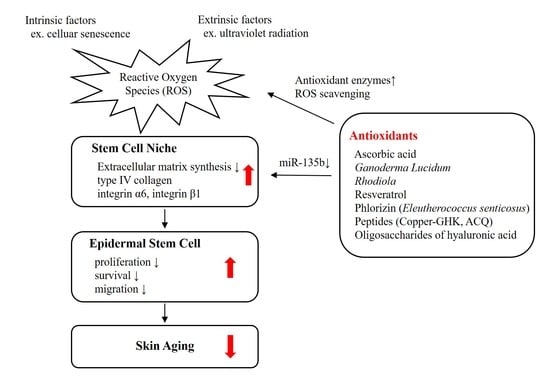
Antioxidants as an Epidermal Stem Cell Activator
Abstract
:1. Introduction
2. Epidermal Stem Cells and Extracellular Matrix
2.1. Interfollicular Epidermal Stem Cells
2.2. Extracellular Matrix and Stem Cell Niche
2.3. Reactive Oxygen Species Regulates Stem Cell Niche
2.4. UV Radiation Affects Epidermal Stem Cells Via ROS Production
3. Antioxidants and Interfollicular Epidermal Stem Cells
3.1. Skin Equivalents
3.2. Ascorbic Acid
3.2.1. Antioxidant Activity
3.2.2. Epigenetic Regulation
3.2.3. Epidermal Stem Cell Activation
3.3. Ganodermal Lucidum
3.3.1. Antioxidant Activity
3.3.2. Epidermal Stem Cell Activation
3.4. Rhodiola
3.4.1. Antioxidant Activity
3.4.2. Epidermal Stem Cell Activation
3.5. Resveratrol
3.5.1. Antioxidant Activity
3.5.2. Epidermal Stem Cell Activation
3.6. Phlorizin (Active Ingredient of Eleutherococcus Senticosus)
3.6.1. Antioxidant Activity
3.6.2. Epidermal Stem Cell Activation
3.7. Human Tripeptide “Copper-GHK”
3.7.1. Antioxidant Activity
3.7.2. Regulation of Gene Expressions
3.7.3. Regulation of ECM Production
3.7.4. Epidermal Stem Cell Activation
3.8. Tripeptide “ACQ: Alanine-Cysteine-Glutamine”
3.9. Hyaluronic Acid
3.9.1. Antioxidant Activity
3.9.2. Epidermal Stem Cell Activation
4. Application in Skin Rejuvenation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALT | serum alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| ASK-1 | apoptosis signal-regulating kinase 1 |
| AST | aspartate aminotransferase |
| ARE | antioxidant response element |
| COX-2 | cyclooxygenase-2 |
| ECM | extracellular matrix |
| ERK | extracellular signal-regulated kinases |
| Fe2+/α-KGDDs | Fe2+- and α-ketoglutarate-dependent dioxygenases |
| FOXO | forkhead box O |
| HaCaT | human skin and human keratinocytes cell line |
| HDAC | histone deacetylase |
| HMWHA | high molecular weight hyaluronic acid |
| iPSC | induced pluripotent stem cell |
| JHDMs | Jumonji C domain-containing histone demethylases |
| JNK | c-Jun n-terminal kinase |
| Keap1 | Kelch-like ECH-associated protein |
| LMWHa | low molecular weight hyaluronic acid |
| MAPK | mitogen-activated protein kinase |
| MMP | matrix metalloproteinase |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| Nrf2 | nuclear factor erythroid 2-related factor |
| PCNA | proliferating cell nuclear antigen |
| ROS | reactive oxygen species |
| SIRT1 | sirtuin 1 |
| SLGT | sodium-linked glucose transporter |
| SOD | superoxide dismutase |
| TET | ten-eleven translocation |
| TIMP | tissue inhibitor of metalloproteinase |
| TNF-α | tumor necrosis factor-α |
| UV | ultraviolet |
References
- Diaz-Flores, L., Jr.; Madrid, J.F.; Gutierrez, R.; Varela, H.; Valladares, F.; Alvarez-Arguelles, H.; Diaz-Flores, L. Adult stem and transit-amplifying cell location. Histol. Histopathol. 2006, 21, 995–1027. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, T. Stem cell niche: Structure and function. Annu. Rev. Cell Dev. Biol. 2005, 21, 605–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Li, Y.; Fan, H.; Liu, Z.; Pestell, R.G. miRNAs regulate stem cell self-renewal and differentiation. Front. Genet. 2012, 3, 191. [Google Scholar] [CrossRef] [Green Version]
- Youn, S.W.; Kim, D.S.; Cho, H.J.; Jeon, S.E.; Bae, I.H.; Yoon, H.J.; Park, K.C. Cellular senescence induced loss of stem cell proportion in the skin in vitro. J. Dermatol. Sci. 2004, 35, 113–123. [Google Scholar] [CrossRef]
- Watt, F.M.; Driskell, R.R. The therapeutic potential of stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Watt, F.M.; Fujiwara, H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.R.; Kang, Y.A.; Shin, J.W.; Na, J.I.; Huh, C.H.; Park, K.C. Redox status is critical for stemness in skin equivalents. Oxid. Med. Cell Longev. 2012, 2012, 819623. [Google Scholar] [CrossRef] [Green Version]
