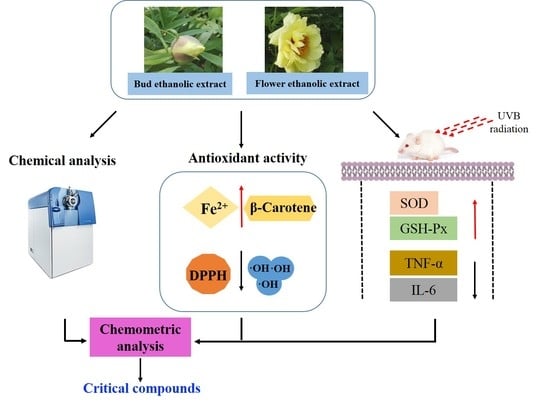
Comparison of Chemical Compositions, Antioxidant, and Anti-Photoaging Activities of Paeonia suffruticosa Flowers at Different Flowering Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Sample Preparation
2.4. Total Phenolic Content Assay
2.5. Total Flavonoid Content Assay
2.6. Antioxidant Assay
2.6.1. DPPH Radical Scavenging Activity Assay
2.6.2. Hydroxyl Radical Scavenging Activity Assay
2.6.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6.4. Inhibition of β-Carotene Bleaching Assay
2.7. Animal Treatment and UV Irradiation
2.8. Histological Analysis
2.9. Biochemical Analysis
2.10. UFLC-DAD-Q-TOF-MS Analysis
2.10.1. System and Conditions
2.10.2. Establishment of Tentative Peak Assignment
2.11. HPLC-DAD Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic and Flavonoid Contents
3.2. Antioxidant Activity In Vitro
3.3. Effects on the Morphology in Mouse Skin
3.4. Effects on the Activities of SOD and GSH-Px in Mouse Skin
3.5. Effects on the Leveles of TNF-α and IL-6 in Mouse Skin
3.6. Chemical Compositions of Ethanolic Extracts of P. suffruticosa Flowers
3.7. Determination of Phytochemicals in Ethanolic Extracts of P. suffruticosa Flowers
3.8. Pearson’s Correlation Analysis between Phytochemicals and Bioactivities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Muzaffer, U.; Paul, V.I.; Prasad, N.R.; Karthikeyan, R.; Agilan, B. Protective effect of Juglans regia L. against ultraviolet B radiation induced inflammatory responses in human epidermal keratinocytes. Phytomedicine 2018, 42, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Tiraravesit, N.; Yakaew, S.; Rukchay, R.; Luangbudnark, W.; Viennet, C.; Humbert, P.; Viyoch, J. Artocarpus altilis heartwood extract protects skin against UVB in vitro and in vivo. J. Ethnopharmacol. 2015, 175, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Divya, S.P.; Wang, X.; Pratheeshkumar, P.; Son, Y.O.; Roy, R.V.; Kim, D.; Dai, J.; Hitron, J.A.; Wang, L.; Asha, P.; et al. Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-κB signaling pathways in SKH-1 mice skin. Toxicol. Appl. Pharm. 2015, 284, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013, 2013, 930163. [Google Scholar] [CrossRef] [PubMed]
- Han, C.V.; Bhat, R. In vitro control of food-borne pathogenic bacteria by essential oils and solvent extracts of underutilized flower buds of Paeonia suffruticosa (andr.). Ind. Crop. Prod 2014, 54, 203–208. [Google Scholar] [CrossRef]
- Zhu, S.; Shirakawa, A.; Shi, Y.; Yu, X.; Tamura, T.; Shibahara, N.; Yoshimatsu, K.; Komatsu, K. Impact of different post-harvest processing methods on the chemical compositions of peony root. J. Nat. Med. 2018, 72, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; He, C.; Peng, Y.; Chen, F.; Xiao, P. Origins, phytochemistry, pharmacology, analytical methods and safety of Cortex Moutan (Paeonia suffruticosa Andrew): A systematic review. Molecules 2017, 22, 946. [Google Scholar] [CrossRef]
- Chen, L.J.; Games, D.E.; Jones, J. Isolation and identification of four flavonoid constituents from the seeds of Oroxylum indicum by high-speed counter-current chromatography. J. Chromatogr. A 2003, 988, 95–105. [Google Scholar] [CrossRef]
- Ogawa, K.; Nakamura, S.; Sugimoto, S.; Tsukioka, J.; Hinomaru, F.; Nakashima, S.; Matsumoto, T.; Ohta, T.; Fujimoto, K.; Yoshikawa, M.; et al. Constituents of flowers of Paeoniaceae plants, Paeonia suffruticosa and Paeonia lactiflora. Phytochem. Lett. 2015, 12, 98–104. [Google Scholar] [CrossRef]
