Perturbations of Lipids and Oxidized Phospholipids in Lipoproteins of Patients with Postmenopausal Osteoporosis Evaluated by Asymmetrical Flow Field-Flow Fractionation and Nanoflow UHPLC–ESI–MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plasma Samples
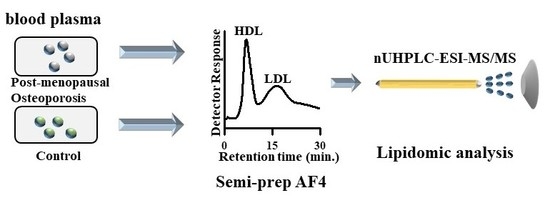
2.3. Separation of HDL and LDL by AF4
2.4. Lipid Extraction
2.5. Nanoflow UHPLC–ESI–MS/MS
3. Results and Discussion
3.1. Size Separation of Lipoproteins by Semi-Prep AF4
3.2. SRM-Based Quantification of Lipids in Each Lipoprotein
3.3. Lipid Alterations in Each Lipoprotein from Patients with PMOp
3.4. Quantification of Ox-PLs from the LDL of Patients with PMOp
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kanis, J.A.; Melton, L.J.; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Lock, C.A.; Lecouturier, J.; Mason, J.M.; Dickinson, H.O. Lifestyle interventions to prevent osteoporotic fractures: A systematic review. Osteoporos. Int. 2006, 17, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Hsu, Y.H.; Terwedow, H.; Tang, G.; Liu, X.; Jiang, S.; Xu, X.; Xu, X. Association of the methylenetetrahydrofolate reductase C677T polymorphism and fracture risk in Chinese postmenopausal women. Bone 2007, 40, 737–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Anazi, A.F.; Qureshi, V.F.; Javaid, K.; Qureshi, S. Preventive effects of phytoestrogens against postmenopausal osteoporosis as compared to the available therapeutic choices: An overview. J. Nat. Sci. Biol. Med. 2011, 2, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef] [Green Version]
- Roholl, P.J.M.; Blauw, E.; Zurcher, C.; Dormans, J.A.M.A.; Theuns, H.M. Evidence for a Diminished Maturation of Preosteoblasts into Osteoblasts during Aging in Rats—An Ultrastructural Analysis. J. Bone Miner. Res. 1994, 9, 355–366. [Google Scholar] [CrossRef]
- Garrett, I.R.; Boyce, B.F.; Oreffo, R.O.C.; Bonewald, L.; Poser, J.; Mundy, G.R. Oxygen-Derived Free-Radicals Stimulate Osteoclastic Bone-Resorption in Rodent Bone Invitro and Invivo. J. Clin. Investig. 1990, 85, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Maggio, D.; Barabani, M.; Pierandrei, M.; Polidori, M.C.; Catani, M.; Mecocci, P.; Senin, U.; Pacifici, R.; Cherubini, A. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J. Clin. Endocrinol. Metab. 2003, 88, 1523–1527. [Google Scholar] [CrossRef] [Green Version]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Zhang, D.; Chen, H.; Chao, Y.; Wu, K.; Dong, X.; Su, J. Integrative Bone Metabolomics—Lipidomics Strategy for Pathological Mechanism of Postmenopausal Osteoporosis Mouse Model. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sugimoto, T.; Yano, S.; Yamauchi, M.; Sowa, H.; Chen, Q.X.; Chihara, K. Plasma lipids and osteoporosis in postmenopausal women. Endocr. J. 2002, 49, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera, D.; Kruger, M.; Wolber, F.M.; Roy, N.C.; Totman, J.J.; Henry, C.J.; Cameron-Smith, D.; Fraser, K. Association of plasma lipids and polar metabolites with low bone mineral density in Singaporean-Chinese menopausal women: A pilot study. Int. J. Environ. Res. Public Health 2018, 15, 1045. [Google Scholar] [CrossRef] [Green Version]
