Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders?
Abstract
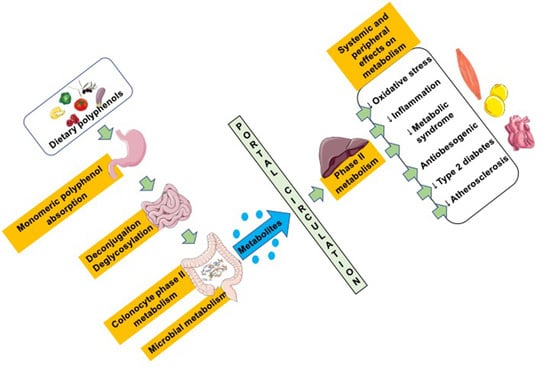
:1. Introduction
2. Polyphenols: Classification, Structure, and Bioavailability
2.1. Classification and Structure of Polyphenols
2.2. Bioavailability of Polyphenols
2.2.1. Factors Affecting Polyphenol Absorption
2.2.2. Absorption of Polyphenols and Derivatives
2.2.3. Gastric Uptake
2.2.4. Small Intestine Uptake
Duodenum
Jejunum and Ileum
Colon
3. Interaction between Polyphenols and the Colon Microbiota
3.1. Polyphenol–Microbiota Interaction
3.2. Impact of Microbiota on Polyphenol Metabolism
4. Polyphenols and Metabolic Syndrome
4.1. Antioxidative Effects of Polyphenols
4.2. Anti-Inflammatory Effects of Polyphenols
4.3. Epigenetic Control of Polyphenols
5. Polyphenol Metabolites
5.1. Flavonoid Metabolites and Their Antioxidant and Anti-Inflammatory Effects
5.1.1. Flavonol Quercetin Metabolites
5.1.2. Flavones and Flavanones Metabolites
5.1.3. Isoflavone Daidzein and Daidzin Metabolites
5.1.4. Flavanol Catechin Metabolites
5.1.5. Tannin Ellagitannin and Urolithin Metabolites
5.2. Non-Flavonoids
5.2.1. Lignan Metabolites
5.2.2. Resveratrol Metabolites
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Chen, Y.; Huang, L.; Liang, X.; Dai, P.; Zhang, Y.; Li, B.; Lin, X.; Sun, C. Enhancement of polyphenolic metabolism as an adaptive response of lettuce (Lactuca sativa) roots to aluminum stress. Environ. Pollut. 2020, 261, 114230. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Sytykiewicz, H.; Durak, R.; Borowiak-Sobkowiak, B.; Chrzanowski, G. Role of phenolic compounds during antioxidative responses of winter triticale to aphid and beetle attack. Plant Physiol. Biochem. 2017, 118, 529–540. [Google Scholar] [CrossRef]
- Mestar, N.G.; Boudiaf, M.N.; Lahcene, S.; Abbaci, H.; Aiche, G.I.; Metna, B.; Saadoun, N.S.; Taibi, F.; Houali, K. Bio-insecticidal effects of Oleaster leaves aqueous extracts against Psylla larvae (Euphyllura olivina (Costa)), a primary pest of Olea europaea L. Cell Mol. Biol. 2018, 64, 35–40. [Google Scholar] [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of polyphenols and evaluation of antioxidant capacity in grape pomace of the cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef]
- Anhe, F.F.; Roy, D.; Pilon, G.; Dudonne, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [Green Version]
- D’Amore, S.; Vacca, M.; Cariello, M.; Graziano, G.; D’Orazio, A.; Salvia, R.; Sasso, R.C.; Sabba, C.; Palasciano, G.; Moschetta, A. Genes and miRNA expression signatures in peripheral blood mononuclear cells in healthy subjects and patients with metabolic syndrome after acute intake of extra virgin olive oil. Biochim. Biophys. Acta 2016, 1861, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Kantartzis, K.; Fritsche, L.; Bombrich, M.; Machann, J.; Schick, F.; Staiger, H.; Kunz, I.; Schoop, R.; Lehn-Stefan, A.; Heni, M.; et al. Effects of resveratrol supplementation on liver fat content in overweight and insulin-resistant subjects: A randomized, double-blind, placebo-controlled clinical trial. Diabet. Obes. Metab. 2018, 20, 1793–1797. [Google Scholar] [CrossRef]
- Paquette, M.; Medina Larque, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonne, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef] [Green Version]
- Babu, P.V.A.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef] [Green Version]
- Dominguez Avila, J.A.; Rodrigo Garcia, J.; Gonzalez Aguilar, G.A.; de la Rosa, L.A. The Antidiabetic Mechanisms of Polyphenols Related to Increased Glucagon-Like Peptide-1 (GLP1) and Insulin Signaling. Molecules 2017, 22, 903. [Google Scholar] [CrossRef] [Green Version]
