41b | 15.46 ± 0.10c | |
| Kaempferol-3-O-rutinoside (KAE-3-RUT) | FP | 1639.34 ± 56.63d | 511.06 ± 15.67a | 1100.85 ± 31.49c | 3618.65 ± 111.97e | 979.31 ± 50.05b | 1077.41 ± 21.29c |
| BP | 212.33 ± 6.29c | 215.77 ± 13.92c | 229.35 ± 20.47d | 43.34 ± 1.34a | 161.17 ± 13.32b | 230.85 ± 16.41d | |
| TP | 1851.67 ± 54.34d | 726.83 ± 14.66a | 1330.20 ± 12.43b | 3661.99 ± 111.75d | 1140.48 ± 49.60c | 1146.31 ± 37.59c | |
| Quercetin (QUE) | FP | 262.14 ± 3.35b | 872.93 ± 21.23d | 328.32 ± 8.74c | 1379.54 ± 33.82e | 144.05 ± 1.68a | 141.03 ± 2.03a |
| BP | 49.51 ± 0.38a | 52.56 ± 2.91b | 47.99 ± 0.16a | 58.35 ± 0.17c | 48.38 ± 0.03a | 47.96 ± 0.11a | |
| TP | 311.65 ± 3.69b | 925.49 ± 21.13d | 376.30 ± 8.61c | 1437.89 ± 33.96e | 192.43 ± 1.71a | 188.99 ± 2.10a | |
| Apigenin (API) | FP | 110.25 ± 1.31b | 108.97 ± 0.03a | 108.86 ± 0.13a | 114.07 ± 0.58b | 113.94 ± 2.47b | 113.70 ± 2.87b |
| BP | 70.58 ± 2.28a | 77.41 ± 6.94b | 79.33 ± 2.38b | 82.69 ± 0.53b | 82.53 ± 0.88b | 79.25 ± 1.09b | |
| TP | 180.83 ± 3.57a | 186.38 ± 6.92b | 188.19 ± 2.33b | 196.76 ± 0.62c | 196.48 ± 2.55c | 192.95 ± 3.66b | |
| Kaempferol (KAE) | FP | 106.24 ± 0.21a | 139.15 ± 1.38b | 108.39 ± 0.39a | 140.10 ± 1.12b | 101.03 ± 0.05a | 100.90 ± 0.08a |
| BP | 64.98 ± 0.31a | 64.20 ± 0.17a | 62.36 ± 0.07a | 62.90 ± 0.02a | 62.37 ± 0.02a | 63.75 ± 0.17a | |
| TP | 171.22 ± 0.52b | 203.35 ± 1.22c | 170.75 ± 0.42b | 203.00 ± 1.10c | 163.40 ± 0.07a | 164.65 ± 0.09a | |
| Antioxidant Activities | Status | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|---|
| DPPH (μmol TE/g DW) | FP | 44.97 ± 1.86d | 17.55 ± 3.10a | 37.35 ± 3.46c | 114.02 ± 0.36e | 26.69 ± 2.24b | 39.38 ± 1.29 c |
| BP | 4.30 ± 0.51a | 7.68 ± 0.20d | 5.65 ± 0.43b | 5.54 ± 1.16b | 6.46 ± 1.84c | 6.40 ± 0.35c | |
| TP | 49.27 ± 2.37d | 25.23 ± 3.30a | 43.00 ± 3.89c | 119.56 ± 1.52e | 33.15 ± 4.08b | 45.78 ± 1.64c | |
| ABTS+ (μmol TE/g DW) | FP | 119.12 ± 1.32d | 69.19 ± 0.30a | 93.50 ± 1.09c | 175.66 ± 1.57e | 75.80 ± 2.28b | 92.12 ± 1.31c |
| BP | 9.01 ± 0.27a | 11.54 ± 0.20c | 9.75 ± 0.69a | 8.92 ± 0.42a | 7.12 ± 0.44b | 10.62 ± 0.20c | |
| TP | 128.13 ± 1.59c | 80.73 ± 0.50a | 103.5 ± 1.78b | 184.58 ± 1.99d | 82.92 ± 2.72a | 102.74 ± 1.51b | |
| OH• (μmol TE/g DW) | FP | 53.69 ± 0.84c | 56.26 ± 1.29c | 32.92 ± 1.90a | 82.64 ± 1.70d | 36.13 ± 3.16a | 42.02 ± 3.44b |
| BP | 14.17 ± 0.55a | 14.54 ± 0.43a | 13.26 ± 0.50a | 13.23 ± 0.74a | 13.90 ± 0.29a | 13.43 ± 0.71a | |
| TP | 67.86 ± 1.39b | 70.80 ± 1.72b | 46.18 ± 2.40a | 95.87 ± 2.44c | 50.03 ± 3.45a | 56.45 ± 4.15a | |
| FRAP (mM FeS(II) E/g DW) | FP | 43.77 ± 1.48c | 31.70 ± 0.33a | 38.82 ± 0.22b | 84.72 ± 3.29d | 29.58 ± 1.31a | 37.06 ± 1.79b |