- Tintut, Y.; Demer, L.L. Effects of bioactive lipids and lipoproteins on bone. Trends Endocrinol. Metab. 2014, 25, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrogini, E.; Que, X.C.; Wang, S.L.; Yamaguchi, F.; Weinstein, R.S.; Tsimikas, S.; Manolagas, S.C.; Witztum, J.L.; Jilka, R.L. Oxidation-specific epitopes restrain bone formation. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.M.; Suoniemi, M.; Kardys, I.; Vihervaara, T.; de Boer, S.P.; Akkerhuis, K.M.; Sysi-Aho, M.; Ekroos, K.; Garcia-Garcia, H.M.; Oemrawsingh, R.M.; et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Atherosclerosis 2015, 243, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Alshehry, Z.H.; Mundra, P.A.; Barlow, C.K.; Mellett, N.A.; Wong, G.; McConville, M.J.; Simes, J.; Tonkin, A.M.; Sullivan, D.R.; Barnes, E.H.; et al. Plasma Lipidomic Profiles Improve on Traditional Risk Factors for the Prediction of Cardiovascular Events in Type 2 Diabetes Mellitus. Circulation 2016, 134, 1637–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syme, C.; Czajkowski, S.; Shin, J.; Abrahamowicz, M.; Leonard, G.; Perron, M.; Richer, L.; Veillette, S.; Gaudet, D.; Strug, L.; et al. Glycerophosphocholine Metabolites and Cardiovascular Disease Risk Factors in Adolescents: A Cohort Study. Circulation 2016, 134, 1629–1636. [Google Scholar] [CrossRef]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. the road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Gleissner, C.A.; Leitinger, N.; Ley, K. Effects of native and modified low-density lipoproteins on monocyte recruitment in atherosclerosis. Hypertension 2007, 50, 276–283. [Google Scholar] [CrossRef]
- Giddings, J.C. Field flow fractionation. A versatile method for the characterization of macromolecular and particulate materials. Anal. Chem. 1981, 53, 1170A–1178A. [Google Scholar] [CrossRef]
- Nilsson, M.; Birnbaum, S.; Wahlund, K.G. Determination of relative amounts of ribosome and subunits in Escherichia coli using asymmetrical flow field-flow fractionation. J. Biochem. Biophys. Methods 1996, 33, 9–23. [Google Scholar] [CrossRef]
- Yang, J.S.; Lee, J.Y.; Moon, M.H. High Speed Size Sorting of Subcellular Organelles by Flow Field-Flow Fractionation. Anal. Chem. 2015, 87, 6342–6348. [Google Scholar] [CrossRef]
- Yang, J.S.; Lee, J.C.; Byeon, S.K.; Rha, K.H.; Moon, M.H. Size Dependent Lipidomic Analysis of Urinary Exosomes from Patients with Prostate Cancer by Flow Field-Flow Fractionation and Nanoflow Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2017, 89, 2488–2496. [Google Scholar] [CrossRef]
- Moon, M.H. Flow field-flow fractionation: Recent applications for lipidomic and proteomic analysis. TrAC Trends Anal. Chem. 2019. [Google Scholar] [CrossRef]