- Yuan, J.; Hao, L.J.; Wu, G.; Wang, S.; Duan, J.A.; Xie, G.Y.; Qin, M.J. Effects of drying methods on the phytochemicals contents and antioxidant properties of chrysanthemum flower heads harvested at two developmental stages. J. Funct. Foods 2015, 19, 786–795. [Google Scholar] [CrossRef]
- Zeng, H.; Su, S.; Xiang, X.; Sha, X.; Zhu, Z.; Wang, Y.; Guo, S.; Yan, H.; Qian, D.; Duan, J. Comparative analysis of the major chemical constituents in Salvia miltiorrhiza roots, stems, leaves and flowers during different growth periods by UPLC-TQ-MS/MS and HPLC-ELSD methods. Molecules 2017, 22, 771. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Zhang, Y.H.; Ma, N.; Zhang, X.L.; Liu, M.H.; Fu, W.M. Comparative analysis of multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities. J. Funct. Foods 2016, 27, 29–41. [Google Scholar] [CrossRef]
- Liu, M.H.; Ko, C.H.; Ma, N.; Tan, P.W.; Fu, W.M.; He, J.Y. Chemical profiles, antioxidant and anti-obesity effects of extract of Bambusa textilis McClure leaves. J. Funct. Foods 2016, 22, 533–546. [Google Scholar] [CrossRef]
- Hassan, S.; Hussein, A.J.; Saeed, A.K. Role of green tea in reducing epidermal thickness upon ultraviolet light-B injury in BALB/c mice. Adv. Biol. 2015, 2015. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Mahasneh, M.A.; Ereifej, K.; Al-Karaki, G.; Al-Duais, M.; Andrade, J.E.; Tranchant, C.C.; Kubow, S.; et al. Profiles of free and bound phenolics extracted from Citrus fruits and their roles in biological systems: Content, and antioxidant, anti-diabetic and anti-hypertensive properties. Food Funct. 2017, 8, 3187–3197. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Interdonato, R.; Rosa, M.; Nieva, C.B.; González, J.A.; Hilal, M.; Prado, F.E. Effects of low UV-B doses on the accumulation of UV-B absorbing compounds and total phenolics and carbohydrate metabolism in the peel of harvested lemons. Environ. Exp. Bot. 2011, 70, 204–211. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Xie, Y.Q.; Chen, F.; Zhao, Y.Y.; Luo, C.X.; Gao, Y.Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Lee, H.J.; Im, A.R.; Kim, S.M.; Kang, H.S.; Lee, J.D.; Chae, S. The flavonoid hesperidin exerts anti-photoaging effect by downregulating matrix metalloproteinase (MMP)-9 expression via mitogen activated protein kinase (MAPK)-dependent signaling pathways. Bmc Complement. Altern. Med. 2018, 18, 39. [Google Scholar] [CrossRef]
- Huang, C.Y.; Lin, Y.T.; Kuo, H.C.; Chiou, W.F.; Lee, M.H. Compounds isolated from Eriobotrya deflexa leaves protect against ultraviolet radiation B-induced photoaging in human fibroblasts. J. Photochem. Photobiol. B 2017, 175, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [Green Version]
- Wang, X.F.; Huang, Y.F.; Wang, L.; Xu, L.Q.; Yu, X.T.; Liu, Y.H.; Li, C.L.; Zhan, J.Y.; Su, Z.R.; Chen, J.N.; et al. Photo-protective activity of pogostone against UV-induced skin premature aging in mice. Exp. Gerontol. 2016, 77, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Fattori, V.; Saito, P.; Melo, C.B.P.S.; Borghi, M.; Pinto, I.C.; Bussmann, A.J.C.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; et al. Lipoxin A4 inhibits UV radiation-induced skin inflammation and oxidative stress in mice. J. Dermatol. Sci. 2018, 91, 164–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Lu, X.; Wei, T.; Dong, Y.; Cai, Z.; Tang, L.; Liu, M. Asperuloside and asperulosidic acid exert an anti-Inflammatory effect via suppression of the NF-κB and MAPK signaling pathways in LPS-induced RAW 264.7 macrophages. Int. J. Mol. Sci. 2018, 19, 2027. