Linking What We Eat to Our Mood: A Review of Diet, Dietary Antioxidants, and Depression
Abstract
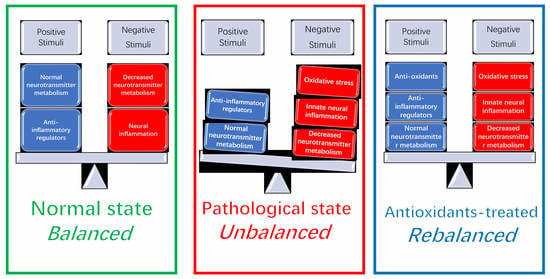
:1. Introduction
2. Definition and Some Hypothetical Mechanism of Depression
3. Diets and Depression
3.1. Dietary Patterns and Depression
3.2. Specific Foods and Depression
3.2.1. Fish Consumption and Depression
3.2.2. Fresh Fruit and/or Vegetable Consumption and Depression
3.2.3. Sugar-Sweetened Drink Consumption and Depression
3.3. Food Addiction and Depression: Extend Your Menu to Enrich Your Brain Health
4. Nutrients and Depression: Focusing on Dietary Antioxidants
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders, Global Health Estimates (No. WHO/MSD/MER/2017.2); World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Holden, C. Global survey examines impact of depression. Science 2000, 288, 39–40. [Google Scholar] [CrossRef]
- Reddy, M.S. Depression, The disorder and the burden. Indian J. Psychol. Med. 2001, 32, 1. [Google Scholar] [CrossRef]
- Lai, J.S.; Hiles, S.; Bisquera, A.; Hure, A.J.; McEvoy, M.; Attia, J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 2013, 99, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Panjwani, M.; Dossa, K.; Ali, Q.Q.; Jummani, D.D.; Jiwani, A. Depression in Adolescence. I-Manag. J. Nurs. 2014, 4, 6. [Google Scholar] [CrossRef]
- Levin, K.A.; Kirby, J.; Currie, C.; Inchley, J. Trends in adolescent eating behavior: A multilevel cross-sectional study of 11–15 year olds in Scotland, 2002–2010. J. Public Health 2012, 34, 523–531. [Google Scholar] [CrossRef]
- Noval-Aldaco, E.; Ruiz-Torres, M.; López-Gil, J.; Payá-González, J. Adolescent Depression, Psychopathology in Women; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 19–29. [Google Scholar]
- Gunnell, D.; Ashby, D. Antidepressants and suicide: What is the balance of benefit and harm. Br. Med. J. 2004, 329, 34–38. [Google Scholar] [CrossRef]
- Banjari, I.; Vukoje, I.; Mandić, M.L. Brain food: How nutrition alters our mood and behaviour. Hrana U Zdr. I Boles. 2014, 3, 13–21. [Google Scholar]
- Boonlert, W.; Benya-aphikul HUmka Welbat, J.; Rodsiri, R. Ginseng Extract G115 Attenuates Ethanol-Induced Depression in Mice by Increasing Brain BDNF Levels. Nutrients 2017, 9, 931. [Google Scholar] [CrossRef]
- Jang, H.M.; Lee, K.E.; Kim, D.H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients 2019, 11, 819. [Google Scholar] [CrossRef]
- Cass, W.A.; Smith, M.P.; Peters, L.E. Calcitriol protects against the dopamine-and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann. N. Y. Acad. Sci. 2006, 1074, 261–271. [Google Scholar] [CrossRef]
- Spedding SVitamin, D.; Depression, A. Systematic Review and Meta-Analysis Comparing Studies with and without Biological Flaws. Nutrients 2014, 6, 1501–1518. [Google Scholar] [CrossRef]
- Sarandol, A.; Sarandol, E.; Eker, S.S.; Erdinc, S.; Vatansever, E.; Kirli, S. Major depressive disorder is accompanied with oxidative stress: Short-term antidepressant treatment does not alter oxidative–antioxidative systems. Hum. Psychopharmacol. Clin. Exp. 2007, 22, 67–73. [Google Scholar] [CrossRef]
- Cabout, M.; Brouwer, I.A.; Visser, M. The Mood food project: Prevention of depression through nutritional strategies. Nutr. Bull. 2017, 42, 94–103. [Google Scholar] [CrossRef]
- Angst, J.A.; Dobler-Mikola, A. The definition of depression. J. Psychiatry Res. 1984, 18, 401–406. [Google Scholar] [CrossRef]
- Verboom, C.E.; Sijtsema, J.J.; Verhulst, F.C.; Penninx, B.W.; Ormel, J. Longitudinal associations between depressive problems, academic performance, and social functioning in adolescent boys and girls. Dev. Psychol. 2014, 50, 247. [Google Scholar] [CrossRef]
- Robaczewska, J.; Kędziora-Kornatowska, K.; Kucharski, R.; Nowak, M.; Muszalik, M.; Kornatowski, M.; Kędziora, J. Decreased expression of heme oxygenase is associated with depressive symptoms and may contribute to depressive and hypertensive comorbidity. Redox Rep. 2016, 21, 209. [Google Scholar] [CrossRef]
- Lu, D.Y.; Tsao, Y.Y.; Leung, Y.M.; Su, K.P. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: Implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology 2010, 35, 2238. [Google Scholar] [CrossRef]
