Memory-Enhancing Effects of Origanum majorana Essential Oil in an Alzheimer’s Amyloid beta1-42 Rat Model: A Molecular and Behavioral Study
Abstract
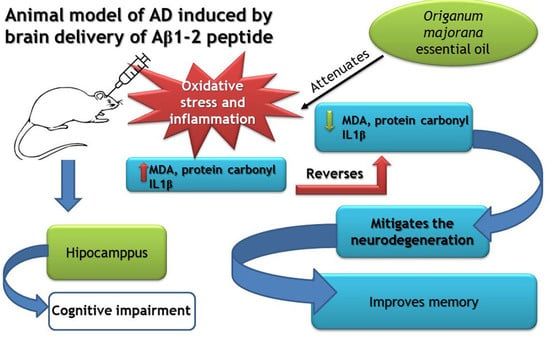
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.3. Animals
2.4. Experimental Protocol for Generating the AD Rat Model
2.5. Drug Treatment and Experimental Design
2.6. Biochemical Parameters Assay
2.7. Apoptotic State Evaluation
2.8. RNA Isolation and Hippocampal Real-Time Quantitative PCR (qRT-PCR)
2.9. Proteomics Pilot Experiment
2.9.1. Generation of Peptide Solutions for Proteome Analysis
2.9.2. Mass Spectrometric Proteome Analysis
2.9.3. Database Assembly and Proteome Data Analysis
2.10. Behavioral Analysis
2.10.1. Y-maze
2.10.2. Radial Arm Maze
2.11. Statistical Analysis
3. Results and Discussions
3.1. Phytochemical Profile of the Origanum Majorana Essential Oil
3.2. Proteome Analysis of the Rat Brains Containing Hippocampi
3.3. Differential Activities of Biological Processes Determined by Functional Assays
3.3.1. Neuroinflammation
3.3.2. Apoptosis
3.3.3. Oxidative Stress
3.3.4. Cognitive Function
3.4. Behavioral Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cooke, B.; Ernst, E. Aromatherapy: A systematic review. Br. J. Gen. Pract. 2000, 50, 493–496. [Google Scholar]
- Sánchez-Vidaña, D.I.; Ngai, S.P.-C.; He, W.; Chow, J.K.-W.; Lau, B.W.-M.; Tsang, H.W.H. The Effectiveness of Aromatherapy for Depressive Symptoms: A Systematic Review. Evid. Based Complement. Altern. Med. 2017, 2017, 5869315. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Dhany, A.L.; Mitchell, T.; Foy, C. Aromatherapy and Massage Intrapartum Service Impact on Use of Analgesia and Anesthesia in Women in Labor: A Retrospective Case Note Analysis. J. Altern. Complement. Med. 2012, 18, 932–938. [Google Scholar] [CrossRef]
- Jun, Y.S.; Kang, P.; Min, S.S.; Lee, J.-M.; Kim, H.-K.; Seol, G.H. Effect of Eucalyptus Oil Inhalation on Pain and Inflammatory Responses after Total Knee Replacement: A Randomized Clinical Trial. Evid. Based Complement. Altern. Med. 2013, 2013, 502727. [Google Scholar] [CrossRef] [PubMed]
- Ayan, M.; Taş, U.; Sogut, E.; Suren, M.; Gurbuzler, L.; Koyuncu, F. Investigating the Effect of Aromatherapy in Patients with Renal Colic. J. Altern. Complement. Med. 2013, 19, 329–333. [Google Scholar] [CrossRef]
- Perry, N.; Perry, E. Aromatherapy in the Management of Psychiatric Disorders. CNS Drugs 2006, 20, 257–280. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.-H.; Hou, W.-H.; Kao, C.-C.; Chang, M.-L.; Yu, L.-F.; Wu, C.-C.; Chen, C. The Anxiolytic Effect of Aromatherapy on Patients Awaiting Ambulatory Surgery: A Randomized Controlled Trial. Evid. Based Complement. Altern. Med. 2013, 2013, 927419. [Google Scholar] [CrossRef]
- Conrad, P.; Adams, C. The effects of clinical aromatherapy for anxiety and depression in the high risk postpartum woman—A pilot study. Complement. Ther. Clin. Pract. 2012, 18, 164–168. [Google Scholar] [CrossRef]
- Bina, F.; Rahimi, R. Sweet Marjoram: A Review of Ethnopharmacology, Phytochemistry, and Biological Activities. J. Evid. Based Integr. Med. 2016, 22, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Erenler, R.; Sen, O.; Akşit, H.; Demirtaş, I.; Yağlıoğlu, A.Ş.; Elmastas, M.; Telci, I.; Yaglioglu, A.S. Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. J. Sci. Food Agric. 2015, 96, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.; Caputo, L. Antimicrobial and Phytotoxic Activity of Origanum heracleoticum and O. majorana Essential Oils Growing in Cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, J.D.L.; Volcão, L.M.; Funck, G.D.; Kroning, I.S.; Da Silva, W.P.; Fiorentini, Â.M.; Ribeiro, G.A. Antimicrobial activity of essential oils of Origanum vulgare L. and Origanum majorana L. against Staphylococcus aureus isolated from poultry meat. Ind. Crop. Prod. 2015, 77, 444–450. [Google Scholar] [CrossRef]