- Na, J.I.; Shin, J.W.; Choi, H.R.; Kwon, S.H.; Park, K.C. Resveratrol as a multifunctional topical hypopigmenting agent. Int. J. Mol. Sci. 2019, 20, 956. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.R.; Nam, K.M.; Lee, H.S.; Yang, S.H.; Kim, Y.S.; Lee, J.; Date, A.; Toyama, K.; Park, K.C. Phlorizin, an active ingredient of Eleutherococcus senticosus, increases proliferative potential of keratinocytes with inhibition of MiR135b and increased expression of type IV collagen. Oxid. Med. Cell Longev. 2016, 2016, 3859721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.A.; Choi, H.R.; Na, J.I.; Huh, C.H.; Kim, M.J.; Youn, S.W.; Kim, K.H.; Park, K.C. Copper-GHK increases integrin expression and p63 positivity by keratinocytes. Arch. Dermatol. Res. 2009, 301, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; Shin, J.W.; Na, J.I.; Nam, K.M.; Lee, H.S.; Park, K.C. Novel antioxidant tripeptide “ACQ” can prevent UV-induced cell death and preserve the number of epidermal stem cells. Oxid. Med. Cell Longev. 2015, 2015, 359740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.R.; Kang, Y.A.; Na, J.I.; Huh, S.Y.; Huh, C.H.; Kim, K.H.; Park, K.C. Oligosaccharides of hyaluronic acid increased epidermal cell stemness by modulation of integrin expression. J. Cosmet. Dermatol. 2012, 11, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Choi, H.R.; Nam, K.M.; Lee, H.S.; Kim, S.A.; Joe, H.J.; Kazumi, T.; Park, K.C. The Co-expression pattern of p63 and HDAC1: A potential way to disclose stem cells in interfollicular epidermis. Int. J. Mol. Sci. 2017, 18, 1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; De Luca, M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161. [Google Scholar] [CrossRef] [Green Version]
- Candi, E.; Amelio, I.; Agostini, M.; Melino, G. MicroRNAs and p63 in epithelial stemness. Cell Death Differ. 2015, 22, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Koster, M.I.; Kim, S.; Roop, D.R. P63 deficiency: A failure of lineage commitment or stem cell maintenance? J. Investig. Dermatol. Symp. Proc. 2005, 10, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Bravo, R.; Frank, R.; Blundell, P.A.; Macdonald-Bravo, H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature 1987, 326, 515–517. [Google Scholar] [CrossRef]
- Breitkreutz, D.; Koxholt, I.; Thiemann, K.; Nischt, R. Skin basement membrane: The foundation of epidermal integrity--BM functions and diverse roles of bridging molecules nidogen and perlecan. Biomed. Res. Int. 2013, 2013, 179784. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.R.; Byun, S.Y.; Kwon, S.H.; Park, K.C. Niche interactions in epidermal stem cells. World J. Stem Cells 2015, 7, 495–501. [Google Scholar] [CrossRef]
- Sonnenberg, A.; Calafat, J.; Janssen, H.; Daams, H.; van der Raaij-Helmer, L.M.; Falcioni, R.; Kennel, S.J.; Aplin, J.D.; Baker, J.; Loizidou, M.; et al. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J. Cell Biol. 1991, 113, 907–917. [Google Scholar] [CrossRef]
- Grose, R.; Hutter, C.; Bloch, W.; Thorey, I.; Watt, F.M.; Fassler, R.; Brakebusch, C.; Werner, S. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 2002, 129, 2303–2315. [Google Scholar] [PubMed]
- Kretzschmar, K.; Watt, F.M. Markers of epidermal stem cell subpopulations in adult mammalian skin. Cold Spring Harb. Perspect. Med. 2014, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.J.; Bae, I.H.; Chung, H.J.; Kim, D.S.; Kwon, S.B.; Cho, Y.J.; Youn, S.W.; Park, K.C. Effects of hair follicle dermal sheath cells in the reconstruction of skin equivalents. J. Dermatol. Sci. 2004, 35, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Upton, Z.; Cuttle, L.; Noble, A.; Kempf, M.; Topping, G.; Malda, J.; Xie, Y.; Mill, J.; Harkin, D.G.; Kravchuk, O.; et al. Vitronectin: Growth factor complexes hold potential as a wound therapy approach. J. Investig. Dermatol. 2008, 128, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, T.L.; Van Lonkhuyzen, D.R.; Dawson, R.A.; Kimlin, M.G.; Upton, Z. Insulin-like growth factor-I and UVB photoprotection in human keratinocytes. Exp. Dermatol. 2015, 24, 235–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, A.J.; Haase, I.; Watt, F.M. Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 6728–6733. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.R.; Nam, K.M.; Park, S.J.; Kim, D.S.; Huh, C.H.; Park, W.Y.; Park, K.C. Suppression of miR135b increases the proliferative potential of normal human keratinocytes. J. Investig. Dermatol. 2014, 134, 1161–1164. [Google Scholar] [CrossRef] [Green Version]
- Peerani, R.; Rao, B.M.; Bauwens, C.; Yin, T.; Wood, G.A.; Nagy, A.; Kumacheva, E.; Zandstra, P.W. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007, 26, 4744–4755. [Google Scholar] [CrossRef] [Green Version]