- Levinson, D.F. The genetics of depression: A review. Biol. Psychiatry 2006, 60, 84–92. [Google Scholar] [CrossRef]
- Haggerty, J.J.; Stern, R.A.; Mason, G.A.; Beckwith, J.; Morey, C.E.; Prange, A.J. Subclinical hypothyroidism: A modifiable risk factor for depression? Am. J. Psychiatry 1993, 150, 508. [Google Scholar]
- Pelletier, G.; Verhoef, M.J.; Khatri, N.; Hagen, N. Quality of life in brain tumor patients: The relative contributions of depression, fatigue, emotional distress, and existential issues. J. Neuro-Oncol. 2002, 57, 41–49. [Google Scholar] [CrossRef]
- Armstrong, D.J.; Meenagh, G.K.; Bickle, I.; Lee, A.S.H.; Curran, E.S.; Finch, M.B. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin. Rheumatol. 2002, 26, 551–554. [Google Scholar] [CrossRef]
- Bjelland, I.; Tell, G.S.; Vollset, S.E.; Refsum, H.; Ueland, P.M. Folate, vitamin B12, homocysteine, and the MTHFR 677C→ T polymorphism in anxiety and depression: The Hordaland Homocysteine Study. Arch. Gen. Psychiatry 2003, 60, 618–626. [Google Scholar] [CrossRef]
- Slavich, G.M.; Irwin, M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 2014, 140, 774. [Google Scholar] [CrossRef]
- Leonard, B.; Maes, M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012, 36, 764–785. [Google Scholar] [CrossRef]
- Nie, X.; Kitaoka, S.; Tanaka, K.; Segi-Nishida, E.; Imoto, Y.; Ogawa, A.; Nakano, F.; Tomohiro, A.; Nakayama, K.; Taniguchi, M.; et al. The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron 2018, 99, 464–479. [Google Scholar] [CrossRef]
- Steiner, J.; Bielau, H.; Brisch, R.; Danos, P.; Ullrich, O.; Mawrin, C.; Bernstein, H.; Bogerts, B. Immunological aspects in the neurobiology of suicide: Elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatry. Res. 2008, 42, 151–157. [Google Scholar] [CrossRef]
- Steiner, J.; Mawrin, C.; Ziegeler, A.; Bielau, H.; Ullrich, O.; Bernstein, H.G.; Bogerts, B. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol. 2006, 112, 305–316. [Google Scholar] [CrossRef]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef]
- Musselman, D.L.; Miller, A.H.; Porter, M.R.; Manatunga, A.; Gao, F.; Penna, S.; Pearce, B.D.; Landry, J.; Glover, S.; McDaniel, S.; et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: Preliminary findings. Am. J. Psychiatry 2001, 158, 1252–1257. [Google Scholar] [CrossRef]
- Tiemeier, H.; Hofman, A.; van Tuijl, H.R.; Kiliaan, A.J.; Meijer, J.; Breteler, M.M. Inflammatory proteins and depression in the elderly. Epidemiology 2003, 14, 103–107. [Google Scholar] [CrossRef]
- Hayley, S.; Poulter, M.O.; Merali, Z.; Anisman, H. The pathogenesis of clinical depression: Stressor-and cytokine-induced alterations of neuroplasticity. Neuroscience 2005, 135, 659–678. [Google Scholar] [CrossRef]
- Pace, T.W.; Hu, F.; Miller, A.H. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 2007, 21, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46. [Google Scholar] [CrossRef]
- Yarlagadda, A.; Alfson, E.; Clayton, A.H. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont) 2009, 6, 18. [Google Scholar]
- Bus, B.A.A.; Molendijk, M.L.; Tendolkar, I.; Penninx, B.W.J.H.; Prickaerts, J.; Elzinga, B.M.; Voshaar, R.C.O. Chronic depression is associated with a pronounced decrease in serum brain-derived neurotrophic factor over time. Mol. Psychiatry 2015, 20, 602. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Bus, B.A.A.; Spinhoven, P.; Penninx, B.W.; Kenis, G.; Prickaerts, J.; Oude Voshaar, R.C.; Elzinga, B.M. Serum levels of brain-derived neurotrophic factor in major depressive disorder: State–trait issues, clinical features and pharmacological treatment. Mol. Psychiatry 2011, 16, 1088. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Spinhoven, P.; Polak, M.; Bus, B.A.A.; Penninx, B.W.J.H.; Elzinga, B.M. Serum BDNF concentrations as peripheral manifestations of depression: Evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol. Psychiatry 2014, 19, 791. [Google Scholar] [CrossRef]