- Mossa, A.-T.H.; Refaie, A.; Ramadan, A.; Bouajila, J. Amelioration of Prallethrin-Induced Oxidative Stress and Hepatotoxicity in Rat by the Administration of Origanum majorana Essential Oil. BioMed Res. Int. 2013, 2013, 859085. [Google Scholar] [CrossRef] [Green Version]
- Mossa, A.-T.H.; Nawwar, G.A.M. Free radical scavenging and antiacetylcholinesterase activities of Origanum majorana L. essential oil. Hum. Exp. Toxicol. 2011, 30, 1501–1513. [Google Scholar] [CrossRef]
- Al Dhaheri, Y.; Attoub, S.; Arafat, K.; AbuQamar, S.F.; Viallet, J.; Saleh, A.; Al Agha, H.; Eid, A.H.; Iratni, R. Anti-Metastatic and Anti-Tumor Growth Effects of Origanum majorana on Highly Metastatic Human Breast Cancer Cells: Inhibition of NFκB Signaling and Reduction of Nitric Oxide Production. PLoS ONE 2013, 8, e68808. [Google Scholar] [CrossRef] [Green Version]
- Abbasi-Maleki, S.; Kadkhoda, Z.; Taghizad-Farid, R. The antidepressant-like effects of Origanum majorana essential oil on mice through monoaminergic modulation using the forced swimming test. J. Tradit. Complement. Med. 2019, 10, 327–335. [Google Scholar] [CrossRef]
- Merino, J.J.; Parmigiani-Izquierdo, J.M.; López-Oliva, M.E.; Cabaña-Muñoz, M.E. Origanum majorana Essential Oil Inhalation during Neurofeedback Training Reduces Saliva Myeloperoxidase Activity at Session-1 in Bruxistic Patients. J. Clin. Med. 2019, 8, 158. [Google Scholar] [CrossRef] [Green Version]
- Jungbauer, A.; Medjakovic, S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas 2012, 71, 227–239. [Google Scholar] [CrossRef]
- Arranz, E.; Jaime, L.; López de las Hazas, M.C.; Reglero, G.; Santoyo, S. Supercritical fluid extraction as an alternative process to obtain essential oils with anti-inflammatory properties from marjoram and sweet basil. Ind. Crop. Prod. 2015, 67, 121–129. [Google Scholar] [CrossRef]
- Cardenas-Aguayo, M.D.C.; Silva-Lucero, M.D.C.; Cortes-Ortiz, M.; Jimnez-Ramos, B.; Gmez-Virgilio, L.; Ramrez-Rodrguez, G.; Arroyo, E.V.; Fiorentino-Prez, R.; Garca, U.; Luna-Muoz, J.; et al. Physiological Role of Amyloid Beta in Neural Cells: The Cellular Trophic Activity. In Neurochemistry; Heinbockel, T., Ed.; IntechOpen: London, UK, 2014; pp. 257–281. [Google Scholar] [CrossRef] [Green Version]
- Mcintee, F.L.; Giannoni, P.; Blais, S.; Sommer, G.; Neubert, T.A.; Rostagno, A.; Ghiso, J. In vivo Differential Brain Clearance and Catabolism of Monomeric and Oligomeric Alzheimer’s Aβ protein. Front. Aging Neurosci. 2016, 8, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Browne, A.; DiVito, J.R.; Stevenson, J.A.; Romano, D.; Dong, Y.; Xie, Z.; Tanzi, R.E. Amyloid-β Production via Cleavage of Amyloid-β Protein Precursor Is Modulated by Cell Density. J. Alzheimer’s Dis. 2010, 22, 683–694. [Google Scholar] [CrossRef] [Green Version]
- Morgese, M.G.; Colaianna, M.; Mhillaj, E.; Zotti, M.; Schiavone, S.; D’Antonio, P.; Harkin, A.; Gigliucci, V.; Campolongo, P.; Trezza, V.; et al. Soluble beta amyloid evokes alteration in brain norepinephrine levels: Role of nitric oxide and interleukin-1. Front. Mol. Neurosci. 2015, 9, 428. [Google Scholar] [CrossRef] [Green Version]
- Mucke, L.; Selkoe, D.J. Neurotoxicity of Amyloid β-Protein: Synaptic and Network Dysfunction. Cold Spring Harb. Perspect. Med. 2012, 2, a006338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puzzo, D.; Privitera, L.; Leznik, E.; Fà, M.; Staniszewski, A.; Palmeri, A.; Arancio, O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci. 2008, 28, 14537–14545. [Google Scholar] [CrossRef]