- Kim, S.H.; Yang, J.S.; Lee, J.C.; Lee, J.Y.; Lee, J.Y.; Kim, E.; Moon, M.H. Lipidomic alterations in lipoproteins of patients with mild cognitive impairment and Alzheimer’s disease by asymmetrical flow field-flow fractionation and nanoflow ultrahigh performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2018, 1568, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.K.; Lee, J.Y.; Moon, M.H. Optimized extraction of phospholipids and lysophospholipids for nanoflow liquid chromatography-electrospray ionization-tandem mass spectrometry. Analyst 2012, 137, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Byeon, S.K.; Lee, J.Y.; Moon, M.H. Computational approach to structural identification of phospholipids using raw mass spectra from nanoflow liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Mass Spectrom. 2012, 47, 1004–1014. [Google Scholar] [CrossRef]
- Park, I.; Paeng, K.J.; Yoon, Y.; Song, J.H.; Moon, M.H. Separation and selective detection of lipoprotein particles of patients with coronary artery disease by frit-inlet asymmetrical flow field-flow fractionation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 780, 415–422. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, D.; Johan, C.; Moon, M.H. Improvement of lipoprotein separation with a guard channel prior to asymmetrical flow field-flow fractionation using fluorescence detection. J. Chromatogr. A 2011, 1218, 4144–4148. [Google Scholar] [CrossRef]
- Broniec, A.; Klosinski, R.; Pawlak, A.; Wrona-Krol, M.; Thompson, D.; Sarna, T. Interactions of plasmalogens and their diacyl analogs with singlet oxygen in selected model systems. Free Radic. Biol. Med. 2011, 50, 892–898. [Google Scholar] [CrossRef] [Green Version]
- Sims, S.M.; Panupinthu, N.; Lapierre, D.M.; Pereverzev, A.; Dixon, S.J. Lysophosphatidic acid: A potential mediator of osteoblast-osteoclast signaling in bone. Bba Mol. Cell Biol. Lipids 2013, 1831, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Li, J.; Chen, K.; Rong, X.; Guo, J. Untargeted Metabolomics Reveals the Protective Effect of Fufang Zhenshu Tiaozhi (FTZ) on Aging-Induced Osteoporosis in Mice. Front. Pharmacol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Y.; Wang, H.Y.; Bai, Y.F.; Zhao, J.X.; Zhang, X.H.; Liang, L.; Chen, Y.; Ye, C.F.; Li, Y.Q.; et al. Integrated Lipidomics and Transcriptomics Characterization upon Aging-Related Changes of Lipid Species and Pathways in Human Bone Marrow Mesenchymal Stem Cells. J. Proteome Res. 2019, 18, 2065–2077. [Google Scholar] [CrossRef]
- Zhan, Q.P.; Dai, Y.F.; Wang, F.; Mai, X.Y.; Fu, M.; Wang, P.; Wang, J.F. Metabonomic analysis in investigating the anti-osteoporotic effect of sialoglycoprotein isolated from eggs of carassius auratus on ovariectomized mice. J. Funct. Foods 2019, 61. [Google Scholar] [CrossRef]