| BP | 2.90 ± 0.18a | 4.35 ± 0.31c | 3.41 ± 0.16b | 2.95 ± 0.12a | 2.23 ± 0.14a | 3.84 ± 0.03b | |
| TP | 46.67 ± 1.66b | 36.05 ± 0.64a | 43.23 ± 0.38b | 87.67 ± 3.41c | 31.81 ± 1.45a | 40.90 ± 1.82b |
| Main Phenolics | C-Score | T-Score | PMF-Score | CHEM-Score | G-Score | D-Score |
|---|---|---|---|---|---|---|
| Quercetin | 5 | 6.37 | −137.893 | −27.110 | −173.998 | −143.148 |
| Rutin | 4 | 5.94 | −260.712 | −31.241 | −310.716 | −278.108 |
| Kaempferol-3-O-rutinoside | 4 | 4.68 | −147.036 | −26.576 | −210.020 | −220.257 |
| Dihydromyricetin | 4 | 5.50 | −167.849 | −29.871 | −287.447 | −247.370 |
| Analytes | Correlations Matrix | ||||
|---|---|---|---|---|---|
| DPPH | ABTS+ | OH• | FRAP | GIA (IC50) | |
| FP | 0.990*** | 0.983** | 0.851* | 0.998*** | −0.671* |
| BP | 0.583* | 0.932** | 0.803* | 0.947** | −0.604* |
| FF | 0.994*** | 0.981** | 0.765* | 0.974** | −0.723* |
| BF | 0.731* | 0.572* | 0.686* | 0.601* | −0.622* |
| GA | 0.933** | 0.931** | 0.924** | 0.970** | −0.585 |
| 4-HA | 0.455 | 0.513 | 0.546* | 0.535* | −0.478 |
| 5-CA | 0.545 | 0.587* | 0.750* | 0.577* | −0.324 |
| SA | 0.208 | 0.371 | 0.198 | 0.151 | −0.401 |
| DIM | 0.934** | 0.938** | 0.877* | 0.912** | −0.765* |
| RUT | 0.959** | 0.963** | 0.633* | 0.921** | −0.803* |
| FA | 0.550* | 0.251 | 0.299 | 0.092 | −0.396 |
| KAE-3-O-RUT | 0.992** | 0.985** | 0.804* | 0.982** | −0.551* |
| QUE | 0.952* | 0.699* | 0.895** | 0.895* | −0.715* |
| API | 0.478 | 0.375 | 0.245 | 0.382 | −0.348 |
| KAE | 0.453 | 0.402 | 0.800* | 0.558 | −0.618* |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Liu, S.; Yao, L.; Wang, L.; Li, C. Free and Bound Phenolics of Buckwheat Varieties: HPLC Characterization, Antioxidant Activity, and Inhibitory Potency towards α-Glucosidase with Molecular Docking Analysis. Antioxidants 2019, 8, 606. https://doi.org/10.3390/antiox8120606
Zhu H, Liu S, Yao L, Wang L, Li C. Free and Bound Phenolics of Buckwheat Varieties: HPLC Characterization, Antioxidant Activity, and Inhibitory Potency towards α-Glucosidase with Molecular Docking Analysis. Antioxidants. 2019; 8(12):606. https://doi.org/10.3390/antiox8120606
Chicago/Turabian StyleZhu, Huilan, Sixin Liu, Linling Yao, Lu Wang, and Congfa Li. 2019. "Free and Bound Phenolics of Buckwheat Varieties: HPLC Characterization, Antioxidant Activity, and Inhibitory Potency towards α-Glucosidase with Molecular Docking Analysis" Antioxidants 8, no. 12: 606. https://doi.org/10.3390/antiox8120606
APA StyleZhu, H., Liu, S., Yao, L., Wang, L., & Li, C. (2019). Free and Bound Phenolics of Buckwheat Varieties: HPLC Characterization, Antioxidant Activity, and Inhibitory Potency towards α-Glucosidase with Molecular Docking Analysis. Antioxidants, 8(12), 606. https://doi.org/10.3390/antiox8120606