- Les, F.; Casedas, G.; Gomez, C.; Moliner, C.; Valero, M.S.; Lopez, V. The role of anthocyanins as antidiabetic agents: From molecular mechanisms to in vivo and human studies. J. Physiol. Biochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Macho-Gonzalez, A.; Lopez-Oliva, M.E.; Merino, J.J.; Garcia-Fernandez, R.A.; Garcimartin, A.; Redondo-Castillejo, R.; Bastida, S.; Sanchez-Muniz, F.J.; Benedi, J. Carob fruit extract-enriched meat improves pancreatic beta-cell dysfunction, hepatic insulin signaling and lipogenesis in late-stage type 2 diabetes mellitus model. J. Nutr. Biochem. 2020, 84, 108461. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.J.; Lee, H.G.; Seo, K.H.; Yokoyama, W.; Kim, H. Antiobesity Effect of Prebiotic Polyphenol-Rich Grape Seed Flour Supplemented with Probiotic Kefir-Derived Lactic Acid Bacteria. J. Agric. Food. Chem. 2018, 66, 12498–12511. [Google Scholar] [CrossRef]
- Sandoval, V.; Sanz-Lamora, H.; Arias, G.; Marrero, P.F.; Haro, D.; Relat, J. Metabolic Impact of Flavonoids Consumption in Obesity: From Central to Peripheral. Nutrients 2020, 12, 2393. [Google Scholar] [CrossRef]
- Anhe, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonne, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef] [Green Version]
- McKay, D.L.; Blumberg, J.B. Cranberries (Vaccinium macrocarpon) and cardiovascular disease risk factors. Nutr. Rev. 2007, 65, 490–502. [Google Scholar] [CrossRef]
- Ruel, G.; Couillard, C. Evidences of the cardioprotective potential of fruits: The case of cranberries. Mol. Nutr. Food Res. 2007, 51, 692–701. [Google Scholar] [CrossRef]
- Ruel, G.; Lapointe, A.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Evidence that cranberry juice may improve augmentation index in overweight men. Nutr. Res. 2013, 33, 41–49. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Ferrari, C.K.B. Anti-atherosclerotic and cardiovascular protective benefits of Brazilian nuts. Front. Biosci. Schol Ed. 2020, 12, 38–56. [Google Scholar] [CrossRef]
- Perez de Vega, M.J.; Moreno-Fernandez, S.; Pontes-Quero, G.M.; Gonzalez-Amor, M.; Vazquez-Lasa, B.; Sabater-Munoz, B.; Briones, A.M.; Aguilar, M.R.; Miguel, M.; Gonzalez-Muniz, R. Characterization of Novel Synthetic Polyphenols: Validation of Antioxidant and Vasculoprotective Activities. Antioxidants 2020, 9, 787. [Google Scholar] [CrossRef]
- Devi, S.A.; Chamoli, A. Polyphenols as an Effective Therapeutic Intervention Against Cognitive Decline During Normal and Pathological Brain Aging. Adv. Exp. Med. Biol. 2020, 1260, 159–174. [Google Scholar]
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macri, R.; et al. The Potential for Natural Antioxidant Supplementation in the Early Stages of Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 2618. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.F.M.; Pogacnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Bensalem, J.; Dudonne, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Laye, S.; Lafenetre, P.; Pallet, V. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018, 7, e19. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Xiao, X.; Chen, D.; Yu, B.; He, J. Tea and Its Components Prevent Cancer: A Review of the Redox-Related Mechanism. Int. J. Mol. Sci. 2019, 20, 5249. [Google Scholar] [CrossRef] [Green Version]
- Toric, J.; Markovic, A.K.; Brala, C.J.; Barbaric, M. Anticancer effects of olive oil polyphenols and their combinations with anticancer drugs. Acta Pharm. 2019, 69, 461–482. [Google Scholar] [CrossRef] [Green Version]