- Carrillo-Mora, P.; Luna, R.; Colín-Barenque, L. Amyloid Beta: Multiple Mechanisms of Toxicity and Only Some Protective Effects? Oxidative Med. Cell. Longev. 2014, 2014, 795375. [Google Scholar] [CrossRef]
- Poon, W.W.; Carlos, A.J.; Aguilar, B.L.; Berchtold, N.C.; Kawano, C.K.; Zograbyan, V.; Yaopruke, T.; Shelanski, M.; Cotman, C.W. β-Amyloid (Aβ) Oligomers Impair Brain-derived Neurotrophic Factor Retrograde Trafficking by Down-regulating Ubiquitin C-terminal Hydrolase, UCH-L1. J. Boil. Chem. 2013, 288, 16937–16948. [Google Scholar] [CrossRef] [Green Version]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Boil. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Gupta, P.; Sil, S.; Ghosh, R.; Ghosh, A.; Ghosh, T. Intracerebroventricular Aβ-Induced Neuroinflammation Alters Peripheral Immune Responses in Rats. J. Mol. Neurosci. 2018, 66, 572–586. [Google Scholar] [CrossRef]
- Carret-Rebillat, A.-S.; Pace, C.; Gourmaud, S.; Ravasi, L.; Montagne-Stora, S.; Longueville, S.; Tible, M.; Sudol, E.; Chang, R.C.-C.; Paquet, C.; et al. Neuroinflammation and Aβ Accumulation Linked To Systemic Inflammation Are Decreased By Genetic PKR Down-Regulation. Sci. Rep. 2015, 5, 8489. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Pub Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Postu, P.A.; Sadiki, F.Z.; El Idrissi, M.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L. Pinus halepensis essential oil attenuates the toxic Alzheimer’s amyloid beta (1-42)-induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharmacother. 2019, 112, 108673. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxiccoordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2005. [Google Scholar]
- Bate, S.T.; Clark, R.A. The Design and Statistical Analysis of Animal Experiments; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Smith, P.; Krohn, R.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.; Goeke, N.; Olson, B.; Klenk, D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Postu, P.A.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Mihasan, M.; Ciorpac, M.; Gorgan, L.D.; Petre, B.A.; Hritcu, L. Lactuca capensis reverses memory deficits in Aβ1-42-induced an animal model of Alzheimer’s disease. J. Cell. Mol. Med. 2017, 22, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Bantscheff, M.; Ringel, B.; Mádi, A.; Schnabel, R.; Glocker, M.O.; Thiesen, H.-J. Differential proteome analysis and mass spectrometric characterization of germ line development-related proteins of Caenorhabditis elegans. Proteomics 2004, 4, 2283–2295. [Google Scholar] [CrossRef] [PubMed]
- Just, T.; Gafumbegete, E.; Gramberg, J.; Prüfer, I.; Mikkat, S.; Ringel, B.; Pau, H.W.; Glocker, M.O. Differential proteome analysis of tonsils from children with chronic tonsillitis or with hyperplasia reveals disease-associated protein expression differences. Anal. Bioanal. Chem. 2006, 384, 1134–1144. [Google Scholar] [CrossRef]
- Röwer, C.; George, C.; Reimer, T.; Stengel, B.; Radtke, A.; Gerber, B.; Glocker, M.O. Distinct Ezrin Truncations Differentiate Metastases in Sentinel Lymph Nodes from Unaffected Lymph Node Tissues, from Primary Breast Tumors, and from Healthy Glandular Breast Tissues. Transl. Oncol. 2017, 11, 1–10. [Google Scholar] [CrossRef]
- Masuda, T.; Tomita, M.; Ishihama, Y. Phase Transfer Surfactant-Aided Trypsin Digestion for Membrane Proteome Analysis. J. Proteome Res. 2008, 7, 731–740. [Google Scholar] [CrossRef]
- Pappesch, R.; Warnke, P.; Mikkat, S.; Normann, J.; Wisniewska-Kucper, A.; Huschka, F.; Wittmann, M.; Khani, A.; Schwengers, O.; Oehmcke-Hecht, S.; et al. The Regulatory Small RNA MarS Supports Virulence of Streptococcus pyogenes. Sci. Rep. 2017, 7, 12241. [Google Scholar] [CrossRef]