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.L.; Lai, C.W.; Cheng, J.T. Antihyperglycemic effects of paeoniflorin and 8-debenzoylpaeoniflorin, glucosides from the root of Paeonia lactiflora. Planta Med. 1997, 63, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Yang, L.; Zeng, X.; Zhang, M.; Wang, Z.T. Characterization of compounds in the Chinese herbal drug Mu-Dan-Pi by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 3275–3288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Geng, Y.; Cui, L.; Duan, W.; Wang, X.; Yan, H. Flavonoids from flowers of Paeonia suffruticosa Andr. Mod. Chin. Med. 2016, 18, 303–306. [Google Scholar]
- Akimoto, N.; Ara, T.; Nakajima, D.; Suda, K.; Ikeda, C.; Takahashi, S.; Muneto, R.; Yamada, M.; Suzuki, H.; Shibata, D.; et al. Flavonoidsearch: A system for comprehensive flavonoid annotation by mass spectrometry. Sci. Rep. 2017, 7, 1243. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wu, J.; Ji, K.X.; Zeng, Q.Y.; Wang, L.S. Methylation mediated by an anthocyanin, O-methyltransferase, is involved in purple flower coloration in Paeonia. J. Exp. Bot. 2015, 66, 6563. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Zeng, R.; Qu, Y.; Huang, L.F. Rapid identification on chemical constituents in roots of Paeonia delavayi var. Lutea by UPLC-Q-TOF-MSE combined with UNIFI informatics platform. Chin. Tradit. Herb. Drugs 2017, 48, 1529–1536. [Google Scholar]
- Yu, S.; Du, S.; Yuan, J.; Hu, Y. Fatty acid profile in the seeds and seed tissues of Paeonia, L. species as new oil plant resources. Sci. Rep. 2016, 6, 26944. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, Y.; Wu, Y.; Wu, Z. Impact of fermentation degree on phenolic compositions and bioactivities during the fermentation of guava leaves with Monascus anka and Bacillus sp. J. Funct. Foods 2018, 41, 183–190. [Google Scholar] [CrossRef]
- Asnaashari, M.; Farhoosh, R.; Sharif, A. Antioxidant activity of gallic acid and methyl gallate in triacylglycerols of Kilka fish oil and its oil-in-water emulsion. Food Chem. 2014, 159, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, T.M.; Hung, T.M.; Thuong, P.T.; Kim, J.C.; Min, B.S. Antioxidative activities of galloyl glucopyranosides from the stem-bark of Juglans mandshurica. Biosci. Biotechnol. Biochem. 2008, 72, 2158–2163. [Google Scholar] [CrossRef]
- Chen, P.; Chen, Y.; Wang, Y.; Cai, S.; Deng, L.; Liu, J.; Zhang, H. Comparative evaluation of hepatoprotective activities of geniposide, crocins and crocetin by CCl4-induced liver injury in mice. Biomol. Ther. 2016, 24, 156. [Google Scholar] [CrossRef]
- Zhang, M.H.; Feng, L.; Zhu, M.M.; Gu, J.F.; Wu, C.; Jia, X.B. Antioxidative and anti-inflammatory activities of paeoniflorin and oxypaeoniflora on AGEs-induced mesangial cell damage. Planta Med. 2013, 79, 1319–1323. [Google Scholar] [CrossRef]
- Senthamizhselvan, O.; Manivannan, J.; Silambarasan, T.; Raja, B. Diosmin pretreatment improves cardiac function and suppresses oxidative stress in rat heart after ischemia/reperfusion. Eur. J. Pharmacol. 2014, 736, 131–137. [Google Scholar] [CrossRef]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.; Iqbal, M.; Anwer, M.K.; Al-Hoshani, A.R.; Attia, S.M.; Ahmad, S.F. Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-κB activation against LPS-induced acute lung injury in mice. Pharmacol. Res. 2015, 102, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jun, C.D.; Suk, K.; Choi, B.J.; Lim, H.; Park, S.; Lee, S.H.; Shin, H.Y.; Kim, D.K.; Shin, T.Y. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol. Sci. 2005, 91, 123–131. [Google Scholar] [CrossRef] [PubMed]






