Polydatin Encapsulated Poly [Lactic-co-glycolic acid] Nanoformulation Counteract the 7,12-Dimethylbenz[a] Anthracene Mediated Experimental Carcinogenesis through the Inhibition of Cell Proliferation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Polydatin Encapsulated PLGA Nanoparticles [POL-PLGA-NPs]
2.3. Characterization of Nanoparticles
2.4. Determination of Encapsulation and Loading Efficiency of POL-PLGA-NPs
2.5. In Vitro Releasing Profile of Polydatin
2.6. Animals
2.7. Treatment Protocol
2.8. Histological Studies
2.9. Biochemical Estimations
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Physiochemical Analysis of Polydatin Loaded Nanoparticles for the Determination of Size, Potential, and Morphological Features
3.2. Elemental Analysis of FTIR and XRD Analysis
3.3. Encapsulation Efficiency, Drug Loading, and Drug Releasing Profile of POL-PLGA-NPs
3.4. POL-PLGA-NPs Suppress the DMBA Induced Neoplastic Changes
3.5. POL-PLGA-NPs Enhances the Lipid Peroxidative Byproducts
3.6. Enzymic and Non Enzymic Antioxidant Status
3.7. Xenobiotic Metabolizing Enzymes
3.8. Effect of POL-PLGA-NPs on the Histopathological Features of the DMBA Induced Buccal Pouch Carcinogenesis
3.9. Effect of POL-PLGA-NPs on Apoptotic and Proliferative Marker Expressions in DMBA Induced Buccal Pouch Carcinogenesis
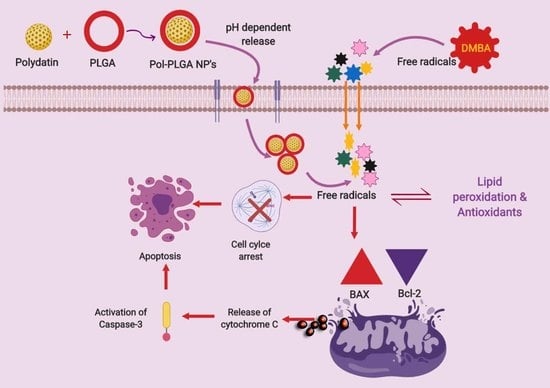
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunasheela, D.; Menon, J.; Ashwin, N. Strategies for fertility preservation in young patients with cancer. Onco Fertil. J. 2018, 1, 86. [Google Scholar] [CrossRef]
- Arem, H.; Loftfield, E. Cancer Epidemiology: A Survey of modifiable risk factors for prevention and survivorship. Am. J. Lifestyle Med. 2017, 12, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Jethwa, A.R.; Khariwala, S.S. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017, 36, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Romero-Perez, A.I.; Ibern-Gomez, M.; Lamuela-Raventos, R.M.; de La Torre-Boronat, M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 1999, 47, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef]
- Chen, S.; Tao, J.; Zhong, F.; Jiao, Y.; Xu, J.; Shen, Q.; Wang, H.; Fan, S.; Zhang, Y. Polydatin down-regulates the phosphorylation level of Creb and induces apoptosis in human breast cancer cell. PLoS ONE 2017, 12, e0176501. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, Z.; Meng, Q.; Jiao, Y.; Xu, J.; Fan, S. Polydatin inhibits growth of lung cancer cells by inducing apoptosis and causing cell cycle arrest. Oncol. Lett. 2014, 7, 295–301. [Google Scholar] [CrossRef]
- Hu, W.H.; Wang, H.Y.; Kong, X.P.; Xiong, Q.P.; Poon, K.K.; Xu, L.; Duan, R.; Chan, G.K.; Dong, T.T.; Tsim, K.W. Polydatin suppresses VEGF-induced angiogenesis through binding with VEGF and inhibiting its receptor signaling. FASEB J. 2018, 33, 532–544. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, S. Polydatin regulates proliferation, apoptosis and autophagy in multiple myeloma cells through mTOR/p70s6k pathway. Onco Targets Ther. 2017, 10, 935–944. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Andima, M.; Costabile, G.; Isert, L.; Ndakala, A.; Derese, S.; Merkel, O. Evaluation of β-Sitosterol loaded PLGA and PEG-PLA nanoparticles for effective treatment of breast cancer: Preparation, physicochemical characterization, and antitumor activity. Pharmaceutics 2018, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Nassir, A.M.; Shahzad, N.; Ibrahim, I.A.; Ahmad, I.; Md, S.; Ain, M.R. Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm. J. 2018, 26, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Bhatnagar, P.; Mishra, S.; Kumar, P.; Shukla, Y.; Gupta, K.C. PLGA-encapsulated tea polyphenols enhance the chemotherapeutic efficacy of cisplatin against human cancer cells and mice bearing Ehrlich ascites carcinoma. Int. J. Nanomed. 2015, 10, 6789. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, Z.; Xiao, Z. Preparation, characterization and thermal stability of β-cyclodextrin/soybean lecithin inclusion complex. Carbohydr. Polym. 2014, 101, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interface Sci. 2010, 351, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.; Fernandez-Fernandez, A.; Nagesetti, A.; McGoron, A.J. Preparation and characterization of a polymeric (PLGA) nanoparticulate drug delivery system with simultaneous incorporation of chemotherapeutic and thermo-optical agents. Colloids Surf. B Biointerfaces 2010, 75, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Breslow, N.E.; Beckwith, J.B.; Takashima, J.; Kelalis, P.; D’Angio, G.J. Treatment outcomes in patients less than 2 years of age with small, stage I, favorable-histology Wilms’ tumors: A report from the National Wilms’ Tumor Study. J. Clin. Oncol. 1993, 11, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxides in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Rao, K.S.; Recknagel, R.O. Early onset of lipoperoxidation in rat liver after carbon tetrachloride administration. Exp. Mol. Pathol. 1968, 9, 271–278. [Google Scholar] [PubMed]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A Modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 131–132. [Google Scholar]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulphydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Palan, P.R.; Mikhail, B.S.; Basu, J.; Romney, S.L. Plasma levels of antioxidant betacarotene and alpha-tocopherol in uterine cervix dysplasias and cancer. Nutr. Cancer 1973, 15, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Omaye, S.T.; Turbull, T.P.; Sauberchich, H.C. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979, 62, 3–11. [Google Scholar] [PubMed]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar]
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar]
- Liu, B.; Li, Y.; Xiao, H.; Liu, Y.; Mo, H.; Ma, H.; Liang, G. Characterization of the super molecular structure of polydatin/6-O-α-maltosyl-β-cyclodextrin inclusion complex. J. Food Sci. 2015, 80, C1156–C1161. [Google Scholar] [CrossRef] [PubMed]
- Briviba, K.; Abrahamse, S.L.; Pool-Zobel, B.L.; Rechkemmer, G. Neurotensin-and EGF-induced metabolic activation of colon carcinoma cells is diminished by dietary flavonoid cyanidin but not by its glycosides. Nutr. Cancer 2001, 41, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, D.; Molski, M. Quantitative structure-antioxidant activity relationship of trans-resveratrol oligomers, trans-4,4′-dihydroxystilbene dimer, trans-resveratrol-3-O-glucuronide, glucosides: Trans-piceid, cis-piceid, trans-astringin and trans-resveratrol-4′-O-beta-d-glucopyranoside. Eur. J. Med. Chem. 2010, 45, 2366–2380. [Google Scholar] [PubMed]
- Mukerjee, A.; Vishwanatha, J.K. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009, 29, 3867–3875. [Google Scholar]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar]
- Lozano, O.; Lazaro-Alfaro, A.; Silva-Platas, C.; Oropeza-Almazan, Y.; Torres-Quintanilla, A.; Bernal-Ramirez, J.; Alves-Figueiredo, H.; Garcia-Rivas, G. Nanoencapsulated quercetin improves cardioprotection during hypoxia-reoxygenation injury through preservation of mitochondrial function. Oxidative Med. Cell. Longev. 2019, 2019, 7683051. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Arya, G.; Das, M.; Sahoo, S.K. Evaluation of curcumin loaded chitosan/PEG blended PLGA nanoparticles for effective treatment of pancreatic cancer. Biomed. Pharmacother. 2018, 102, 555–566. [Google Scholar] [CrossRef]
- Wilson, N.M.; Christou, M.; Turner, C.R.; Wrighton, S.A.; Jefcoate, C.R. Binding and metabolism of benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene by seven purified forms of cytochrome P-450. Carcinogenesis 1984, 5, 1475–1483. [Google Scholar] [CrossRef]
- Anand, M.A.; Suresh, K. Biochemical profiling and chemopreventive activity of phloretin on 7, 12-Dimethylbenz (a) anthracene induced oral carcinogenesis in male golden Syrian hamsters. Toxicol. Int. 2014, 21, 179–185. [Google Scholar]