- Mohyeldin, A.; Garzon-Muvdi, T.; Quinones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Pervaiz, S.; Taneja, R.; Ghaffari, S. Oxidative stress regulation of stem and progenitor cells. Antioxid. Redox Sign. 2009, 11, 2777–2789. [Google Scholar] [CrossRef]
- Ji, A.R.; Ku, S.Y.; Cho, M.S.; Kim, Y.Y.; Kim, Y.J.; Oh, S.K.; Kim, S.H.; Moon, S.Y.; Choi, Y.M. Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage. Exp. Mol. Med. 2010, 42, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Park, H.H.; Choi, H.; Kim, Y.S.; Yu, H.J.; Lee, K.Y.; Lee, Y.J.; Kim, S.H.; Koh, S.H. Coenzyme Q10 protects neural stem cells against hypoxia by enhancing survival signals. Brain Res. 2012, 1478, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Carr, W.J.; Oberley-Deegan, R.E.; Zhang, Y.; Oberley, C.C.; Oberley, L.W.; Dunnwald, M. Antioxidant proteins and reactive oxygen species are decreased in a murine epidermal side population with stem cell-like characteristics. Histochem. Cell Biol. 2011, 135, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhie, G.; Shin, M.H.; Seo, J.Y.; Choi, W.W.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C.; Chung, J.H. Aging-and photoaging-dependent changes of enzymic and nonenzymic antioxidants in the epidermis and dermis of human skin in vivo. J. Investig. Dermatol. 2001, 117, 1212–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [Green Version]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef]
- Cesarini, J.P.; Michel, L.; Maurette, J.M.; Adhoute, H.; Bejot, M. Immediate effects of UV radiation on the skin: Modification by an antioxidant complex containing carotenoids. Photodermatol. Photoimmunol. Photomed. 2003, 19, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Halliday, G.M. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat. Res. 2005, 571, 107–120. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef] [Green Version]
- Pence, B.C.; Naylor, M.F. Effects of single-dose ultraviolet radiation on skin superoxide dismutase, catalase, and xanthine oxidase in hairless mice. J. Investig. Dermatol. 1990, 95, 213–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shindo, Y.; Witt, E.; Packer, L. Antioxidant defense mechanisms in murine epidermis and dermis and their responses to ultraviolet light. J. Investig. Dermatol. 1993, 100, 260–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sander, C.S.; Chang, H.; Salzmann, S.; Muller, C.S.; Ekanayake-Mudiyanselage, S.; Elsner, P.; Thiele, J.J. Photoaging is associated with protein oxidation in human skin in vivo. J. Investig. Dermatol. 2002, 118, 618–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.S.; Cho, H.J.; Choi, H.R.; Kwon, S.B.; Park, K.C. Isolation of human epidermal stem cells by adherence and the reconstruction of skin equivalents. Cell. Mol. Life Sci. 2004, 61, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [Green Version]
- Peterkofsky, B. Ascorbate requirement for hydroxylation and secretion of procollagen: Relationship to inhibition of collagen synthesis in scurvy. Am. J. Clin. Nutr. 1991, 54, 1135S–1140S. [Google Scholar] [CrossRef]
- Sram, R.J.; Binkova, B.; Rossner, P., Jr. Vitamin C for DNA damage prevention. Mutat. Res. 2012, 733, 39–49. [Google Scholar] [CrossRef]
- Savini, I.; D’Angelo, I.; Ranalli, M.; Melino, G.; Avigliano, L. Ascorbic acid maintenance in HaCaT cells prevents radical formation and apoptosis by UV-B. Free Radic. Biol. Med. 1999, 26, 1172–1180. [Google Scholar] [CrossRef]
- Stewart, M.S.; Cameron, G.S.; Pence, B.C. Antioxidant nutrients protect against UVB-induced oxidative damage to DNA of mouse keratinocytes in culture. J. Investig. Dermatol. 1996, 106, 1086–1089. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, S.; Funakoshi, T.; Sato, Y.; Saito, N.; Ohsawa, H.; Kurita, K.; Nagata, K.; Yoshida, M.; Ishigami, A. Protective effect of pre- and post-vitamin C treatments on UVB-irradiation-induced skin damage. Sci. Rep. 2018, 8, 16199. [Google Scholar] [CrossRef]