- Yefremova, Y.; Opuni, K.F.M.; Danquah, B.D.; Thiesen, H.-J.; Glocker, M.O. Intact Transition Epitope Mapping (ITEM). J. Am. Soc. Mass Spectrom. 2017, 28, 1612–1622. [Google Scholar] [CrossRef]
- Wölter, M.; Okai, C.A.; Smith, D.S.; Ruß, M.; Rath, W.; Pecks, U.; Borchers, C.H.; Glocker, M.O. Maternal Apolipoprotein B100 Serum Levels are Diminished in Pregnancies with Intrauterine Growth Restriction and Differentiate from Controls. Proteom. Clin. Appl. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; James, B.L.; Ruß, M.; Mikkat, S.; Suresh, A.; Kämmerer, P.W.; Glocker, M.O. Proteome analysis reveals that de novo regenerated mucosa over fibula flap-reconstructed mandibles resembles mature keratinized oral mucosa. Oral Oncol. 2018, 78, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Geromanos, S.J.; Vissers, J.P.; Silva, J.C.; Dorschel, C.A.; Li, G.-Z.; Gorenstein, M.V.; Bateman, R.H.; Langridge, J.I. The detection, correlation, and comparison of peptide precursor and product ions from data independent LC-MS with data dependant LC-MS/MS. Proteomics 2009, 9, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Shliaha, P.V.; Bond, N.J.; Gatto, L.; Lilley, K.S. Effects of Traveling Wave Ion Mobility Separation on Data Independent Acquisition in Proteomics Studies. J. Proteome Res. 2013, 12, 2323–2339. [Google Scholar] [CrossRef]
- Distler, U.; Kuharev, J.; Navarro, P.; Levin, Y.; Schild, H.; Tenzer, S. Drift time-specific collision energies enable deep-coverage data-independent acquisition proteomics. Nat. Methods 2013, 11, 167–170. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Llinares, M.B.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2018, 47, D442–D450. [Google Scholar] [CrossRef]
- Jackson, L.L. VTE on an elevated T-maze. J. Comp. Psychol. 1943, 36, 99–107. [Google Scholar] [CrossRef]
- Sadiki, F.Z.; El Idrissi, M.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L.; Postu, P.A. Tetraclinis articulata essential oil mitigates cognitive deficits and brain oxidative stress in an Alzheimer’s disease amyloidosis model. Phytomedicine 2018, 56, 57–63. [Google Scholar] [CrossRef]
- Olton, D.S.; Samuelson, R.J. Remembrance of places passed: Spatial memory in rats. J. Exp. Psychol. Anim. Behav. Process. 1976, 2, 97–116. [Google Scholar] [CrossRef]
- Olton, D.S.; Schlosberg, P. Food-searching strategies in young rats: Win-shift predominates over win-stay. J. Comp. Physiol. Psychol. 1978, 92, 609–618. [Google Scholar] [CrossRef]
- Timberlake, W.; White, W. Winning isn’t everything: Rats need only food deprivation and not food reward to efficiently traverse a radial arm maze. Learn. Motiv. 1990, 21, 153–163. [Google Scholar] [CrossRef]
- Bannerman, D.; Sprengel, R.; Sanderson, D.J.; McHugh, S.B.; Rawlins, J.N.P.; Monyer, H.; Seeburg, P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014, 15, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athamneh, K.; Alneyadi, A.H.; Alsamri, H.; Alrashedi, A.; Palakott, A.; El-Tarabily, K.A.; Eid, A.; Al-Dhaheri, Y.; Iratni, R. Origanum majorana Essential Oil Triggers p38 MAPK-Mediated Protective Autophagy, Apoptosis, and Caspase-Dependent Cleavage of P70S6K in Colorectal Cancer Cells. Biomolecules 2020, 10, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amor, G.; Caputo, L.; La Storia, A.; De Feo, V.; Mauriello, G.; Fechtali, T. Chemical Composition and Antimicrobial Activity of Artemisia herba-alba and Origanum majorana Essential Oils from Morocco. Molecules 2019, 24, 4021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurzyńska-Wierdak, R.; Zawiślak, G.; Kowalski, R. The Content and Composition of Essential Oil of Origanum majorana L. Grown in Poland Depending on Harvest Tme and Method of Raw Material Preparation. J. Essent. Oil Bear. Plants 2015, 18, 1482–1489. [Google Scholar] [CrossRef]