- Zhou, C.; Zhong, J.; Zou, B.; Fang, L.; Chen, J.; Deng, X.; Zhang, L.; Zhao, X.; Qu, X.; Lei, Y.; et al. Meta-analyses of comparative efficacy of antidepressant medications on peripheral BDNF concentration in patients with depression. PLoS ONE 2017, 12, e0172270. [Google Scholar] [CrossRef]
- Kishi, T.; Yoshimura, R.; Ikuta, T.; Iwata, N. Brain-derived neurotrophic factor and major depressive disorder: Evidence from meta-analyses. Front. Psychiaty 2018, 8, 308. [Google Scholar] [CrossRef]
- Connor, T.J.; Leonard, B.E. Depression, stress and immunological activation: The role of cytokines in depressive disorders. Life Sci. 1998, 62, 583–606. [Google Scholar] [CrossRef]
- Schiepers, O.J.; Wichers, M.C.; Maes, M. Cytokines and major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 201–217. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238. [Google Scholar] [CrossRef]
- Pezawas, L.; Meyer-Lindenberg, A.; Drabant, E.M.; Verchinski, B.A.; Munoz, K.E.; Kolachana, B.S.; Egan, M.F.; Mattay, V.S.; Hariri, A.R.; Weinberger, D.R. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat. Neurosci. 2005, 8, 828. [Google Scholar] [CrossRef]
- Malemud, C.J.; Miller, A.H. Pro-inflammatory cytokine-induced SAPK/MAPK and JAK/STAT in rheumatoid arthritis and the new anti-depression drugs. Expert Opin. Ther. Targets 2008, 12, 171–183. [Google Scholar] [CrossRef]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef] [Green Version]
- Karege, F.; Bondolfi, G.; Gervasoni, N.; Schwald, M.; Aubry, J.M.; Bertschy, G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatry 2005, 57, 1068–1072. [Google Scholar] [CrossRef]
- Davies, C.H.; Davies, S.N.; Collingridge, G.L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J. Physiol. 1990, 424, 513–531. [Google Scholar] [CrossRef]
- Luscher, B.; Shen, Q.; Sahir, N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 2011, 16, 383. [Google Scholar] [CrossRef]
- Loftis, J.M.; Huckans, M.; Morasco, B.J. Neuroimmune mechanisms of cytokine-induced depression: Current theories and novel treatment strategies. Neurobiol. Dis. 2010, 37, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Prasad, C. Food, mood and health: A neurobiologic outlook. Braz. J. Med. Biol. Res. 1998, 31, 1517–1527. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Marventano, S.; Castellano, S.; Mistretta, A.; Pajak, A.; Galvano, F. Dietary n-3 PUFA, fish consumption and depression, A systematic review and meta-analysis of observational studies. J. Affect. Disord. 2016, 205, 269. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Zhang, D. Fish consumption and risk of depression: A meta-analysis. J. Epidemiol. Community Health 2016, 70, 299–304. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Castellano, S.; Pajak, A.; Galvano, F. Coffee, tea, caffeine and risk of depression: A systematic review and dose–response meta-analysis of observational studies. Mol. Nutr. Food Res. 2016, 60, 223–234. [Google Scholar] [CrossRef]
- Biro, G.; Hulshof, K.F.A.M.; Ovesen, L.; Cruz, J.A. Selection of methodology to assess food intake. Eur. J. Clin. Nutr. 2002, 56, S25. [Google Scholar] [CrossRef]
- Garcíatoro, M.; Vicenspons, E.; Gili, M.; Roca, M.; Serrano-Ripoll, M.J.; Vives, M.; Leiva, A.; Yáñez, A.M.; Bennasar-Veny, M.; Oliván-Blázquez, B. Obesity, metabolic syndrome and Mediterranean diet, Impact on depression outcome. J. Affect. Disord. 2016, 194, 105–108. [Google Scholar] [CrossRef]
- Rienks, J.; Dobson, A.J.; Mishra, G.D. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: Results from a large community-based prospective study. Eur. J. Clin. Nutr. 2013, 67, 75. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Salem Jr, N. Dietary polyunsaturated fatty acids and depression: When cholesterol does not satisfy. Am. J. Clin. Nutr. 1995, 62, 1–9. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Delgado-Rodríguez, M.; Alonso, A.; Schlatter, J.; Lahortiga, F.; Majem, L.S.; Martínez-González, M.A. The Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch. Gen. Psychiatry 2009, 66, 1090–1098. [Google Scholar] [CrossRef]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsanga, T.; Blunden, S.; Segal, L.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression, A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Covas, M.I.; Arós, F.; Romaguera, D.; Gómez-Gracia, E.; Lapetra, J.; et al. Mediterranean dietary pattern and depression: The PREDIMED randomized trial. BMC Med. 2013, 11, 1–12. [Google Scholar] [CrossRef]