- Cimmino, L.; Neel, B.G.; Aifantis, I. Vitamin C in Stem Cell Reprogramming and Cancer. Trends Cell. Biol. 2018, 28, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Gorres, K.L.; Raines, R.T. Prolyl 4-hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, K.; Zeng, X.; Yang, J.; Wu, Y.; Shi, X.; Qin, B.; Zeng, L.; Esteban, M.A.; Pan, G.; et al. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell 2011, 9, 575–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, C.; Jia, G.; Hou, G.; Dai, Q.; Zhang, W.; Zheng, G.; Jian, X.; Yang, C.G.; Cui, Q.; He, C. Iron-catalysed oxidation intermediates captured in a DNA repair dioxygenase. Nature 2010, 468, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Gerken, T.; Girard, C.A.; Tung, Y.C.; Webby, C.J.; Saudek, V.; Hewitson, K.S.; Yeo, G.S.; McDonough, M.A.; Cunliffe, S.; McNeill, L.A.; et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007, 318, 1469–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-Martinez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A.; et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Mao, S.Q.; Zhao, B.; Chong, Z.; Yang, Y.; Zhao, C.; Zhang, D.; Huang, H.; Gao, J.; Li, Z.; et al. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J. Am. Chem. Soc. 2013, 135, 10396–10403. [Google Scholar] [CrossRef]
- Chung, T.L.; Brena, R.M.; Kolle, G.; Grimmond, S.M.; Berman, B.P.; Laird, P.W.; Pera, M.F.; Wolvetang, E.J. Vitamin C promotes widespread yet specific DNA demethylation of the epigenome in human embryonic stem cells. Stem Cells 2010, 28, 1848–1855. [Google Scholar] [CrossRef]
- Fujisawa, K.; Hara, K.; Takami, T.; Okada, S.; Matsumoto, T.; Yamamoto, N.; Sakaida, I. Evaluation of the effects of ascorbic acid on metabolism of human mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 93. [Google Scholar] [CrossRef]
- Minor, E.A.; Court, B.L.; Young, J.I.; Wang, G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J. Biol. Chem. 2013, 288, 13669–13674. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Chen, Y.; Bian, C.; Fujiki, R.; Yu, X. TET2 promotes histone O-GlcNAcylation during gene transcription. Nature 2013, 493, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lan, J.; Liu, D.; Backman, L.J.; Zhang, W.; Zhou, Q.; Danielson, P. Ascorbic acid promotes the stemness of corneal epithelial stem/progenitor cells and accelerates epithelial wound healing in the cornea. Stem Cells Transl. Med. 2017, 6, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Rembe, J.D.; Fromm-Dornieden, C.; Stuermer, E.K. Effects of vitamin B complex and vitamin C on human skin cells: Is the perceived effect measurable? Adv. Skin Wound Care 2018, 31, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lin, J.; Yin, Y.; Zhao, J.; Sun, X.; Tang, K. Ganodermataceae: Natural products and their related pharmacological functions. Am. J. Chin. Med. 2007, 35, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Li, L.D.; Mao, P.W.; Shao, K.D.; Bai, X.H.; Zhou, X.W. Ganoderma proteins and their potential applications in cosmetics. Appl. Microbiol. Biotechnol. 2019, 103, 9239–9250. [Google Scholar] [CrossRef]
- Smina, T.P.; Mathew, J.; Janardhanan, K.K.; Devasagayam, T.P. Antioxidant activity and toxicity profile of total triterpenes isolated from Ganoderma lucidum (Fr.) P. Karst occurring in South India. Environ. Toxicol. Pharmacol. 2011, 32, 438–446. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, B.; Ren, H. Preventive and therapeutic effect of Ganoderma (Lingzhi) on skin diseases and care. Adv. Exp. Med. Biol. 2019, 1182, 311–321. [Google Scholar] [CrossRef]
- You, Y.H.; Lin, Z.B. Antioxidant effect of Ganoderma polysaccharide peptide. Yao Xue Xue Bao 2003, 38, 85–88. [Google Scholar]
- You, Y.H.; Lin, Z.B. Protective effects of Ganoderma lucidum polysaccharides peptide on injury of macrophages induced by reactive oxygen species. Acta Pharmacol. Sin. 2002, 23, 787–791. [Google Scholar]
- Zhonghui, Z.; Xiaowei, Z.; Fang, F. Ganoderma lucidum polysaccharides supplementation attenuates exercise-induced oxidative stress in skeletal muscle of mice. Saudi J. Biol. Sci. 2014, 21, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Zhong, D.; Wang, H.; Liu, M.; Li, X.; Huang, M.; Zhou, H.; Lin, S.; Lin, Z.; Yang, B. Ganoderma lucidum polysaccharide peptide prevents renal ischemia reperfusion injury via counteracting oxidative stress. Sci. Rep. 2015, 5, 16910. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Tang, Y.P.; Xiang, J.; Wua, P.; Jin, H.M.; Wang, Z.; Mori, M.; Cai, D.F. Neuroprotective effects of water-soluble Ganoderma lucidum polysaccharides on cerebral ischemic injury in rats. J. Ethnopharmacol. 