- Abdelgawad, I.Y.; Grant, M.K.O.; Zordoky, B.N. Leveraging the Cardio-Protective and Anticancer Properties of Resveratrol in Cardio-Oncology. Nutrients 2019, 11, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horne, J.R.; Vohl, M.C. Biological plausibility for interactions between dietary fat, resveratrol, ACE2, and SARS-CoV illness severity. Am. J. Physiol. Endocrinol. Metab. 2020, 318, e830–e833. [Google Scholar] [CrossRef] [Green Version]
- Mani, J.S.; Johnson, J.B.; Steel, J.C.; Broszczak, D.A.; Neilsen, P.M.; Walsh, K.B.; Naiker, M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020, 284, 197989. [Google Scholar] [CrossRef]
- Levy, E.; Delvin, E.; Marcil, V.; Spahis, S. May phytotherapy with polyphenols serve as a powerful approach for the prevention and therapy tool of novel coronavirus disease 2019 (Covid-19)? Am. J. Physiol. Endocrinol. Metab. 2020, 319. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [Green Version]
- Rechner, A.R.; Kuhnle, G.; Bremner, P.; Hubbard, G.P.; Moore, K.P.; Rice-Evans, C.A. The metabolic fate of dietary polyphenols in humans. Free Radic. Biol. Med. 2002, 33, 220–235. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [Green Version]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [Green Version]
- Prigent, S.V.; Gruppen, H.; Visser, A.J.; Van Koningsveld, G.A.; De Jong, G.A.; Voragen, A.G. Effects of non-covalent interactions with 5-O-caffeoylquinic acid (chlorogenic acid) on the heat denaturation and solubility of globular proteins. J. Agric. Food. Chem. 2003, 51, 5088–5095. [Google Scholar] [CrossRef]
- Prigent, S.V.; Voragen, A.G.; Li, F.; Visser, A.J.; van Koningsveld, G.A.; Gruppen, H. Covalent interactions between amino acid side chains and oxidation products of caffeoylquinic acid (chlorogenic acid). J. Sci. Food Agric. 2008, 88, 1748–1754. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Kevers, C.; Pincemail, J.; Tabart, J.; Defraigne, J.O.; Dommes, J. Influence of cultivar, harvest time, storage conditions, and peeling on the antioxidant capacity and phenolic and ascorbic acid contents of apples and pears. J. Agric. Food Chem. 2011, 59, 6165–6171. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Rico, A.; Salvador, M.D.; La Greca, M.; Fregapane, G. Phenolic and volatile compounds of extra virgin olive oil (Olea europaea L. Cv. Cornicabra) with regard to fruit ripening and irrigation management. J. Agric. Food Chem. 2006, 54, 7130–7136. [Google Scholar] [CrossRef]
- Bonoli, M.; Bendini, A.; Cerretani, L.; Lercker, G.; Toschi, T.G. Qualitative and semiquantitative analysis of phenolic compounds in extra virgin olive oils as a function of the ripening degree of olive fruits by different analytical techniques. J. Agric. Food Chem. 2004, 52, 7026–7032. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Marti, M.P.; Pantaleon, A.; Rozek, A.; Soler, A.; Valls, J.; Macia, A.; Romero, M.P.; Motilva, M.J. Rapid analysis of procyanidins and anthocyanins in plasma by microelution SPE and ultra-HPLC. J. Sep. Sci. 2010, 33, 2841–2853. [Google Scholar] [CrossRef]
- Denis, M.C.; Dubé, P.; Dudonné, S.; Desjardins, Y.; Matei, C.; Delvin, E.; Levy, E.; Furtos, A. Characterization of bioactive cranberry fractions by mass spectrometry. Can. J. Chem. 2020, in press. [Google Scholar] [CrossRef]