- Opie, R.S.; O’Neil, A.; Jacka, F.N.; Pizzinga, J.; Itsiopoulos, C. A modified Mediterranean dietary intervention for adults with major depression, Dietary protocol and feasibility data from the SMILES trial. Nutr. Neurosci. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Fried, E.I.; Van der Does, W. The SMILES trial: Do undisclosed recruitment practices explain the remarkably large effect? BMC Med. 2018, 16, 243. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Toledo, E.; De Irala, J.; Ruiz-Canela, M.; Pla-Vidal, J.; Martínez-González, M.A. Fast-food and commercial baked goods consumption and the risk of depression. Public Health Nutr. 2012, 15, 424–432. [Google Scholar] [CrossRef]
- Christ, A.; Günther, P.; Lauterbach, M.A.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 2018, 172, 162–175. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Leung, B.M.; Kaplan, B.J. Perinatal depression: Prevalence, risks, and the nutrition link—A review of the literature. J. Am. Diet. Assoc. 2009, 109, 1566–1575. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Chen, K.; Jing, Y.; He, J.; Sun, H.; Hu, X. Dietary inflammatory index and depression: A meta-analysis. Public Health Nutr. 2019, 22, 654–660. [Google Scholar] [CrossRef]
- Miki, T.; Kochi, T.; Kuwahara, K.; Eguchi, M.; Kurotani, K.; Tsuruoka, H.; Ito, R.; Kabe, I.; Kawakami, N.; Mizoue, T.; et al. Dietary patterns derived by reduced rank regression (RRR) and depressive symptoms in Japanese employees: The Furukawa nutrition and health study. Psychiatry Res. 2015, 229, 214–219. [Google Scholar] [CrossRef]
- Vermeulen, E.; Stronks, K.; Visser, M.; Brouwer, I.A.; Snijder, M.B.; Mocking, R.J.T.; Schene, A.H.; Nicolaou, M. Dietary pattern derived by reduced rank regression and depressive symptoms in a multi-ethnic population: The HELIUS study. Eur. J. Clin. Nutr. 2017, 71, 987. [Google Scholar] [CrossRef]
- Hou, F.L.; Lu, Q.Y.; Xu, S.J. Effects of depressive symptoms and emotional eating behaviors on dietary patterns among adolescents. J. School Health 2015, 9, 1289–1293. [Google Scholar]
- Molendijk, M.; Molero, P.; Sánchez-Pedreño, F.O.; Van der Does, W.; Martínez-González, M.A. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 2017, 226, 346. [Google Scholar] [CrossRef]
- Teo, C.; Chia, A.R.; Colega, M.T.; Chen, L.W.; Fok, D.; Pang, W.W.; Godfrey, K.M.; Tan, K.H.; Yap, F.; Shek, L.P.C.; et al. Prospective Associations of Maternal Dietary Patterns and Postpartum Mental Health in a Multi-Ethnic Asian Cohort, The Growing up in Singapore towards Healthy Outcomes (GUSTO) Study. Nutrients 2018, 10, 299. [Google Scholar] [CrossRef]
- MartÃnez-GonzÃlez, M.A.; Sãn, A. Dietary patterns and the prevention of depression. Proc. Nutr. Soc. 2016, 75, 139–146. [Google Scholar] [CrossRef]
- Xu, M.Q.; Cao, H.L.; Wang, W.Q.; Wang, S.; Cao, X.C.; Yan, F.; Wang, B.M. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J. Gastroenterol. 2015, 21, 102. [Google Scholar] [CrossRef]
- Hibbeln, J.R. Fish consumption and major depression. Lancet 1998, 351, 1213. [Google Scholar] [CrossRef]
- Yoshikawa, E.; Nishi, D.; Matsuoka, Y. Fish consumption and resilience to depression in Japanese company workers: a cross-sectional study. Lipids Health Dis. 2015, 14, 51–58. [Google Scholar] [CrossRef]
- Tanskanen, A.; Hibbeln, J.R.; Tuomilehto, J.; Uutela, A.; Haukkala, A.; Viinamäki, H.; Lehtonen, J.; Vartiainen, E. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr. Serv. 2001, 52, 529–531. [Google Scholar] [CrossRef]
- Timonen, M.; Horrobin, D.; Jokelainen, J.; Laitinen, J.; Herva, A.; Räsänen, P. Fish consumption and depression: The Northern Finland 1966 birth cohort study. J. Affect. Disord. 2004, 82, 447–452. [Google Scholar] [CrossRef]