- Ramos, S.; Rojas, L.B.; Lucena, M.E.; Meccia, G.; Usubillaga, A. Chemical Composition and Antibacterial Activity of Origanum majorana L. Essential Oil from the Venezuelan Andes. J. Essent. Oil Res. 2011, 23, 45–49. [Google Scholar] [CrossRef]
- Maass, W.; Parsons, J.; Purao, S.; Storey, V.C.; Woo, C. Data-Driven Meets Theory-Driven Research in the Era of Big Data: Opportunities and Challenges for Information Systems Research. J. Assoc. Inf. Syst. 2018, 19, 1253–1273. [Google Scholar] [CrossRef] [Green Version]
- Pathan, M.; Keerthikumar, S.; Ang, C.-S.; Gangoda, L.; Quek, C.Y.; Williamson, N.A.; Mouradov, D.; Sieber, O.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Soliman, M.M.; Nassan, M.A.; Ismail, T.A. Origanum Majoranum Extract Modulates Gene Expression, Hepatic and Renal Changes in a Rat Model of Type 2 Diabetes. Iran. J. Pharm. Res. 2016, 15, 45–54. [Google Scholar]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harb. Perspect. Boil. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Payan, R.; Aguilar-Medina, M.; Estrada-Parra, S.; González-Y-Merchand, J.A.; Favila-Castillo, L.; Monroy-Ostria, A.; Estrada-Garcia, I.C.E. Quantification of Cytokine Gene Expression Using an Economical Real-Time Polymerase Chain Reaction Method Based on SYBRR Green I. Scand. J. Immunol. 2003, 57, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, S.M.; Tyrrell, P.; Rothwell, N.J. Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 2005, 5, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, N.; Simpson, J.; Taylor, G.; Rafique, S.; Whitehouse, A.; Hiscox, J.A.; Stark, L.A. Nucleolar NF-κB/RelA mediates apoptosis by causing cytoplasmic relocalization of nucleophosmin. Cell Death Differ. 2011, 18, 1889–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, N.D.; Gilmore, T.D. Good cop, bad cop: The different faces of NF-κB. Cell Death Differ. 2006, 13, 759–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afshin-Majd, S.; Khalili, M.; Roghani, M.; Mehranmehr, N.; Baluchnejadmojarad, T. Carnosine Exerts Neuroprotective Effect against 6-Hydroxydopamine Toxicity in Hemiparkinsonian Rat. Mol. Neurobiol. 2014, 51, 1064–1070. [Google Scholar] [CrossRef]
- Morroni, F.; Tarozzi, A.; Sita, G.; Bolondi, C.; Moraga, J.M.Z.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson’s disease. NeuroToxicology 2013, 36, 63–71. [Google Scholar] [CrossRef]
- Strozyk, E.; Pöppelmann, B.; Schwarz, T.; Kulms, D. Differential effects of NF-κB on apoptosis induced by DNA-damaging agents: The type of DNA damage determines the final outcome. Oncogene 2006, 25, 6239–6251. [Google Scholar] [CrossRef] [Green Version]
- Morishima, Y.; Gotoh, Y.; Zieg, J.; Barrett, T.; Takano, H.; Flavell, R.; Davis, R.J.; Shirasaki, Y.; Greenberg, M.E. β-Amyloid Induces Neuronal Apoptosis Via a Mechanism that Involves the c-Jun N-Terminal Kinase Pathway and the Induction of Fas Ligand. J. Neurosci. 2001, 21, 7551–7560. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Reddy, P.H. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: Implications for early intervention and therapeutics. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1359–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.-J.; Hu, Y.-Y.; Yang, Z.-J.; Jiang, L.-P.; Shi, S.-L.; Li, Y.-R.; Guo, M.-Y.; Wu, H.-L.; Wan, Y.