2010, 131, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Q.; Deng, W.; Li, Y.; Xing, G.; Shi, X.; Du, Y. Neuroprotective effect of pretreatment with Ganoderma lucidum in cerebral ischemia/reperfusion injury in rat hippocampus. Neural Regen. Res. 2014, 9, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Meng, X.; Yin, J.; Sun, J.; Huang, Q.; Yin, Z. Ganoderma lucidum polysaccharide peptide attenuates skin flap ischemia-reperfusion injury in a thioredoxin-dependent manner. Plast Reconstr. Surg. 2018, 142, 23e–33e. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhou, F.; Lei, L.; Chen, J.; Lu, J.; Zhou, J.; Cao, K.; Gao, L.; Xia, F.; Ding, S.; et al. Ganoderma lucidum polysaccharides protect fibroblasts against UVB-induced photoaging. Mol. Med. Rep. 2017, 15, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Chiang, H.M.; Chen, H.C.; Wu, C.S.; Wu, P.Y.; Wen, K.C. Rhodiola plants: Chemistry and biological activity. J. Food Drug Anal. 2015, 23, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef]
- Chen, T.S.; Liou, S.Y.; Chang, Y.L. Antioxidant evaluation of three adaptogen extracts. Am. J. Chin. Med. 2008, 36, 1209–1217. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, R.; Upadhyay, N.K.; Pal, K.; Kumar, R.; Sawhney, R.C. Effects of Rhodiola imbricata on dermal wound healing. Planta Med. 2007, 73, 774–777. [Google Scholar] [CrossRef]
- Calcabrini, C.; De Bellis, R.; Mancini, U.; Cucchiarini, L.; Potenza, L.; De Sanctis, R.; Patrone, V.; Scesa, C.; Dacha, M. Rhodiola rosea ability to enrich cellular antioxidant defences of cultured human keratinocytes. Arch. Dermatol. Res. 2010, 302, 191–200. [Google Scholar] [CrossRef]
- Zhou, Q.; Yin, Z.P.; Ma, L.; Zhao, W.; Hao, H.W.; Li, H.L. Free radical-scavenging activities of oligomeric proanthocyanidin from Rhodiola rosea L. and its antioxidant effects in vivo. Nat. Prod. Res. 2014, 28, 2301–2303. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.T.; Chang, T.C.; Lai, F.Y.; Lin, C.S.; Chao, H.L.; Lee, S.Y. Rhodiola crenulata Attenuates gamma-Ray Induced Cellular Injury via Modulation of Oxidative Stress in Human Skin Cells. Am. J. Chin. Med. 2018, 46, 175–190. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Dumont, Y.; Duranton, A.; Vercauteren, F.; Breton, L.; Quirion, R. Protective action of resveratrol in human skin: Possible involvement of specific receptor binding sites. PLoS ONE 2010, 5, e12935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boo, Y.C. Human skin lightening efficacy of resveratrol and its analogs: From in vitro studies to cosmetic applications. Antioxidants (Basel) 2019, 8, 332. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.H.; Choi, H.R.; Kang, Y.A.; Park, K.C. Depigmenting effect of resveratrol is dependent on FOXO3a activation without SIRT1 activation. Int. J. Mol. Sci. 2017, 18, 1213. [Google Scholar] [CrossRef] [Green Version]
- Jagdeo, J.; Adams, L.; Lev-Tov, H.; Sieminska, J.; Michl, J.; Brody, N. Dose-dependent antioxidant function of resveratrol demonstrated via modulation of reactive oxygen species in normal human skin fibroblasts in vitro. J. Drugs Dermatol. 2010, 9, 1523–1526. [Google Scholar]
- Shin, J.W.; Lee, H.S.; Na, J.I.; Huh, C.H.; Park, K.C.; Choi, H.R. Resveratrol inhibits particulate matter-induced inflammatory responses in human keratinocytes. Int. J. Mol. Sci. 2020, 21, 3446. [Google Scholar] [CrossRef]
- Soeur, J.; Eilstein, J.; Lereaux, G.; Jones, C.; Marrot, L. Skin resistance to oxidative stress induced by resveratrol: From Nrf2 activation to GSH biosynthesis. Free Radic. Biol. Med. 2015, 78, 213–223. [Google Scholar] [CrossRef]
- Weller, R. Nitric oxide: A key mediator in cutaneous physiology. Clin. Exp. Dermatol. 2003, 28, 511–514. [Google Scholar] [CrossRef]
- Vitale, N.; Kisslinger, A.; Paladino, S.; Procaccini, C.; Matarese, G.; Pierantoni, G.M.; Mancini, F.P.; Tramontano, D. Resveratrol couples apoptosis with autophagy in UVB-irradiated HaCaT cells. PLoS ONE 2013, 8, e80728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sticozzi, C.; Cervellati, F.; Muresan, X.M.; Cervellati, C.; Valacchi, G. Resveratrol prevents cigarette smoke-induced keratinocytes damage. Food Funct. 2014, 5, 2348–2356. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Lu, S.; Kivlin, R.; Wallin, B.; Card, E.; Bagdasarian, A.; Tamakloe, T.; Chu, W.M.; Guan, K.L.; Wan, Y. AMP-activated protein kinase contributes to UV-and H2O2-induced apoptosis in human skin keratinocytes. J. Biol. Chem. 2010, 285, 14842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ido, Y.; Duranton, A.; Lan, F.; Weikel, K.A.; Breton, L.; Ruderman, N.B. Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS ONE 2015, 10, e0115341. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhao, H.; Huang, B.; Zheng, C.; Peng, W.; Qin, L. Acanthopanax senticosus: Review of botany, chemistry and pharmacology. Pharmazie 2011, 66, 83–97. [Google Scholar]