- Smith, K.J.; Sanderson, K.; McNaughton, S.A.; Gall, S.L.; Dwyer, T.; Venn, A.J. Longitudinal associations between fish consumption and depression in young adults. Am. J. Epidemiol. 2014, 179, 1228–1235. [Google Scholar] [CrossRef]
- Strasser, B.; Gostner, J.M.; Fuchs, D. Mood, food, and cognition: Role of tryptophan and serotonin. Curr. Opinion Clin. Nutr. Metab. Care 2016, 19, 55–61. [Google Scholar] [CrossRef]
- Kingsbury, M.; Dupuis, G.; Jacka, F.; Roy-Gagnon, M.H.; McMartin, S.E.; Colman, I. Associations between fruit and vegetable consumption and depressive symptoms: Evidence from a national Canadian longitudinal survey. J. Epidemiol. Community Health 2016, 70, 155–161. [Google Scholar] [CrossRef]
- Liu, C.; Xie, B.; Chou, C.P.; Koprowski, C.; Zhou, D.; Palmer, P.; Sun, P.; Guo, Q.; Duan, L.; Sun, X.; et al. Perceived stress, depression and food consumption frequency in the college students of China Seven Cities. Physiol. Behav. 2007, 92, 748–754. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Wurtman, J.J. Brain serotonin, carbohydrate-craving, obesity and depression. Obes. Res. 1995, 3, 477S–480S. [Google Scholar] [CrossRef]
- Angelino, D.; Godos, J.; Ghelfi, F.; Tieri, M.; Titta, L.; Lafranconi, A.; Marventano, S.; Alonzo, E.; Gambera, A.; Sciacca, S.; et al. Fruit and vegetable consumption and health outcomes: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2019, 70, 652–667. [Google Scholar] [CrossRef]
- Liu, X.; Yan, Y.; Li, F.; Zhang, D. Fruit and vegetable consumption and the risk of depression: A meta-analysis. Nutrition 2016, 32, 296–302. [Google Scholar] [CrossRef]
- Westover, A.N.; Marangell, L.B. A cross-national relationship between sugar consumption and major depression? Depress. Anxiety 2002, 16, 118–120. [Google Scholar] [CrossRef]
- Yu, B.; He, H.; Zhang, Q.; Wu, H.; Du, H.; Liu, L.; Wang, C.; Shi, H.; Xia, Y.; Guo, X.; et al. Soft drink consumption is associated with depressive symptoms among adults in China. J. Affect. Disord. 2015, 172, 422–427. [Google Scholar] [CrossRef]
- Knüppel, A.; Shipley, M.J.; Llewellyn, C.H.; Brunner, E.J. Sugar intake from sweet food and beverages, common mental disorder and depression: Prospective findings from the Whitehall II study. Sci. Rep. 2017, 7, 6287. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Xiao, Y.; Jing, D.; Huang, Y.; Chen, L.; Luo, D.; Chen, X.; Shen, M. Daily intake of soft drinks is associated with symptoms of anxiety and depression in Chinese adolescents. Public Health Nutr. 2019, 22, 474–487. [Google Scholar] [CrossRef]
- Sanchez-Villegas, A.; Zazpe, I.; Santiago, S.; Perez-Cornago, A.; Martinez-Gonzalez, M.A.; Lahortiga-Ramos, F. Added sugars and sugar-sweetened beverage consumption, dietary carbohydrate index and depression risk in the Seguimiento Universidad de Navarra (SUN) Project. B. J. Nutr. 2018, 119, 211–221. [Google Scholar] [CrossRef]
- Hu, D.; Cheng, L.; Jiang, W. Sugar-sweetened beverages consumption and the risk of depression, A meta-analysis of observational studies. J. Affect. Disord. 2018, 15, 348–355. [Google Scholar] [CrossRef]
- Burrows, T.; Kay-Lambkin, F.; Pursey, K.; Skinner, J.; Dayas, C. Food addiction and associations with mental health symptoms: A systematic review with meta-analysis. J. Hum. Nutr. Diet. 2018, 31, 4. [Google Scholar] [CrossRef]
- Nolan, L.J.; Geliebter, A. Validation of the Night Eating Diagnostic Questionnaire (NEDQ) and its relationship with depression, sleep quality, food addiction, and body mass index. Appetite 2017, 111, 86–95. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, F.; Wang, L.; Yang, T.; Shi, L.; Li, X.; Shen, J.; Xu, W.; Guo, T.; Li, Q. Anti-hyperlipidemic effect of rice bran polysaccharide and its potential mechanism in high-fat diet mice. Food Funct. 2017, 8, 4028–4041. [Google Scholar] [CrossRef]
- Bin, Y.U.; Niu, K. Diet, Nutrition, and Depression. Adv. Psychol. Sci. 2015, 23, 2107. [Google Scholar]
- Pouwer, F.; Nijpels, G.; Beekman, A.T.; Dekker, J.M.; van Dam, R.M.; Heine, R.J.; Snoek, F.J. Fat food for a bad mood. Could we treat and prevent depression in Type 2 diabetes by means of ω-3 polyunsaturated fatty acids? A review of the evidence. Diabet. Med. 2010, 22, 1465–1475. [Google Scholar] [CrossRef]