-Y. Amyloid β-42 induces neuronal apoptosis by targeting mitochondria. Mol. Med. Rep. 2017, 16, 4521–4528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kechko, O.I.; Petrushanko, I.Y.; Brower, C.S.; Adzhubei, A.A.; Moskalev, A.A.; Piatkov, K.I.; Mitkevich, V.A.; Makarov, A.A. Beta-amyloid induces apoptosis of neuronal cells by inhibition of the Arg/N-end rule pathway proteolytic activity. Aging 2019, 11, 6134–6152. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Arguelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Curtis, J.M.; Hahn, W.S.; Long, E.K.; Burrill, J.S.; Arriaga, E.A.; Bernlohr, D.A. Protein carbonylation and metabolic control systems. Trends Endocrinol. Metab. 2012, 23, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Dalle-Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzani, A.D.G. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell. Mol. Med. 2007, 10, 389–406. [Google Scholar] [CrossRef]
- Zuo, L.; Hemmelgarn, B.T.; Chuang, C.-C.; Best, T.M. The Role of Oxidative Stress-Induced Epigenetic Alterations in Amyloid-β Production in Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2015, 2015, 604658. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-Peptide (1–42)-Induced Oxidative Stress in Alzheimer Disease: Importance in Disease Pathogenesis and Progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef] [Green Version]
- Deuschle, R.A.N.; Deuschle, V.C.K.N.; Bonfanti-Azzolin, G.; De Oliveira, J.S.; Sostisso, Q.C.B.; Goulart, J.D.S.; Mayer, M.S.; Horn, R.C.; Golle, D.P. Phytochemical Screening and Antioxidant Activity of Origanum majorana against Oxidative Stress Biomarkers. J. Agric. Sci. 2018, 10, 395. [Google Scholar] [CrossRef] [Green Version]
- Soliman, A.M.; Desouky, S.; Marzouk, M.; Sayed, A.A. Origanum majorana Attenuates Nephrotoxicity of Cisplatin Anticancer Drug through Ameliorating Oxidative Stress. Nutrients 2016, 8, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddam, M.G.; Ansari, I.; Roghani, M.; Moradi, M. The Effects of Origanum Majorana on Oxidative Stress and Histopathology of Renal Tissue among Streptozotocin-Induced Diabetic Rats. Thrita J. Med. Sci. 2013, 2, 29–34. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Morys, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2017, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Cory, S.; Kidane, A.H.; Shirkey, N.J.; Marshak, S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev. Neurobiol. 2010, 70, 271–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Fang, Y.; Lian, Y.; Chen, Y.; Wu, T.; Zheng, Y.; Zong, H.; Sun, L.; Zhang, R.; Wang, Z.; et al. Brain-Derived Neurotrophic Factor Ameliorates Learning Deficits in a Rat Model of Alzheimer’s Disease Induced by Aβ1-42. PLoS ONE 2015, 10, e0122415. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Fang, Y.; Xu, Y.; Lian, Y.; Xie, N.; Wu, T.; Zhang, H.; Sun, L.; Zhang, R.; Wang, Z. Curcumin Improves Amyloid β-Peptide (1-42) Induced Spatial Memory Deficits through BDNF-ERK Signaling Pathway. PLoS ONE 2015, 10, e0131525. [Google Scholar] [CrossRef] [Green Version]
- Gürbüz, P.; Martinez, A.; Pérez, C.; Martínez-González, L.; Göger, F.; Ayran, I. Potential anti-Alzheimer effects of selected Lamiaceae plants through polypharmacology on glycogen synthase kinase-3β, β-secretase, and casein kinase 1δ. Ind. Crop. Prod. 2019, 138, 111431. [Google Scholar] [CrossRef]







| Rank (a) | No. (b) | Protein Name (b) | Cellular Component/Molecular Function/Biological Process (c) | Assay (d) |
|---|---|---|---|---|
| 320 | P30009 | Myristoylated alanine-rich C-kinase substrate | postsynaptic cytoskeleton/calmodulin binding/positive regulation of neuron projection development | cognitive function |