- Ge, Y.W.; Zhu, S.; Yoshimatsu, K.; Komatsu, K. MS/MS similarity networking accelerated target profiling of triterpene saponins in Eleutherococcus senticosus leaves. Food Chem. 2017, 227, 444–452. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Chen, R.; Liu, Z.; Zhao, J.; Chen, R.; Meng, F.; Zhang, M.; Ge, W. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosu. Food Chem. 2011, 127, 434–440. [Google Scholar] [CrossRef]
- Wang, X.; Hai, C.X.; Liang, X.; Yu, S.X.; Zhang, W.; Li, Y.L. The protective effects of Acanthopanax senticosus Harms aqueous extracts against oxidative stress: Role of Nrf2 and antioxidant enzymes. J. Ethnopharmacol. 2010, 127, 424–432. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Malisardi, G.; Scalambra, E.; Andreotti, E.; Romagnoli, C.; Vicentini, C.B.; Manfredini, S.; Vertuani, S. Synthesis, antioxidant and antimicrobial activity of a new phloridzin derivative for dermo-cosmetic applications. Molecules 2012, 17, 13275–13289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calliste, C.A.; Le Bail, J.C.; Trouillas, P.; Pouget, C.; Habrioux, G.; Chulia, A.J.; Duroux, J.L. Chalcones: Structural requirements for antioxidant, estrogenic and antiproliferative activities. Anticancer Res. 2001, 21, 3949–3956. [Google Scholar] [PubMed]
- Rezk, B.M.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A. The antioxidant activity of phloretin: The disclosure of a new antioxidant pharmacophore in flavonoids. Biochem. Biophys. Res. Commun. 2002, 295, 9–13. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A review. Diabet. Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, T.; O’Reilly, J.; West, G.; Tucker, G.; Wiseman, H. Potent antioxidant properties of novel apple-derived flavonoids with commercial potential as food additives. Biochem. Soc. Trans. 1996, 24, 391S. [Google Scholar] [CrossRef] [Green Version]
- Ridgway, T.; O’Reilly, J.; West, G.; Tucker, G.; Wiseman, H. Antioxidant action of novel derivatives of the apple-derived flavonoid phloridzin compared to oestrogen: Relevance to potential cardioprotective action. Biochem. Soc. Trans. 1997, 25, 106S. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Dang, Y.; Gao, W.; Zhang, Y.; Xu, P.; Gu, J.; Ye, X. P38 and JNK signal pathways are involved in the regulation of phlorizin against UVB-induced skin damage. Exp. Dermatol. 2015, 24, 275–279. [Google Scholar] [CrossRef]
- Wang, Y.F.; Xu, X.; Fan, X.; Zhang, C.; Wei, Q.; Wang, X.; Guo, W.; Xing, W.; Yu, J.; Yan, J.L.; et al. A cell-penetrating peptide suppresses inflammation by inhibiting NF-kappaB signaling. Mol. Ther. 2011, 19, 1849–1857. [Google Scholar] [CrossRef] [Green Version]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. The human tripeptide GHK-Cu in prevention of oxidative stress and degenerative conditions of aging: Implications for cognitive health. Oxid. Med. Cell Longev. 2012, 2012, 324832. [Google Scholar] [CrossRef] [Green Version]
- Hureau, C.; Eury, H.; Guillot, R.; Bijani, C.; Sayen, S.; Solari, P.L.; Guillon, E.; Faller, P.; Dorlet, P. X-ray and solution structures of Cu (II) GHK and Cu (II) DAHK complexes: Influence on their redox properties. Chemistry 2011, 17, 10151–10160. [Google Scholar] [CrossRef]
- Pickart, L.; Freedman, J.H.; Loker, W.J.; Peisach, J.; Perkins, C.M.; Stenkamp, R.E.; Weinstein, B. Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells. Nature 1980, 288, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huo, Q.; Kele, P.; Andreopoulos, F.M.; Pham, S.M.; Leblanc, R.M. A new fluorescent chemosensor for copper ions based on tripeptide glycyl-histidyl-lysine (GHK). Org. Lett. 2001, 3, 3277–3280. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S.; Bhattacharjee, A.; Hubbard, A.L. Copper handling machinery of the brain. Metallomics 2010, 2, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Hawk, S.N.; Lanoue, L.; Keen, C.L.; Kwik-Uribe, C.L.; Rucker, R.B.; Uriu-Adams, J.Y. Copper-deficient rat embryos are characterized by low superoxide dismutase activity and elevated superoxide anions. Biol. Reprod. 2003, 68, 896–903. [Google Scholar] [CrossRef] [Green Version]