- Lin, P.Y.; Huang, S.Y.; Su, K.P. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry 2010, 68, 140–147. [Google Scholar] [CrossRef]
- Hibbeln, J.R. Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: A cross-national, ecological analysis. J. Affect. Disord. 2002, 69, 15–29. [Google Scholar] [CrossRef]
- MacFabe, D.F.; Cain, D.P.; Rodriguez-Capote, K.; Franklin, A.E.; Hoffman, J.E.; Boon, F.; Taylor, A.R.; Kavaliers, M.; Ossenkopp, K. Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 2007, 176, 149–169. [Google Scholar] [CrossRef]
- Khanzode, S.D.; Dakhale, G.N.; Khanzode, S.S.; Saoji, A.; Palasodkar, R. Oxidative damage and major depression: The potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Report 2003, 8, 365–370. [Google Scholar] [CrossRef]
- Maes, M.; De Vos, N.; Pioli, R.; Demedts, P.; Wauters, A.; Neels, H.; Christophe, A. Lower serum vitamin E concentrations in major depression: Another marker of lowered antioxidant defenses in that illness. J. Affect. Disord. 2000, 58, 241–246. [Google Scholar] [CrossRef]
- Payne, M.E.; Steck, S.E.; George, R.R.; Steffens, D.C. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J. Acad. Nutr. Diet. 2012, 112, 2022–2027. [Google Scholar] [CrossRef]
- Robinson, D.G.; Gallego, J.A.; John, M.; Hanna, L.A.; Zhang, J.P.; Birnbaum, M.L.; Greenberg, J.; Naraine, M.; Peters, B.D.; McNamara, R.K.; et al. A potential role for adjunctive omega-3 polyunsaturated fatty acids for depression and anxiety symptoms in recent onset psychosis, Results from a 16 week randomized placebo-controlled trial for participants concurrently treated with risperidone. Schizophr. Res. 2019, 204, 295–303. [Google Scholar] [CrossRef]
- Marangell, L.B.; Martinez, J.M.; Zboyan, H.A.; Kertz, B.; Kim, H.F.; Puryear, L.J. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am. J. Psychiatry 2003, 160, 996–998. [Google Scholar] [CrossRef]
- Sublette, M.E.; Hibbeln, J.R.; Galfalvy, H.; Oquendo, M.A.; Mann, J.J. ω-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. China Prescr. Drug 2006, 163, 1100–1102. [Google Scholar] [CrossRef]
- Nemets, B.; Stahl, Z.; Belmaker, R.H. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am. J. Psychiatry 2002, 159, 477–479. [Google Scholar] [CrossRef]
- Jazayeri, S.; Tehranidoost, M.; Keshavarz, S.A.; Hosseini, M.; Djazayery, A.; Amini, H.; Jalali, M.; Peet, M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust. N. Z. J. Psychiatry 2008, 42, 192–198. [Google Scholar] [CrossRef]
- Buydensbranchey, L.; Branchey, M.; Hibbeln, J.R. Higher n-3 fatty acids are associated with more intense fenfluramine-induced acth and cortisol responses among cocaine-abusing men. Psychiatry Res. 2011, 188, 422–427. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894. [Google Scholar] [CrossRef]
- Simopoulos, A.P. ω-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef]
- Su, K.P.; Huang, S.Y.; Chiu, C.C.; Shen, W.W. ω-3 fatty acids in major depressive disorder. a preliminary double-blind, placebo-controlled trial. Eur. Neuropsychopharmacol. 2003, 13, 267–271. [Google Scholar] [CrossRef]
- Gilbody, S.; Lightfoot, T.; Sheldon, T. Is low folate a risk factor for depression? a meta-analysis and exploration of heterogeneity. J. Epidemiol. Community Health 2007, 61, 631–637. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Shroff, M.R.; Beydoun, H.A.; Zonderman, A.B. Serum folate, vitamin B-12 and homocysteine and their association with depressive symptoms among US adults. Psychosom. Med. 2010, 72, 862. [Google Scholar] [CrossRef]
- Forti, P.; Rietti, E.; Pisacane, N.; Olivelli, V.; Dalmonte, E.; Mecocci, P.; Ravaglia, G. Blood homocysteine and risk of depression in the elderly. Arch. Gerontol. Geriatr. 2010, 51, 21–25. [Google Scholar] [CrossRef]
- Sánchezvillegas, A.; Doreste, J.; Schlatter, J.; Pla, J.; Besrastrollo, M.; Martínezgonzález, M.A. Association between folate, vitamin b6 and vitamin b12 intake and depression in the sun cohort study. J. Hum. Nutr. Diet. 2009, 22, 122. [Google Scholar] [CrossRef]