| Items | VC | BHT | Samples | |||
|---|---|---|---|---|---|---|
| BEE | FEE | PEE | SEE | |||
| Extraction yields (%) | -- | -- | 35.46 ± 3.12 | 50.76 ± 1.95 | 42.72 ± 2.48 | 25.77 ± 4.54 |
| Total phenolic content (mg GAE/g ext.) | -- | -- | 191.80 ± 3.44 | 113.84 ± 0.84 | 51.66 ± 0.34 | 77.25 ± 0.58 |
| Total flavonoid content (mg RE/g ext.) | -- | -- | 30.60 ± 0.94 | 49.87 ± 0.82 | 12.98 ± 0.78 | 8.86 ± 0.98 |
| DPPH radical scavenging activity (μg/mL) | 6.16 ± 0.46 | 64.49 ± 4.68 | 34.44 ± 1.35 | 14.83 ± 2.03 | 64.00 ± 5.60 | 42.70 ± 0.79 |
| Hydroxyl radical scavenging activity (mg/mL) | 0.61 ± 0.02 | -- | 1.57 ± 0.04 | 1.79 ± 0.02 | 2.57 ± 0.06 | 2.10 ± 0.004 |
| No. | Rt (min) | Molecular Formula | [M+H]+ (ppm) | [M−H]− (ppm) | Fragments in Positive Mode | Fragments in Negative Mode | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 1.03 | C12H22O11 | 343.12331 (−0.5) | 341.10911 (0.5) | 365.10508 [M+Na]+, 203.0519 [M+Na−C6H10O5]+ | 179.0557 [M−H−C6H10O5]− | Sucrose |
| 2 * | 1.69 | C6H13NO2 | 132.1018 (−0.8) | 130.08735 (0.02) | 86.0986 [M+H−HCOOH]+, 69.0731 [M+H−HCOOH-NH3]+ | 112.0398 [M−H−H2O]−, 86.0338 [M−H−CO2]− | Leucine |
| 3 | 1.77 | C16H24O10 | 377.14221 (−5.3) | 375.12986 (0.5) | 377.14293 [M+NH4]+, 197.0778 [M+H−C6H10O5−H2O]+, 179.0 [M+H−C6H10O5−2H2O]+ | 195.0581 [M−H−C6H10O5−H2O]− | 8-Debenzoylpaeoniflorin |
| 4 | 2.10 | C13H16O10 | 333.08113 (−1.5) | 331.06748 (1.2) | 350.10806 [M+NH4]+ | 169.0138 [M−H−C6H10O5]−, 125.0340 [M−H−C6H10O5−CO2]− | Glucogallin or isomer |
| 5 * | 2.30 | C7H6O5 | 171.02846 (−2) | 169.01513 (5.2) | 153.0181 [M+H−H2O]+, 127.0392 [M+H−CO2]+, 109.1294 [M+H−CO2−H2O]+ | 125.0245 [M−H−CO2]− | Gallic acid |
| 6 | 2.48 | C10H13N5O5 | 284.09891 (−0.1) | 282.08447 (0.3) | 152.0260 [M+H−C5H8O4]+, 135.0293 [M+H−C5H8O4−NH3]+ | 150.0419 [M−H−C5H8O4]−, 133.0159 [M−H−C5H8O4−NH3]− | Guanosine |
| 7 * | 2.70 | C17H26O11 | 405.13989 (−0.8) | 424.18247 [M+H+NH4]+ | 359.1325 [M−H−HCOOH]−, 197.0806 [M−H−HCOOH−C6H10O5]− | Morroniside | |
| 8 * | 2.74 | C10H13N5O4 | 268.10411 (0.3) | 266.08843 (−3.9) | 136.0616 [M+H−C5H8O4]+, 119.0355 [M+H−C5H8O4−NH3]+ | 134.0437 [M−H−C5H8O4]− | Adenosine |
| 9 | 3.20 | C13H16O10 | 331.06755 (1.4) | 331.06745 (1.1) | 315.0683 [M+H−H2O]+, 153.0172 [M+H−H2O−C6H10O5]+ | 241.0325 [M−H−C3H6O3]−, 169.0138 [M−H−C6H10O5]−, 125.0340 [M−H−C6H10O5−CO2]− | Glucogallin or isomer |
| 10 | 3.70 | C19H26O15 | 495.13369 (−1.5) | 493.12022 (0.7) | 333.0772 [M+H−C6H10O5]+, 315.0711 [M+H−C6H12O6]+, 297.0592 [M+H−C6H12O6−H2O]+, 171.0272 [M+H−2C6H10O5]+, 153.0178 [M+H−C6H12O6−C6H10O5]+ | 313.0546 [M−H−C6H12O6]−, 169.0124 [M−H−2C6H10O5]− | Gallic acid-di-O-glucoside |
| 11 | 4.10 | C9H17NO5 | 220.11782 (−0.6) | 218.1036 (0.9) | 202.1071 [M+H−H2O]+, 184.0959 [M+H−2H2O]+, 174.1126 [M+H−HCOOH]+ | 146.0825 [M−H−C4H10O]−, | N-(tert-Butoxycarbonyl)threonine |
| 12 | 4.60 | C14H18O9 | 331.10195 (−1.2) | 329.08812 (0.9) | 169.0427 [M+H−C6H10O5]+ | 167.0341 [M−H−C6H10O5]− | Mudanoside A |
| 13 * | 4.86 | C11H12N2O2 | 205.09701 (−0.7) | 203.08298 (1.9) | 188.0709 [M+H−NH3]+, 118.0657 [M+H−CO2−C2H5N]+ | 159.0911 [M−H−CO2]−, 116.0513 [M−H−CO2−C2H5N]− | Tryptophan |
| 14 | 5.06 | C13H16O9 | 317.08639 (−1) | 315.07227 (0.4) | 155.0321 [M+H−C6H10O5]+, 137.0229 [M+H−C6H10O5−H2O]+ | 153.0189 [M−H−C6H10O5]−, 109.0303 [M−H−C6H10O5−CO2]− | Gentisic acid-5-O-glucoside |