- Younes, R.N.; Noguchi, Y. Pathophysiology of cancer cachexia. Rev. Hosp. Clín. 2000, 55, 181–193. [Google Scholar] [CrossRef]
- Martano, M.; Stiuso, P.; Facchiano, A.; De Maria, S.; Vanacore, D.; Restucci, B.; Lo Muzio, L. Aryl hydrocarbon receptor, a tumor grade associated marker of oral cancer, is directly downregulated by polydatin: A pilot study. Oncol. Rep. 2018, 40, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Manoharan, S.; Vijayaanand, M.A.; Sugunadevi, G. Chemopreventive and antioxidant efficacy of (6)-paradol in 7,12-dimethylbenz (a) anthracene induced hamster buccal pouch carcinogenesis. Pharmacol. Rep. 2010, 62, 1178–1185. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Mariadoss, A.V.; Vinayagam, R.; Xu, B.; Venkatachalam, K.; Sankaran, V.; Vijayakumar, S.; Bakthavatsalam, S.R.; Mohamed, S.A.; David, E. Phloretin loaded chitosan nanoparticles enhance the antioxidants and apoptotic mechanisms in DMBA induced experimental carcinogenesis. Chem. Biol. Interact. 2019, 308, 11–19. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Wang, H.; Agrawal, S.; Zhang, R. Antisense therapy targeting MDM2 oncogene in prostate cancer: Effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc. Natl. Acad. Sci. USA 2003, 100, 11636–11641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarkovic, K.; Uchida, K.; Kolenc, D.; Hlupic, L.J.; Zarkovic, N. Tissue distribution of lipid peroxidation product acrolein in human colon carcinogenesis. Free Radic. Res. 2006, 40, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Zarkovic, K.; Jakovcevic, A.; Zarkovic, N. Contribution of the HNE-immunohistochemistry to modern pathological concepts of major human diseases. Free Radic. Biol. Med. 2017, 111, 110–125. [Google Scholar] [CrossRef]
- Miki, M.; Ramai, H.; Mino, M.; Yamamoto, Y.; Niki, E. Free radical chain oxidation of rat red blood cells by molecular oxygen and its inhibition by α-toxopherol. Arch. Biochem. Biophys. 1987, 258, 373–380. [Google Scholar] [CrossRef]
- Della Rovere, F.; Granata, A.; Saija, A.; Broccio, M.; Tomaino, A.; Zirilli, A.; De Caridi, G.; Broccio, G. SH groups and glutathione in cancer patients. Anticancer Res. 2000, 20, 1595–1598. [Google Scholar]
- Rajalingam, K.; Sugunadevi, G.; Arokia Vijayaanand, M.; Kalaimathi, J.; Suresh, K. Anti-tumour and anti-oxidative potential of diosgenin against 7,12-dimethylbenz(a)anthracene induced experimental oral carcinogenesis. Pathol. Oncol. Res. 2012, 18, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, A.C. Dietary antioxidants during cancer chemotherapy: Impact on chemotherapeutic effectiveness and development of side effects. Nutr. Cancer 2000, 37, 1–18. [Google Scholar]
- Nakagami, K.; Uchida, T.; Okwada, S. Increased choline kinase activity in 1,2 dimethylhydrazine induced rat colon cancer. Jpn. J. Cancer Res. 1990, 90, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Masotti, L.; Casali, E.; Gesmundo, N.; Sartor, G.; Galeotti, T.; Borrello, S.; Piretti, M.V.; Pagliuca, G. Lipid peroxidation in cancer cells: Chemical and physical studies. Ann. N.Y. Acad. Sci. 1988, 551, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Ray, G.; Husain, S.A. Oxidants, antioxidants and carcinogenesis. Indian J. Exp. Biol. 2002, 40, 1213–1232. [Google Scholar] [PubMed]
- Soujanya, J.; Silambujanaki, P.; Krishna, V.L. Anticancer efficacy of Holoptelea integrifolia, Planch. against 7,12-dimethyl benz (a) anthracene induced breast carcinoma in experimental rats. Int. J. Pharm. Pharm. Sci. 2011, 3, 103–106. [Google Scholar]
- Balamurugan, M.; Sivakumar, K.; Mariadoss, A.V.; Suresh, K. Modulating effect of hypneamusciformis (red seaweed) on lipid peroxidation, antioxidants and biotransforming enzymes in 7, 12-Dimethylbenz(a)anthracene induced mammary carcinogenesis in experimental animals. Pharmacogn. Res. 2017, 9, 108–115. [Google Scholar]