- Mattson, M.P.; Shea, T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003, 26, 137–146. [Google Scholar] [CrossRef]
- Penninx, B.W.; Guralnik, J.M.; Ferrucci, L.; Fried, L.P.; Allen, R.H.; Stabler, S.P. Vitamin B12 deficiency and depression in physically disabled older women: Epidemiologic evidence from the Women’s Health and Aging Study. Am. J. Psychiatry 2000, 157, 715–721. [Google Scholar] [CrossRef]
- Murakami, K.; Mizoue, T.; Sasaki, S.; Ohta, M.; Sato, M.; Matsushita, Y.; Mishima, N. Dietary intake of folate, other B vitamins, and ω-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition 2008, 24, 140–147. [Google Scholar] [CrossRef]
- Payne, M.E.; Jamerson, B.D.; Potocky, C.F.; Ashley-Koch, A.E.; Speer, M.C.; Steffens, D.C. Natural food folate and late-life depression. J. Nutr. Elder. 2009, 28, 348–358. [Google Scholar] [CrossRef]
- Skarupski, K.A.; Tangney, C.; Li, H.; Ouyang, B.; Evans, D.A.; Morris, M.C. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am. J. Clin. Nutr. 2010, 92, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. Calcium regulation of neural rhythms, memory and Alzheimer’s disease. J. Physiology 2014, 592, 281–293. [Google Scholar] [CrossRef]
- May, H.T.; Bair, T.L.; Lappé, D.L.; Anderson, J.L.; Horne, B.D.; Carlquist, J.F.; Muhlestein, J.B. Association of vitamin D levels with incident depression among a general cardiovascular population. Am. Heart J. 2010, 159, 1037–1043. [Google Scholar] [CrossRef]
- Hoang, M.T.; Defina, L.F.; Willis, B.L.; Leonard, D.S.; Weiner, M.F.; Brown, E.S. Association between low serum 25-hydroxyvitamin D and depression in a large sample of healthy adults, The Cooper Center longitudinal study. Mayo Clin. Proc. 2011, 86, 1050–1052. [Google Scholar] [CrossRef]
- Kjærgaard, M.; Joakimsen, R.; Jorde, R. Low serum 25-hydroxyvitamin D levels are associated with depression in an adult Norwegian population. Psychiatry Res. 2011, 190, 221–225. [Google Scholar] [CrossRef]
- Umhau, J.C.; George, D.T.; Heaney, R.P.; Lewis, M.D.; Ursano, R.J.; Heilig, M.; Hibben, J.R.; Schwandt, M.L. Low vitamin D status and suicide: A case-control study of active duty military service members. PLoS ONE 2013, 8, e51543. [Google Scholar] [CrossRef]
- Kerr, D.C.R.; Zava, D.T.; Piper, W.T.; Saturn, S.R.; Frei, B.; Gombart, A.F. Associations between Vitamin D Levels and Depressive Symptoms in Healthy Young Adult Women. Psychiatry Res. 2015, 227, 46–51. [Google Scholar] [CrossRef]
- Sikoglu, E.M.; Navarro, A.A.L.; Starr, D.; Dvir, Y.; Nwosu, B.U.; Czerniak, S.M.; Rogan, R.C.; Castro, M.C.; Edden, R.A.E.; Frazier, J.A.; et al. Vitamin D3 supplemental treatment for mania in youth with bipolar spectrum disorders. J. Child Adolesc. Psychopharmacol. 2015, 25, 415–424. [Google Scholar] [CrossRef]
- Stokes, C.S.; Grünhage, F.; Baus, C.; Volmer, D.A.; Wagenpfeil, S.; Riemenschneider, M.; Lammert, F. Vitamin D supplementation reduces depressive symptoms in patients with chronic liver disease. Clin. Nutr. 2016, 35, 950–957. [Google Scholar] [CrossRef]
- Pérez, A.V.; Picotto, G.; Carpentieri, A.R.; Rivoira, M.A.; López, M.E.P.; De Talamoni, N.G.T. Minireview on regulation of intestinal calcium absorption. Digestion 2008, 77, 22–34. [Google Scholar] [CrossRef]
- Wasserman, R.H. Vitamin D and the dual processes of intestinal calcium absorption. J. Nutr. 2004, 134, 3137–3139. [Google Scholar] [CrossRef]
- Berridge, M.J. Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion 2013, 7, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Pham, N.M.; Nanri, A.; Kurotani, K.; Kuwahara, K.; Kume, A.; Sato, M.; Hayabuchi, H.; Mizoue, T. Green tea and coffee consumption is inversely associated with depressive symptoms in a Japanese working population. Public Health Nutr. 2014, 17, 625–633. [Google Scholar] [CrossRef]
- Chang, S.C.; Cassidy, A.; Willett, W.C.; Rimm, E.B.; O’Reilly, E.J.; Okereke, O.I. Dietary flavonoid intake and risk of incident depression in midlife and older women. Am. J. Clin. Nutr. 2016, 104, 704–714. [Google Scholar] [CrossRef]