| 15 * | 5.91 | C7H6O3 | 139.03894 (−0.3) | 137.02588 (10.7) | 121.0279 [M+H−H2O]+, 95.0495 [M+H−CO2]+, 77.0400 [M+H−CO2−H2O]+ | 93.0363 [M−H−CO2]− | p-Hydroxybenzoic acid |
| 16 | 6.30 | C8H8O5 | 185.04434 (−0.6) | 183.03088 (5.4) | 153.0188 [M+H−CH3OH]+, 125.0239 [M+H−CH3COOH]+, 107.0137 [M+H−CH3COOH−H2O]+ | 168.0065 [M−H−CH3]−, 124.0174 [M−H−CH3−CO2]− | Methyl gallate |
| 17 | 6.58 | C23H28O14 | 529.15249 (−5.1) | 527.14061 (0) | 493.1146 [M+H−2H2O]+, 315.0648 [M+H−C7H4O5−2H2O]+, 179.0679 [C10H11O3]+ | 345.1178 [M−H−C8H6O5]−, 313.0594 [M−H−C7H4O5−2H2O]−, 271.0502, 211.0201, 169.0146 [C10H11O3]− | Debenzoylgalloylpaeoniflorin |
| 18 * | 6.76 | C17H26O10 | 391.16024 (0.9) | 389.14563 (0.8) | 343.1424 [M−H−HCOOH]−, 181.0874 [M−H−HCOOH−C6H10O5]−, 163.0764 [M−H−HCOOH−C6H10O5−H2O]− | Loganin | |
| 19 * | 7.15 | C17H24O10 | 387.12994 (0.7) | 341.1357 [M−H−HCOOH]−, 179.0705 [M−H−HCOOH−C6H10O5]− | Geniposide | ||
| 20 | 7.28 | C27H24O18 | 637.10441 (1.4) | 635.08956 (0.9) | 467.0798 [M+H−C7H6O3]+, 297.0615 [M+H−2C7H6O3]+, 279.0415 [M+H−2C7H6O3−H2O]+, 153.0171 [C7H5O4]+ | 465.1075 [M−H−C7H6O3]−, 295.0506 [M−H−2C7H6O3]−, 169.1023 [C7H5O5]− | Trigalloyl glucose isomer |
| 21 * | 7.53 | C23H28O12 | 497.1651 (−0.5) | 495.15083 (0.1) | 479.1557 [M+H−H2O]+, 335.1123 [M+H−C6H10O5]+, 317.1018 [M+H−H2O−C6H10O6]+, 197.0810 [M+H−C6H10O6−C7H6O3]+, 179.0704 [M+H−C6H10O6−C7H6O3−H2O]+, 151.0752 [M+H−C6H10O6−C7H6O3−2H2O]+, 133.0643 [M+H−C6H10O6−C7H6O3−3H2O]+ | 465.1446 [M−H−CH2O]−, 333.0998 [M−H−C6H10O5]−, 195.0657 [M−H−C6H10O6−C7H6O3]−, 165.0549 [M−H−C6H10O6−C7H6O3−CH2O]−, 137.0348 [M−H−C6H10O6−C7H6O3−CH2O−H2O]− | Oxypaeoniflorin |
| 22 | 8.23 | C21H22O11 | 451.12391 (0.9) | 449.10937 (1) | 289.0705 [M+H−C6H10O5]+, 271.0590 [M+H−C6H10O5−H2O]+, 153.0168 | 287.0562 [M−H−C6H10O5]−, 259.0618, 151.0034 | Eriodictyol-7-O-glucoside |
| 23 | 8.45 | C24H30O13 | 525.16213 (1.5) | 544.202 [M+NH4]+, 365.1232 [M+H−C6H10O5]+, 347.1106 [M+H−C6H10O5−H2O]+, 197.0797 [M+H−C6H12O6−C8H6O3]+, 179.0689 [M+H−C6H12O6−C8H6O3−H2O]+ | 495.1558 [M−H−CH2O]−, 345.1044 [M−H−C6H12O6]−, 195.0652 [M−H−C6H12O6−C8H6O3]−, 177.0639 [M−H−C6H12O6−C8H6O3−H2O]− | Mudanpioside E | |
| 24 | 8.64 | C27H24O18 | 635.08955 (0.9) | 654.12968 [M+NH4]+, 619.0922 [M+H−H2O]+, 449.0770 [M+H−H2O−C7H6O5]+, 297.0615 [M+H−H2O−C7H6O5−C7H4O4]+, 279.0476 [M+H−2H2O−C7H6O5−C7H4O4]+ | 465.0666 [M−H−C7H6O5]−, 313.0554 [M−H−C7H6O5−C7H4O4]−, 241.05094 [M−H−C7H6O5−2C7H4O4]− | Trigalloyl glucose | |
| 25 | 8.79 | C8H10O3 | 155.07034 (0.5) | 140.0472 [M+H−CH3]+, 123.0441 [M+H−CH4O]+ | 3,4-Dimethoxyphenol | ||
| 26 | 9.20 | C27H30O17 | 627.15612 (0.9) | 625.14123 (0.3) | 465.1022 [M+H−C6H10O5]+, 303.0497 [M+H−2C6H10O5]+ | 463.0928 [M−H−C6H10O5]−, 301.0358 [M−H−2C6H10O5]− | Quercetin-O-di-glucoside or isomer |
| 27 | 9.67 | C20H28O12 | 461.16556 (0.5) | 459.14963 (−2.6) | 299.1206 [M+H−C6H10O5]+, 167.0707 [M+H−C6H10O5−C5H8O4]+ | 297.0885 [M−H−C9H10O3]−, 165.0551 [M−H−C6H10O5−C5H8O4]− | Paeonolide |
| 28 * | 10.03 | C23H28O11 | 481.17026 (−0.4) | 479.15559 (−0.6) | 319.1260 [M+H−C6H10O5]+, 301.1567 [M+H−C6H10O5−H2O]+, 179.0703 [M+H−C6H10O5−H2O−C7H6O2]+, | 449.1520 [M−H−CH2O]−, 327.1088 [M−H−C7H6O2]−, 165.0551 [M−H−C7H6O2−C6H10O5]− | Paeoniflorin |
| 29 | 10.27 | C27H30O16 | 611.16221 (2.5) | 609.14676 (1.1) | 449.1081 [M+H−C6H10O5]+, 287.0552 [M+H−2C6H10O5]+ | 447.0961 [M−H−C6H10O5]−, 285.0403 [M−H−2C6H10O5]− | Kaempferol-3,7-di-O-glucoside |