- Qi, H.H.; Bao, J.; An, G.H.; Ouyang, G.; Zhang, P.L.; Wang, C.; Ying, H.J.; Ouyang, P.K.; Ma, B.; Zhang, Q. Association between the metabolome and bone mineral density in pre- and post-menopausal Chinese women using GC-MS. Mol. Biosyst. 2016, 12, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Sanders, F.; McNally, B.; Griffin, J.L. Blood triacylglycerols: A lipidomic window on diet and disease. Biochem. Soc. Trans. 2016, 44, 638–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meikle, P.J.; Wong, G.; Barlow, C.K.; Kingwell, B.A. Lipidomics: Potential role in risk prediction and therapeutic monitoring for diabetes and cardiovascular disease. Pharmacol. Ther. 2014, 143, 12–23. [Google Scholar] [CrossRef]
- Fukumoto, S.; Iwamoto, T.; Sakai, E.; Yuasa, K.; Fukumoto, E.; Yamada, A.; Hasegawa, T.; Nonaka, K.; Kato, Y. Current topics in pharmacological research on bone metabolism: Osteoclast differentiation regulated by glycosphingolipids. J. Pharmaco. Sci. 2006, 100, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Brodeur, M.R.; Brissette, L.; Falstrault, L.; Ouellet, P.; Moreau, R. Influence of oxidized low-density lipoproteins (LDL) on the viability of osteoblastic cells. Free Radic. Biol. Med. 2008, 44, 506–517. [Google Scholar] [CrossRef]
- Klein, B.Y.; Rojansky, N.; Ben-Yehuda, A.; Abou-Atta, I.; Abedat, S.; Friedman, G. Cell death in cultured human saos2 osteoblasts exposed to low-density lipoprotein. J. Cell. Biochem. 2003, 90, 42–58. [Google Scholar] [CrossRef]
- Almeida, M.; Ambrogini, E.; Han, L.; Manolagas, S.C.; Jilka, R.L. Increased Lipid Oxidation Causes Oxidative Stress, Increased Peroxisome Proliferator-activated Receptor-gamma Expression, and Diminished Pro-osteogenic Wnt Signaling in the Skeleton. J. Biol. Chem. 2009, 284, 27438–27448. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Class | Chain Structure | m/z | Control (n = 10) | PMOp (n = 10) | P/C | |
|---|---|---|---|---|---|---|
| R1 | R2 | |||||
| PC | 34:5 | 752.5 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.43 ± 0.05 * | |
| (20) | 14:0 | 8:1 COOH | 622.5 | 0.01 ± 0.00 | 0.01 ± 0.00 | 1.16 ± 0.24 ** |
| 14:0 | 5:0 COOH | 582.5 | 0.01 ± 0.00 | 0.02 ± 0.00 | 1.45 ± 0.37 | |
| 38:5 | 808.5 | 9.25 ± 0.65 | 6.20 ± 0.36 | 0.65 ± 0.06 * | ||
| 16:0 | 22:5 + O | 824.5 | 0.02 ± 0.00 | 0.05 ± 0.01 | 2.04 ± 0.38 | |
| 22:5+OO | 840.5 | 0.02 ± 0.00 | 0.03 ± 0.00 | 1.91 ± 0.24 ** | ||
| 19:4 CHO | 782.5 | 0.01 ± 0.00 | 0.01 ± 0.00 | 1.21 ± 0.43 * | ||
| 19:4 COOH | 798.5 | 0.01 ± 0.00 | 0.02 ± 0.00 | 2.41 ± 0.36 ** | ||
| 16:3 COOH | 758.5 | 0.02 ± 0.00 | 0.02 ± 0.00 | 1.24 ± 0.21 | ||
| 13:2 COOH | 718.5 | 0.01 ± 0.00 | 0.02 ± 0.00 | 1.26 ± 0.37 | ||
| 10:1 COOH | 678.5 | 0.04 ± 0.00 | 0.05 ± 0.00 | 1.34 ± 0.19 | ||
| 7:0 COOH | 638.5 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.96 ± 0.43 * | ||
| 16:1 | 22:4 + O | 824.5 | 0.02 ± 0.00 | 0.05 ± 0.00 | 1.92 ± 0.30 | |
| 16:2 COOH | 758.5 | 0.03 ± 0.00 | 0.03 ± 0.00 | 1.02 ± 0.11 ** | ||
| 18:0 | 20:5 + O | 824.5 | 0.02 ± 0.00 | 0.04 ± 0.01 | 1.87 ± 0.36 | |
| 20:5 + OO | 840.5 | 0.02 ± 0.00 | 0.03 ± 0.00 | 1.93 ± 0.31 ** | ||