| 487 | Q08163 | Adenylyl cyclase-associated protein 1 | cortical actin cytoskeleton/actin binding/actin cytoskeleton organization | n.a. |
| 602 | M0RBL8 | Transcription elongation factor A (SII)-like 6 | nucleus/WW domain binding/n.d. | n.a. |
| 620 | P31232 | Transgelin | cytoplasm/actin filament binding/epithelial cell differentiation | n.a. |
| 657 | Q9EPH2 | MARCKS-related protein | anchored component of presynaptic membrane/calmodulin binding/positive regulation of cell population proliferation | cognitive function |
| 761 | P84083 | ADP-ribosylation factor 5 | perinuclear region of cytoplasm/GTP binding/vesicle-mediated transport | n.a. |
| 782 | P60901 | Proteasome subunit alpha type-6 | cytoplasm/threonine-type endopeptidase activity/positive regulation of NF-κB transcription factor activity | apoptosis a/o neuroinflammation |
| 896 | Q6AY41 | Cell cycle control protein 50A | Golgi apparatus and membrane/aminophospholipid flippase activity/positive regulation of neuron projection development | cognitive function |
| 899 | P34067 | Proteasome subunit beta type-4 | cytoplasm/threonine-type endopeptidase activity/proteasome-mediated ubiquitin-dependent protein catabolic process | oxidative stress |
| 1012 | Q6AXS5 | Plasminogen activator inhibitor 1 RNA-binding protein | cytoplasm/mRNA 3′-UTR binding/regulation of apoptotic process | apoptosis |
| 1021 | P06302 | Prothymosin alpha | nucleus/NF-κB binding/positive regulation of NF-κB transcription factor activity and negative regulation of apoptotic process | apoptosis a/o neuroinflammation |
| 1118 | Q5M821 | Protein phosphatase 1H | n.d./protein serine/threonine phosphatase activity/positive regulation of pyruvate dehydrogenase activity | n.a. |
| 1119 | P01835 | Ig kappa chain C region, B allele | n.d./n.d./n.d. | n.a. |
| 1146 | Q63941 | Ras-related protein Rab-3 | endomembrane system/GTP binding/peptidyl-cysteine methylation | oxidative stress |
| 1150 | P13084 | Nucleophosmin | ribonucleoprotein complex/identical protein binding/positive regulation of NF-κB transcription factor activity | apoptosis a/o neuroinflammation |
| 1159 | Q5XIU9 | Membrane-associated progesterone receptor component 2 | membrane/steroid binding/adipose tissue development | n.a. |
| 1160 | P25093 | Fumarylacetoacetase | n.d./metal ion binding/homogentisate catabolic process | oxidative stress |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postu, P.A.; Gorgan, D.L.; Cioanca, O.; Russ, M.; Mikkat, S.; Glocker, M.O.; Hritcu, L. Memory-Enhancing Effects of Origanum majorana Essential Oil in an Alzheimer’s Amyloid beta1-42 Rat Model: A Molecular and Behavioral Study. Antioxidants 2020, 9, 919. https://doi.org/10.3390/antiox9100919
Postu PA, Gorgan DL, Cioanca O, Russ M, Mikkat S, Glocker MO, Hritcu L. Memory-Enhancing Effects of Origanum majorana Essential Oil in an Alzheimer’s Amyloid beta1-42 Rat Model: A Molecular and Behavioral Study. Antioxidants. 2020; 9(10):919. https://doi.org/10.3390/antiox9100919
Chicago/Turabian StylePostu, Paula Alexandra, Dragos Lucian Gorgan, Oana Cioanca, Manuela Russ, Stefan Mikkat, Michael Otto Glocker, and Lucian Hritcu. 2020. "Memory-Enhancing Effects of Origanum majorana Essential Oil in an Alzheimer’s Amyloid beta1-42 Rat Model: A Molecular and Behavioral Study" Antioxidants 9, no. 10: 919. https://doi.org/10.3390/antiox9100919
APA StylePostu, P. A., Gorgan, D. L., Cioanca, O., Russ, M., Mikkat, S., Glocker, M. O., & Hritcu, L. (2020). Memory-Enhancing Effects of Origanum majorana Essential Oil in an Alzheimer’s Amyloid beta1-42 Rat Model: A Molecular and Behavioral Study. Antioxidants, 9(10), 919. https://doi.org/10.3390/antiox9100919