- Mariadoss, A.V.; Kathiresan, S.; Muthusamy, R.; Kathiresan, S. Protective effects of [6]-paradol on histological lesions and immunohistochemical gene expression in DMBA induced hamster buccal pouch carcinogenesis. Asian Pac. J. Cancer Prev. 2013, 14, 3123–3129. [Google Scholar] [CrossRef]
- Faramarzi, L.; Dadashpour, M.; Sadeghzadeh, H.; Mahdavi, M.; Zarghami, N. Enhanced anti-proliferative and pro-apoptotic effects of metformin encapsulated PLGA-PEG nanoparticles on SKOV3 human ovarian carcinoma cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 737–746. [Google Scholar] [CrossRef] [Green Version]













| Concentration of Polydatin | 1 mg/mL | 3 mg/mL | 5 mg/mL |
|---|---|---|---|
| Loading efficiency (%) | 3.81 ± 0.25 | 7.29 ± 0.63 | 10.71 ± 0.74 |
| Encapsulation efficiency (%) | 22.78 ± 1.37 | 83.15 ± 6.22 | 96.54 ± 8.03 |
| Parameters | Control | DMBA | DMBA+ POL-PLGA-NPs (7.5 mg/kg b.wt.) | DMBA+ POL-PLGA-NPs (15 mg/kg b.wt.) | DMBA+ POL-PLGA-NPs (30 mg/kg b.wt.) | POL-PLGA-NP alone (30 mg/kg b.wt.) |
|---|---|---|---|---|---|---|
| Initial Bodyweight (g) | 125.24 ± 6.47 a | 120.15 ± 3.03 b | 131.15 ± 9.03 b | 130.24 ± 12.16 c | 126.82 ± 8.04 e | 130.47 ± 7.95 a |
| Final Bodyweight (g) | 193.45 ± 7.17 a | 136.73 ± 9.01 b | 149.97 ± 8.61 c | 151.16 ± 9.81 d | 168.33 ± 9.17 e | 183.12 ± 8.07 a |
| Weight Gain(g) | 68.21 ± 6.72 a | 16.54 ± 1.72 b | 18.82 ± 5.72 c | 20.92 ± 6.74 d | 43.51 ± 6.38 e | 52.65 ± 5.21 a |
| Tumor Incidence | - | 100 | 80 | 68 | 20 | - |
| Total number of tumors/animals | - | 12/(6) | 10/(6) | 7/(6) | 2/(6) | - |
| Tumor Burden | - | 2024.76 ± 82.6 * | 1586.2 ± 62.45 | 942.61.54.83 | 105.73 ± 7.11 *** | |
| Tumor Volume | - | 168.73 ± 6.43 * | 158.6 ± 5.84 | 134.3 ± 4.81 | 52.86 ± 1.33 *** | - |
| Keratosis | Not observed | Severe | Moderate | Moderate | Mild | Not observed |
| Hyperplasia | Not observed | Severe | Moderate | Moderate | Mild | Not observed |
| Dysplasia | Not observed | Severe | Moderate | Moderate | Mild | Not observed |
| Squamous cell carcinoma | Not observed | Well differentiated | Severe | Moderate | Mild | Not observed |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayalakshmi, S.; Mariadoss, A.V.A.; Ramachandran, V.; Shalini, V.; Agilan, B.; Sangeetha, C.C.; Balu, P.; Kotakadi, V.S.; Karthikkumar, V.; Ernest, D. Polydatin Encapsulated Poly [Lactic-co-glycolic acid] Nanoformulation Counteract the 7,12-Dimethylbenz[a] Anthracene Mediated Experimental Carcinogenesis through the Inhibition of Cell Proliferation. Antioxidants 2019, 8, 375. https://doi.org/10.3390/antiox8090375
Vijayalakshmi S, Mariadoss AVA, Ramachandran V, Shalini V, Agilan B, Sangeetha CC, Balu P, Kotakadi VS, Karthikkumar V, Ernest D. Polydatin Encapsulated Poly [Lactic-co-glycolic acid] Nanoformulation Counteract the 7,12-Dimethylbenz[a] Anthracene Mediated Experimental Carcinogenesis through the Inhibition of Cell Proliferation. Antioxidants. 2019; 8(9):375. https://doi.org/10.3390/antiox8090375
Chicago/Turabian StyleVijayalakshmi, Sankaran, Arokia Vijaya Anand Mariadoss, Vinayagam Ramachandran, Vijayakumar Shalini, Balupillai Agilan, Casimeer C. Sangeetha, Periyasamy Balu, Venkata Subbaih Kotakadi, Venkatachalam Karthikkumar, and David Ernest. 2019. "Polydatin Encapsulated Poly [Lactic-co-glycolic acid] Nanoformulation Counteract the 7,12-Dimethylbenz[a] Anthracene Mediated Experimental Carcinogenesis through the Inhibition of Cell Proliferation" Antioxidants 8, no. 9: 375. https://doi.org/10.3390/antiox8090375
APA StyleVijayalakshmi, S., Mariadoss, A. V. A., Ramachandran, V., Shalini, V., Agilan, B., Sangeetha, C. C., Balu, P., Kotakadi, V. S., Karthikkumar, V., & Ernest, D. (2019). Polydatin Encapsulated Poly [Lactic-co-glycolic acid] Nanoformulation Counteract the 7,12-Dimethylbenz[a] Anthracene Mediated Experimental Carcinogenesis through the Inhibition of Cell Proliferation. Antioxidants, 8(9), 375. https://doi.org/10.3390/antiox8090375