| 18:1 | 20:4 + O | 824.5 | 0.03 ± 0.00 | 0.05 ± 0.00 | 2.10 ± 0.28 * | |
| 20:4 + OO | 840.5 | 0.02 ± 0.00 | 0.03 ± 0.00 | 1.85 ± 0.26 ** | ||
| 14:2 COOH | 758.5 | 0.02 ± 0.00 | 0.03 ± 0.00 | 1.25 ± 0.26 * | ||
| 18:2 | 17:2 COOH | 798.5 | 0.01 ± 0.00 | 0.02 ± 0.00 | 2.16 ± 0.43 ** | |
| 14:1 COOH | 758.5 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.99 ± 0.11 | ||
| 11:0 COOH | 718.5 | 0.03 ± 0.00 | 0.04 ± 0.01 | 1.22 ± 0.26 | ||
| PA | 16:0 | 18:2 | 671.5 | 0.51 ± 0.06 | 0.17 ± 0.07 | 0.34 ± 0.14 * |
| (6) | 16:0 | 18:2 + O | 687.5 | 0.05 ± 0.01 | 0.04 ± 0.00 | 0.81 ± 0.15 |
| 13:3 CHO | 565.5 | 0.04 ± 0.01 | 0.04 ± 0.01 | 1.05 ± 0.27 | ||
| 10:2 CHO | 525.5 | 0.13 ± 0.02 | 0.10 ± 0.02 | 0.82 ± 0.19 | ||
| 18:1 | 22:6 | 745.5 | 5.29 ± 0.36 | 1.74 ± 0.67 | 0.33 ± 0.13 * | |
| 18:1 | 22:6 + OO | 777.5 | 0.63 ± 0.09 | 0.96 ± 0.06 | 1.54 ± 0.25 * | |
| 19:5 CHO | 719.5 | 0.03 ± 0.00 | 0.06 ± 0.01 | 1.85 ± 0.26 * | ||
| 19:5 COOH | 735.5 | 0.24 ± 0.06 | 0.24 ± 0.04 | 0.98 ± 0.28 | ||
| PI | 18:0 | 18:2 | 861.5 | 0.54 ± 0.07 | 0.36 ± 0.03 | 0.66 ± 0.11 |
| (7) | 18:0 | 18:2 + OO | 893.5 | 0.00 ± 0.00 | 0.01 ± 0.00 | 1.12 ± 0.06 |
| 15:1 COOH | 851.5 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.44 ± 0.16 | ||
| 12:0 COOH | 731.5 | 0.00 ± 0.00 | 0.01 ± 0.00 | 1.74 ± 0.23 | ||
| 18:0 | 22:4 | 913.5 | 0.10 ± 0.03 | 0.07 ± 0.01 | 0.66 ± 0.20 | |
| 18:0 | 16:2 CHO | 847.5 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.91 ± 0.33 | |
| 16:2 COOH | 863.5 | 0.04 ± 0.01 | 0.04 ± 0.00 | 1.06 ± 0.20* | ||
| 15:1 CHO | 835.5 | 0.00 ± 0.00 | 0.00 ± 0.01 | 1.10 ± 0.22 | ||
| 13:1 CHO | 807.5 | 0.02 ± 0.00 | 0.03 ± 0.00 | 1.22 ± 0.05* | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.G.; Lee, G.B.; Yang, J.S.; Moon, M.H. Perturbations of Lipids and Oxidized Phospholipids in Lipoproteins of Patients with Postmenopausal Osteoporosis Evaluated by Asymmetrical Flow Field-Flow Fractionation and Nanoflow UHPLC–ESI–MS/MS. Antioxidants 2020, 9, 46. https://doi.org/10.3390/antiox9010046
Lee KG, Lee GB, Yang JS, Moon MH. Perturbations of Lipids and Oxidized Phospholipids in Lipoproteins of Patients with Postmenopausal Osteoporosis Evaluated by Asymmetrical Flow Field-Flow Fractionation and Nanoflow UHPLC–ESI–MS/MS. Antioxidants. 2020; 9(1):46. https://doi.org/10.3390/antiox9010046
Chicago/Turabian StyleLee, Kang Geun, Gwang Bin Lee, Joon Seon Yang, and Myeong Hee Moon. 2020. "Perturbations of Lipids and Oxidized Phospholipids in Lipoproteins of Patients with Postmenopausal Osteoporosis Evaluated by Asymmetrical Flow Field-Flow Fractionation and Nanoflow UHPLC–ESI–MS/MS" Antioxidants 9, no. 1: 46. https://doi.org/10.3390/antiox9010046
APA StyleLee, K. G., Lee, G. B., Yang, J. S., & Moon, M. H. (2020). Perturbations of Lipids and Oxidized Phospholipids in Lipoproteins of Patients with Postmenopausal Osteoporosis Evaluated by Asymmetrical Flow Field-Flow Fractionation and Nanoflow UHPLC–ESI–MS/MS. Antioxidants, 9(1), 46. https://doi.org/10.3390/antiox9010046