- Arul, V.; Gopinath, D.; Gomathi, K.; Jayakumar, R. Biotinylated GHK peptide incorporated collagenous matrix: A novel biomaterial for dermal wound healing in rats. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 73, 383–391. [Google Scholar] [CrossRef]
- Sakuma, S.; Ishimura, M.; Yuba, Y.; Itoh, Y.; Fujimoto, Y. The peptide glycyl-L-histidyl-L-lysine is an endogenous antioxidant in living organisms, possibly by diminishing hydroxyl and peroxyl radicals. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 132–138. [Google Scholar]
- Miller, D.M.; DeSilva, D.; Pickart, L.; Aust, S.D. Effects of glycyl-histidyl-lysyl chelated Cu (II) on ferritin dependent lipid peroxidation. Adv. Exp. Med. Biol. 1990, 264, 79–84. [Google Scholar] [CrossRef]
- Hong, Y.; Downey, T.; Eu, K.W.; Koh, P.K.; Cheah, P.Y. A ‛metastasis-prone’ signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin. Exp. Metastasis 2010, 27, 83–90. [Google Scholar] [CrossRef]
- Maquart, F.X.; Pickart, L.; Laurent, M.; Gillery, P.; Monboisse, J.C.; Borel, J.P. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. FEBS Lett. 1988, 238, 343–346. [Google Scholar] [CrossRef] [Green Version]
- Maquart, F.X.; Bellon, G.; Chaqour, B.; Wegrowski, J.; Patt, L.M.; Trachy, R.E.; Monboisse, J.C.; Chastang, F.; Birembaut, P.; Gillery, P.; et al. In vivo stimulation of connective tissue accumulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ in rat experimental wounds. J. Clin. Investig. 1993, 92, 2368–2376. [Google Scholar] [CrossRef]
- Wegrowski, Y.; Maquart, F.X.; Borel, J.P. Stimulation of sulfated glycosaminoglycan synthesis by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. Life Sci. 1992, 51, 1049–1056. [Google Scholar] [CrossRef]
- Simeon, A.; Wegrowski, Y.; Bontemps, Y.; Maquart, F.X. Expression of glycosaminoglycans and small proteoglycans in wounds: Modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu (2+). J. Investig. Dermatol. 2000, 115, 962–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hocking, A.M.; Shinomura, T.; McQuillan, D.J. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998, 17, 1–19. [Google Scholar] [CrossRef]
- Simeon, A.; Monier, F.; Emonard, H.; Gillery, P.; Birembaut, P.; Hornebeck, W.; Maquart, F.X. Expression and activation of matrix metalloproteinases in wounds: Modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. J. Investig. Dermatol. 1999, 112, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Simeon, A.; Emonard, H.; Hornebeck, W.; Maquart, F.X. The tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fibroblast cultures. Life Sci. 2000, 67, 2257–2265. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sudha, P.N.; Rose, M.H. Beneficial effects of hyaluronic acid. Adv. Food Nutr. Res. 2014, 72, 137–176. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Ferlazzo, A.M.; Calatroni, A. The antioxidant and antifibrogenic effects of the glycosaminoglycans hyaluronic acid and chondroitin-4-sulphate in a subchronic rat model of carbon tetrachloride-induced liver fibrogenesis. Chem. Biol. Interact. 2004, 148, 125–138. [Google Scholar] [CrossRef]
- Halicka, H.D.; Mitlitski, V.; Heeter, J.; Balazs, E.A.; Darzynkiewicz, Z. Attenuation of the oxidative burst-induced DNA damage in human leukocytes by hyaluronan. Int. J. Mol. Med. 2009, 23, 695–699. [Google Scholar] [CrossRef] [Green Version]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic acid in inflammation and tissue regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Ye, J.; Zhang, H.; Wu, H.; Wang, C.; Shi, X.; Xie, J.; He, J.; Yang, J. Cytoprotective effect of hyaluronic acid and hydroxypropyl methylcellulose against DNA damage induced by thimerosal in Chang conjunctival cells. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Foschi, D.; Castoldi, L.; Radaelli, E.; Abelli, P.; Calderini, G.; Rastrelli, A.; Mariscotti, C.; Marazzi, M.; Trabucchi, E. Hyaluronic acid prevents oxygen free-radical damage to granulation tissue: A study in rats. Int. J. Tissue React. 1990, 12, 333–339. [Google Scholar] [PubMed]
- Trabucchi, E.; Pallotta, S.; Morini, M.; Corsi, F.; Franceschini, R.; Casiraghi, A.; Pravettoni, A.; Foschi, D.; Minghetti, P. Low molecular weight hyaluronic acid prevents oxygen free radical damage to granulation tissue during wound healing. Int. J. Tissue React. 2002, 24, 65–71. [Google Scholar] [PubMed]