| 30 | 10.48 | C30H32O16 | 649.17332 (−4.6) | 647.1606 (−1.8) | 511.1395 [M+H−C7H6O3]+, 315.0712 [M+H−C17H18O7]+, 153.0174 [M+H−C17H18O7−C6H10O5]+ | 509.1335 [M−H−C7H6O3]−, 313.0559 [M−H−C17H18O7]−, | Galloyloxypaeoniflorin |
| 31 | 10.78 | C34H28O22 | 789.11272 (−2.3) | 787.10067 (0.9) | 771.1010 [M+H−H2O]+, 619.0877 [M+H−C7H4O4−H2O]+, 449.0694 [M+H−2C7H4O4−2H2O]+, 279.0476 [M+H−3C7H4O4−3H2O]+ | 635.0985 [M−H−C7H4O4]−, 617.0932 [M−H−C7H4O4−H2O]−, 465.0734 [M−H−2C7H4O4−H2O]−, 295.0473 [M−H−3C7H4O4−2H2O]−, | Teragalloyl glucose |
| 32 | 11.28 | C28H32O18 | 657.16586 (−0.4) | 655.135 (2.9) | 495.118 [M+H−C6H10O5]+, 333.0606 [M+H−2C6H10O5]+ | 331.0490 [M−H−2C6H10O5]−, | Patuletin-3,5-di-O-glucoside |
| 33 | 11.69 | C27H30O17 | 627.1546 (−1.6) | 625.1419 (1.4) | 465.1038 [M+H− C6H10O5]+, 303.0500 [M+H−2 C6H10O5]+ | 301.0337 [M−H− C6H10O5]−, 271.0245 [M−H−2 C6H10O5−CH2O]− | Quercetin-di-O-glucoside or isomer |
| 34 | 12.21 | C28H32O17 | 641.17086 (−0.6) | 639.15895 (3.6) | 479.1168 [M+H−C6H10O5]+, 317.0652 [M+H−2C6H10O5]+ | 315.0507 [M−H−2C6H10O5]− | Isorhamnetin-3,7-di-O-glucoside |
| 35 * | 12.33 | C41H32O26 | 941.12626 (0.8) | 939.11461 (3.9) | 771.110 [M+H−C7H6O5]+, 431.0623 [M+H−3C7H6O5]+, 279.0462 [M+H−3C7H6O5−C6H10O5]+, | 769.1070 [M−H−C7H6O5]−, 617.0931 [M−H− C7H6O5−C7H4O4]−, 465.07051 [M−H− C7H6O5−2C7H4O4]−, 295.0430 [M−H−2 C7H6O5−2C7H4O4]− | 1,2,3,4,6-O-Pentagalloyl glucose |
| 36 | 12.36 | C30H32O15 | 633.18077 (−1) | 631.16737 (0.8) | 315.0721 [M+H−C7H6O2−C10H12O10]+, 179.0689 [C10H11O3]+ | 613.1706 [M−H−H2O]−, 509.1421 [M−H− C7H6O2]−, 313.1568 [M−H− C7H6O2− C10H12O10]−, 271.0539 [M−H− C7H6O2− C10H12O10−C2H2O]− | Galloylpaeoniflorin |
| 37 | 12.47 | C29H34O18 | 671.18123 (−0.8) | 669.16952 (3.4) | 509.1271 [M+H−C6H10O5]+, 347.0753 [M+H−2C6H10O5]+, | 345.0623 [M−H−2C6H10O5]−, 301.0361 [M−H−2C6H10O5−CO2]− | 6,3′-Dimethoxyquercetin-di-O-glucoside |
| 38 | 12.98 | C29H34O17 | 655.18661 (−0.4) | 653.17535 (4.6) | 509.1293 [M+H−C5H8O4]+, 347.0763 [M+H−C5H8O4−C6H10O5]+ | 345.0552 [M−H−C5H8O4−C6H10O5]−, 301.0335 [M−H−C5H8O4−C6H10O5−C2H4O]− | Monoxerutin |
| 39 | 13.11 | C21H20O11 | 449.10826 () | 447.09399 (1.6) | 287.0555 [M+H−C6H10O5]+, 153.0181 | 285 [M−H−C6H10O5]− | Kaempferol-3-O-glucoside |
| 40 * | 13.48 | C21H20O12 | 463.08881 (1.3) | 301.0354 [M−H−C6H10O5]−, 151.0026 | Quercetin-3-O-glucoside | ||
| 41 | 13.59 | C27H30O15 | 595.16617 (0.7) | 593.15348 (3.9) | 287.0549 [M+H−C12H20O9]+ | 285.0425 [M−H−C12H20O9]− | Kaempferol-3-O-rutinoside |
| 42 | 13.66 | C30H32O14 | 617.18694 (0.7) | 615.17285 (1.5) | 599.1479 [M+H−H2O]+, 479.1685 [M+H−C7H6O3]+, 443.1294 [M+H−C7H6O3−2H2O]+, 317.1017 [M+H−C7H6O3−C6H10O5]+, 179.0703 [C10H11O3]+ | 431.1396 [M−H−CH2O2−C7H6O3]−, 281.06836 [M−H−CH2O2−C7H6O3−C7H6O2−CH2O]− | Mudanploside H |
| 43 | 13.94 | C28H24O15 | 601.11847 (−0.5) | 599.10555 (2.2) | 287.0544 [M+H−C13H14O9]+ | 285.0413 [M−H−C13H14O9]− | Kaempferol-3-O- (2′’-O-galloyl)-glucoside |
| 44 | 14.28 | C22H22O12 | 479.1188 (0.8) | 477.10532 (3.1) | 317.0661 [M+H−C6H10O5]+ | 315.0537 [M−H− C6H10O5]−, 299.0214 [M−H−C6H10O6]−, 271.0272 [M−H−C6H10O6−H2O]−, 255.0335, 199.0371, 171.0587 | Isorhamnetin-3-O-glucoside |
| 45 | 14.31 | C21H20O10 | 433.11321 (0.7) | 431.10019 (4.2) | 271.0600 [M+H−C6H10O5]+, 153.0176 | 269.0449 [M−H−C6H10O5]− | Apigenin-7-O-glucoside |
| 46 * | 14.61 | C27H30O14 | 579.1711 (0.5) | 577.15834 (3.6) | 433.1131 [M+H−C5H8O4]+, 271.0606 [M+H−C5H8O4−C6H10O5]+, 153.0177 | 269.0462 [M+H−C5H8O4−C6H10O5]7− | Rhoifolin |