- Godos, J.; Castellano, S.; Ray, S.; Grosso, G.; Galvano, F. Dietary polyphenol intake and depression, Results from the mediterranean healthy eating, lifestyle and aging (meal) study. Molecules 2018, 23, 999. [Google Scholar] [CrossRef]
- Mofrad, M.D.; Siassi, F.; Guilani, B.; Bellissimo, N.; Azadbakht, L. Association of dietary phytochemical index and mental health in women: A cross-sectional study. Br. J. Nutr. 2019, 121, 1049–1056. [Google Scholar] [CrossRef]
- Khalid, S.; Barfoot, K.; May, G.; Lamport, D.; Reynolds, S.; Williams, C. Effects of acute blueberry flavonoids on mood in children and young adults. Nutrients 2017, 9, 158. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Furukawa, S.; Arakawa, M. Soy isoflavone intake and prevalence of depressive symptoms during pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. Eur. J. Nutr. 2018, 57, 441–450. [Google Scholar] [CrossRef]
- Losi, G.; Puia, G.; Garzon, G.; de Vuono, M.C.; Baraldi, M. Apigenin modulates GABAergic and glutamatergic transmission in cultured cortical neurons. Eur. J. Pharmacol. 2004, 502, 41–46. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, F.M.; Qiang, L.Q.; Zhang, D.M.; Kong, L.D. Icariin attenuates chronic mild stress-induced dysregulation of the LHPA stress circuit in rats. Psychoneuroendocrinology 2010, 35, 272–283. [Google Scholar] [CrossRef]
- Yang, X.H.; Song, S.Q.; Xu, Y. Resveratrol ameliorates chronic unpredictable mild stress-induced depression-like behavior: Involvement of the HPA axis, inflammatory markers, BDNF, and Wnt/β-catenin pathway in rats. Neuropsychiatr. Dis. Treat. 2017, 13, 2727. [Google Scholar] [CrossRef]
- Hao, C.W.; Lai, W.S.; Ho, C.T.; Sheen, L.Y. Antidepressant-like effect of lemon essential oil is through a modulation in the levels of norepinephrine, dopamine, and serotonin in mice: Use of the tail suspension test. J. Funct. Foods 2013, 5, 370–379. [Google Scholar] [CrossRef]
- Paderin, N.M.; Popov, S.V. The effect of dietary pectins on object recognition memory, depression-like behaviour, and IL-6 in mouse hippocampi. J. Funct. Foods 2018, 43, 131–138. [Google Scholar] [CrossRef]
- Hall, S.; Arora, D.; Anoopkumar-Dukie, S.; Grant, G.D. Effect of coffee in lipopolysaccharide-induced indoleamine 2,3-dioxygenase activation and depressive-like behavior in mice. J. Agric. Food Chem. 2016, 64, 8745–8754. [Google Scholar] [CrossRef]
- Kang, A.; Xie, T.; Zhu, D.; Shan, J.; Di, L.; Zheng, X. Suppressive effect of ginsenoside Rg3 against lipopolysaccharide-induced depression-like behavior and neuroinflammation in mice. J. Agric. Food Chem. 2017, 65, 6861–6869. [Google Scholar] [CrossRef]
- Wei, I.H.; Chen, K.T.; Tsai, M.H.; Wu, C.H.; Lane, H.Y.; Huang, C.C. Acute amino acid D-serine administration, similar to ketamine, produces antidepressant-like effects through identical mechanisms. J. agric. food chem. 2017, 65, 10792–10803. [Google Scholar] [CrossRef]
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef]
- Special Issue Anti-Inflammatory and Antioxidant Effects of Dietary Supplementation and Lifestyle Factors. Available online: https://www.mdpi.com/journal/antioxidants/special_issues/anti-inflammatory_antioxidant_effects (accessed on 9 July 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Liu, H.; Suzuki, K.; Ma, S.; Liu, C. Linking What We Eat to Our Mood: A Review of Diet, Dietary Antioxidants, and Depression. Antioxidants 2019, 8, 376. https://doi.org/10.3390/antiox8090376
Huang Q, Liu H, Suzuki K, Ma S, Liu C. Linking What We Eat to Our Mood: A Review of Diet, Dietary Antioxidants, and Depression. Antioxidants. 2019; 8(9):376. https://doi.org/10.3390/antiox8090376
Chicago/Turabian StyleHuang, Qingyi, Huan Liu, Katsuhiko Suzuki, Sihui Ma, and Chunhong Liu. 2019. "Linking What We Eat to Our Mood: A Review of Diet, Dietary Antioxidants, and Depression" Antioxidants 8, no. 9: 376. https://doi.org/10.3390/antiox8090376
APA StyleHuang, Q., Liu, H., Suzuki, K., Ma, S., & Liu, C. (2019). Linking What We Eat to Our Mood: A Review of Diet, Dietary Antioxidants, and Depression. Antioxidants, 8(9), 376. https://doi.org/10.3390/antiox8090376