- Kim, J.K.; Srinivasan, P.; Kim, J.H.; Choi, J.I.; Park, H.J.; Byun, M.W.; Lee, J.W. Structural and antioxidant properties of gamma irradiated hyaluronic acid. Food Chem. 2008, 109, 763–770. [Google Scholar] [CrossRef]
- Ke, C.; Sun, L.; Qiao, D.; Wang, D.; Zeng, X. Antioxidant acitivity of low molecular weight hyaluronic acid. Food Chem. Toxicol. 2011, 49, 2670–2675. [Google Scholar] [CrossRef]
- Kanitakis, J.; Ramirez-Bosca, A.; Reano, A.; Viac, J.; Roche, P.; Thivolet, J. Filaggrin expression in normal and pathological skin. A marker of keratinocyte differentiation. Virchows Arch. A Pathol. Anat. Histopathol. 1988, 412, 375–382. [Google Scholar] [CrossRef]
- Godic, A.; Poljsak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Maione-Silva, L.; de Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci. Rep. 2019, 9, 522. [Google Scholar] [CrossRef] [Green Version]
- Kumano, Y.; Sakamoto, T.; Egawa, M.; Tanaka, M.; Yamamoto, I. Enhancing effect of 2-O-alpha-D-glucopyranosyl-L-ascorbic acid, a stable ascorbic acid derivative, on collagen synthesis. Biol. Pharm. Bull. 1998, 21, 662–666. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, M.; Arai, N.; Kohno, K.; Ushio, S.; Fukuda, S. Anti-oxidative and anti-aging activities of 2-O-alpha-glucopyranosyl-L-ascorbic acid on human dermal fibroblasts. Eur. J. Pharmacol. 2012, 674, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.I.; Park, J.I.; Kim, H.J.; Kim, D.W.; Kim, S.S. A novel L-ascorbic acid and peptide conjugate with increased stability and collagen biosynthesis. BMB Rep. 2009, 42, 743–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.M.; An, H.S.; Bae, J.S.; Kim, J.Y.; Choi, C.H.; Kim, J.Y.; Lim, J.H.; Choi, J.H.; Song, H.; Moon, S.H.; et al. Effects of palmitoyl-KVK-L-ascorbic acid on skin wrinkles and pigmentation. Arch. Dermatol. Res. 2017, 309, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.J.; Seok, J.K.; Kim, S.Y.; Park, W.; Baek, J.H.; Kim, Y.M.; Boo, Y.C. Human skin-depigmenting effects of resveratryl triglycolate, a hybrid compound of resveratrol and glycolic acid. Int. J. Cosmet. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, J.H.; Suh, H.J.; Lee, I.C.; Koh, J.; Boo, Y.C. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch. Dermatol. Res. 2014, 306, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Seok, J.K.; An, S.M.; Baek, J.H.; Koh, J.S.; Boo, Y.C. A study of the human skin-whitening effects of resveratryl triacetate. Arch. Dermatol. Res. 2015, 307, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.B.; Yang, S.H.; Byun, S.Y.; Choi, H.R.; Shin, J.W.; Park, K.C. The effects of hydroporation on melasma with anti-aging cocktail. J. Cosmet. Dermatol. 2017, 16, e15–e20. [Google Scholar] [CrossRef]
- Byun, S.Y.; Chae, J.B.; Na, J.I.; Park, K.C. Significant improvement in crow’s feet after treatment with Jet-M and a mixed solution of copper-GHK, oligo-hyaluronic acid, rhodiolar extract, tranexamic acid, and beta-glucan (GHR formulation). J. Cosmet. Laser Ther. 2016, 18, 293–295. [Google Scholar] [CrossRef]
- Shin, J.W.; Choi, H.R.; Yang, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Park, K.C. The increase of interfollicular epidermal stem cells and regulation of aryl hydrocarbon receptor and its repressors in the skin through hydroporation with anti-aging cocktail. J. Cosmet. Dermatol. 2019, 18, 1133–1139. [Google Scholar] [CrossRef]
- Lorencini, M.; Brohem, C.A.; Dieamant, G.C.; Zanchin, N.I.; Maibach, H.I. Active ingredients against human epidermal aging. Ageing Res. Rev. 2014, 15, 100–115. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.-H.; Park, K.-C. Antioxidants as an Epidermal Stem Cell Activator. Antioxidants 2020, 9, 958. https://doi.org/10.3390/antiox9100958
Kwon S-H, Park K-C. Antioxidants as an Epidermal Stem Cell Activator. Antioxidants. 2020; 9(10):958. https://doi.org/10.3390/antiox9100958
Chicago/Turabian StyleKwon, Soon-Hyo, and Kyoung-Chan Park. 2020. "Antioxidants as an Epidermal Stem Cell Activator" Antioxidants 9, no. 10: 958. https://doi.org/10.3390/antiox9100958
APA StyleKwon, S. -H., & Park, K. -C. (2020). Antioxidants as an Epidermal Stem Cell Activator. Antioxidants, 9(10), 958. https://doi.org/10.3390/antiox9100958