| 47 | 14.91 | C28H32O15 | 0.5 | 607.17007 (5.3) | 463.1220 [M+H−C6H10O4]+, 301.0703 [M+H−C6H10O4−C6H10O5]+, 286.0461 [M+H−C6H10O4−C6H10O5−CH3]+ | 443.0939 [M−H−C6H12O5]−, 299.0546 [M−H−C6H10O4−C6H10O5]−, 284.0301 [M−H−C5H8O4−C6H10O5−CH3]− | Diosmin |
| 48 | 14.95 | C22H22O12 | 479.11832 (−0.2) | 477.10464 (1.7) | 317.0668 [M+H−C6H10O5]+ | 285.0372 [M−H−C6H10O5−CH2O]− | Isorhamnetin-7-O-glucoside |
| 49 | 15.34 | C30H32O13 | 601.18754 (−6.7) | 599.17976 (4.6) | 461.1447 [M+H−C7H6O2−H2O]+, 443.1269 [M+H−C7H6O2−2H2O]+, 301.1056 [M+H−C7H4O2−C7H4O3−CO2]+, 283.08136 [M+H−C7H4O2−C7H4O3−CO2−H2O]+, 179.0695 [C10H11O3]+ | 447.1527 [M−H−CH2O−C7H6O2]−, 431.1379 [M−H−CH2O−C7H6O3]−, 281.0682 [M−H−C7H4O2−C7H4O3−CO2−H2O]−, 179.0329 [C10H11O3]− | Mudanpioside C |
| 50 * | 15.69 | C9H10O3 | 167.06995 (−1.9) | 165.05663 (5.5) | 149.0737 [M+H−H2O]+, 121.0616 [M+H−H2O−CO]+ | 150.0318 [M−H−CH3]−, 135.0098 [M−H−CH2O]−, 122.0377 [M−H−CH3−CO]− | Paeonol |
| 51 | 15.78 | C30H32O13 | 599.18003 (5) | 569.1748 [M−H−CH2O]−, 447.1519 [M−H−CH2O−C7H6O2]− | Benzoyloxypaeoniflorin | ||
| 52 | 16.14 | C31H34O14 | 631.20136 (−1.2) | 629.19181 (6.7) | 457.1797 [M+H−C8H6O4−H2O]+, 317.1009 [M+H−C7H6O2−C8H6O4−H2O]+, 297.1009 [M+H−C7H6O2−C8H6O4−2H2O]+, 279.1108 [M+H−C7H6O2−C8H6O4−3H2O]+, 179.0692 [C10H11O3]+ | 477.1511 [M−H−C8H6O3]−, 333.1206 [M−H−C7H6O2−C8H6O4]− | Mudanpioside J |
| 53 * | 16.95 | C15H10O6 | 285.04116 (2.4) | 175.0407, 133.0318 | 151.0031, 133.0278 | Luteolin | |
| 54 * | 17.47 | C30H32O12 | 585.19501 (−2.8) | 583.1816 (−0.9) | 445.1496 [M+H−C7H6O2−H2O]+, 427.1407 [M+H−C7H6O2−2H2O]+, 179.0698 [C10H11O3]+ | 461.1775 [M−H−C7H6O2]−, 343.1562 [M−H−C7H4O2−C7H4O3]− | Benzoylpaeoniflorin |
| 55 * | 18.27 | C15H10O5 | 269.0465 (3.5) | 225.0553, 149.0235, 117.0346 | Apigenin | ||
| 56 | 18.49 | C16H12O6 | 301.07102 (1.2) | 299.05704 (3.1) | 286.0479 [M+H−CH3]+, 258.0531 [M+H−CH3−CO]+ 153.0158 | 284.0352 [M−H−CH3]−, 256.0408 [M−H−CH3−CO]− | Chrysoeriol |
| 57 | 19.66 | C18H32O5 | 329.23227 (0.1) | 327.21889 (3.7) | 293.1959 [M+H−2H2O]+, 275.1959 [M+H−2H2O]+, 225.1488, 185.1171, 161.1375, 119.0830, 105.0700, | 309.2062 [M−H−H2O]−, 291,1961 [M−H−2H2O]−, 229.1448, 211.1341, 171.1038 | Malyngic acid |
| 58 | 20.57 | C18H34O5 | 331.2482 (0.9) | 329.23479 (4.4) | 313.2713 [M+H−H2O]+, 295.2205 [M+H−2H2O]+, 277.2121 [M+H−3H2O]+, 213.1447, 195.1343, 171.1285 | 311.1223 [M−H−H2O]−, 229.1447, 211.1327, 171.023 | 9,12,13-Trihydroxyoctadec-10-enoic acid |
| 59 | 21.15 | C16H32O4 | 289.23763 (1.0) | 287.22468 (6.6) | 271.2277 [M+H−H2O]+, 253.2155 [M+H−2H2O]+, 235.2064 [M+H−3H2O]+, 217.1897 [M+H−4H2O]+, 161.1364, 135.1159, 111.1162 | 269.2127 [M−H−H2O]−, 241.2167 [M−H−HCOOH]− | 3,12-Dihydroxyhexadecanoic acid |
| 60 | 24.79 | C18H30O3 | 295.22674 (−0.1) | 293.21325 (3.5) | 277.2145 [M+H−H2O]+, 231.1648 [M+H−3H2O]+, 207.1426, 171.1093, 147.1158 | 275.2024 [M−H−H2O]−, 223.134, 195.1395 | 9-Oxooctadeca-10,12-dienoic acid |
| 61 | 25.57 | C18H32O3 | 297.24039 (−6.8) | 295.22859 (2.4) | 281.0520 [M+H−H2O]+, 191.0009,133.0103 | 277.21992 [M−H−H2O]−, 195.1402 | 13-Hydroxy-9,11-octadecadienoic acid |
| 62 | 26.63 | C30H48O4 | 473.36163 (−1.9) | 471.34891 (2) | 427.3607 [M+H−HCOOH]+ | 425.2233 [M−H−HCOOH]− | Hederagenin |
| 63 | 28.34 | C30H48O3 | 457.36722 (−0.9) | 455.35374 (1.5) | 439.3579 [M+H−H2O]+, 411.3613 [M+H−HCOOH]+, 393.3485 [M+H−HCOOH−H2O]+ | 409.2504 [M−H−HCOOH]− | Oleanolic acid |
| 64 * | 29.21 | C16H32O2 | 257.24755 (0.2) | 255.23339 (1.7) | 229.2057 [M+H−CO]+ | 237.1230 [M−H−H2O]− | Hexadecanoic acid |
| 65 | 29.42 | C22H44O3 | 357.33656 (0.7) | 355.32323 (4.1) | 339.3238 [M+H−H2O]+, 321.3230 [M+H−2H2O]+, 303.3031 [M+H−3H2O]+ | 309.3120 [M−H−HCOOH]− | 2-Hydroxybehenic acid |
| 66 * | 29.50 | C18H34O2 | 283.26342 (0.9) | 281.24868 (0.3) | 265.2497 [M+H−H2O]+, 247.2429 [M+H−2H2O]+, 237.5873 [M+H−HCOOH]+ | 263.0356 [M−H−H2O]− | 9-Octadecenoic acid |
| 67 | 29.38 | C27H44O2 | 401.34101 (−1) | 399.32889 (5.1) | 283.3072 [M+H−H2O]+ | 355.3114 [M−H−CO2]− | Dehydrotocopherol |
| 68 * | 29.65 | C18H36O2 | 283.26451 (0.9) | 265.2659 [M−H−H2O]− | Octadecanoic acid |
| Compounds | Regression Equation | Correlation Coefficient (r) | Linear Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) | Intraday Precision (RSD%, n = 6) | Interday Precision (RSD%, n = 6) | Accuracy (%, n = 6) |
|---|---|---|---|---|---|---|---|---|
| Gallic acid (5) | y = 9579.15x − 281.13 | 0.99938 | 1.61–807.0 | 0.48 | 1.61 | 1.77 | 2.60 | 97.05 ± 4.64 |
| Oxypaeoniflorin (21) | y = 17106.22x − 36.29 | 0.99952 | 1.44–72.00 | 0.43 | 1.44 | 1.96 | 5.42 | 97.79 ± 4.90 |
| Paeoniflorin (28) | y = 2380.45x − 11.64 | 0.99987 | 0.98–246.0 | 0.29 | 0.98 | 1.67 | 3.24 | 98.02 ± 4.01 |
| 1,2,3,4,6-O-Pentagalloyl glucose (35) | y = 10624.06x + 58.48 | 0.99957 | 1.06–532.0 | 0.32 | 1.06 | 2.57 | 4.22 | 96.86 ± 2.27 |
| Luteolin (53) | y = 20195.32x − 2.47 | 0.99999 | 0.76–19.00 | 0.23 | 0.76 | 1.19 | 3.93 | 99.14 ± 3.96 |
| Apigenin (55) | y = 17107.34x + 12.70 | 0.99987 | 1.72–43.00 | 0.52 | 1.72 | 1.72 | 2.39 | 101.5 ± 3.17 |
| Benzoylpaeoniflorin (54) | y = 2765.39x − 0.54 | 0.99999 | 1.56–39.00 | 0.47 | 1.56 | 1.70 | 3.50 | 101.8 ± 5.17 |
| Paeonol (50) | y = 17767.49x − 3.55 | 0.99978 | 1.96–49.00 | 0.59 | 1.96 | 4.39 | 3.63 | 97.74 ± 1.58 |
| Compounds | BEE (mg/g ext.) | FEE (mg/g ext.) | PEE (mg/g ext.) | SEE (mg/g ext.) |
|---|---|---|---|---|
| Gallic acid (5) | 159.99 ± 5.06 | 46.98 ± 0.92 | 32.31 ± 0.80 | 40.39 ± 1.82 |
| Oxypaeoniflorin (21) | nd | 10.46 ± 0.20 | 6.93 ± 0.17 | 11.19 ± 0.50 |
| Paeoniflorin (28) | 1.76 ± 0.06 | 19.61 ± 0.38 | 16.19 ± 0.40 | 10.84 ± 0.49 |
| 1,2,3,4,6-O-Pentagalloyl glucose (35) | 197.20 ± 6.24 | 38.72 ± 0.76 | 26.25 ± 0.65 | 21.44 ± 0.97 |
| Luteolin (53) | 0.14 ± 0.004 | 0.16 ± 0.003 | 1.23 ± 0.03 | 0.14 ± 0.01 |
| Apigenin (55) | nd | nd | 1.48 ± 0.04 | nd |
| Benzoylpaeoniflorin (54) | 1.30 ± 0.04 | nd | 0.48 ± 0.01 | nd |
| Paeonol (50) | 1.68 ± 0.05 | 1.12 ± 0.02 | 2.60 ± 0.06 | 0.36 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Dong, Y.; Liu, X.; Wan, Y.; Gu, T.; Zhou, X.; Liu, M. Comparison of Chemical Compositions, Antioxidant, and Anti-Photoaging Activities of Paeonia suffruticosa Flowers at Different Flowering Stages. Antioxidants 2019, 8, 345. https://doi.org/10.3390/antiox8090345
He J, Dong Y, Liu X, Wan Y, Gu T, Zhou X, Liu M. Comparison of Chemical Compositions, Antioxidant, and Anti-Photoaging Activities of Paeonia suffruticosa Flowers at Different Flowering Stages. Antioxidants. 2019; 8(9):345. https://doi.org/10.3390/antiox8090345
Chicago/Turabian StyleHe, Jingyu, Yaqian Dong, Xiaoyan Liu, Yiling Wan, Tanwei Gu, Xuefeng Zhou, and Menghua Liu. 2019. "Comparison of Chemical Compositions, Antioxidant, and Anti-Photoaging Activities of Paeonia suffruticosa Flowers at Different Flowering Stages" Antioxidants 8, no. 9: 345. https://doi.org/10.3390/antiox8090345
APA StyleHe, J., Dong, Y., Liu, X., Wan, Y., Gu, T., Zhou, X., & Liu, M. (2019). Comparison of Chemical Compositions, Antioxidant, and Anti-Photoaging Activities of Paeonia suffruticosa Flowers at Different Flowering Stages. Antioxidants, 8(9), 345. https://doi.org/10.3390/antiox8090345