- Meng, X.; Maliakal, P.; Lu, H.; Lee, M.J.; Yang, C.S. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J. Agric. Food Chem. 2004, 52, 935–942. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomas-Barberan, F.; Garcia-Viguera, C. Health-promoting compounds in broccoli as influenced by refrigerated transport and retail sale period. J. Agric. Food Chem. 2003, 51, 3029–3034. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Total Phenolic, Phenolic Acid, Anthocyanin, Flavan-3-ol, and Flavonol Profiles and Antioxidant Properties of Pinto and Black Beans (Phaseolus vulgaris L.) as Affected by Thermal Processing. J. Agric. Food Chem. 2009, 57, 4754–4764. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.; Eguchi, S.; Yamaguchi, T.; Takamura, H.; Matoba, T. Effect of Thermal Treatment on Radical-scavenging Activity of Some Spices. Food Sci. Technol. Res. 2006, 12, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Azuma, K.; Ippoushi, K.; Ito, H.; Higashio, H.; Terao, J. Combination of lipids and emulsifiers enhances the absorption of orally administered quercetin in rats. J. Agric. Food Chem. 2002, 50, 1706–1712. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Grande, S.; Colonnelli, K.; Patelli, C.; Galli, G.; Caruso, D. Hydroxytyrosol excretion differs between rats and humans and depends on the vehicle of administration. J. Nutr. 2003, 133, 2612–2615. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Jiménez, J.; Hubert, J.; Hooper, L.; Cassidy, A.; Manach, C.; Williamson, G.; Scalbert, A. Urinary metabolites as biomarkers of polyphenol intake in humans: A systematic review. Am. J. Clin. Nutr. 2010, 92, 801–809. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Gonthier, M.P.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of dietary procyanidins in rats. Free Radic. Biol. Med. 2003, 35, 837–844. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, S.; Li, L.; Jiang, H. Metabolism of flavonoids in human: A comprehensive review. Curr. Drug Metab. 2014, 15, 48–61. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Ifie, I.; Gonzalez-Aguilar, G.A.; Roopchand, D.E. The Gastrointestinal Tract as Prime Site for Cardiometabolic Protection by Dietary Polyphenols. Adv. Nutr. 2019, 10, 999–1011. [Google Scholar] [CrossRef]
- Spencer, J.P.; Chaudry, F.; Pannala, A.S.; Srai, S.K.; Debnam, E.; Rice-Evans, C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem. Biophys. Res. Commun. 2000, 272, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Y.; Holt, R.R.; Lazarus, S.A.; Ensunsa, J.L.; Hammerstone, J.F.; Schmitz, H.H.; Keen, C.L. Stability of the flavan-3-ols epicatechin and catechin and related dimeric procyanidins derived from cocoa. J. Agric. Food Chem. 2002, 50, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Tzounis, X.; Assunta Dessi, M.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P. The fate of olive oil polyphenols in the gastrointestinal tract: Implications of gastric and colonic microflora-dependent biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Macia, A.; Romero, M.P.; Valls, J.; Blade, C.; Arola, L.; Motilva, M.J. Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef] [Green Version]
- Kahle, K.; Kempf, M.; Schreier, P.; Scheppach, W.; Schrenk, D.; Kautenburger, T.; Hecker, D.; Huemmer, W.; Ackermann, M.; Richling, E. Intestinal transit and systemic metabolism of apple polyphenols. Eur. J. Nutr. 2011, 50, 507–522. [Google Scholar] [CrossRef]
- Van Rymenant, E.; Salden, B.; Voorspoels, S.; Jacobs, G.; Noten, B.; Pitart, J.; Possemiers, S.; Smagghe, G.; Grootaert, C.; Van Camp, J. A Critical Evaluation of In Vitro Hesperidin 2S Bioavailability in a Model Combining Luminal (Microbial) Digestion and Caco-2 Cell Absorption in Comparison to a Randomized Controlled Human Trial. Mol. Nutr. Food Res. 2018, 62, e1700881. [Google Scholar] [CrossRef]
- Hu, X.P.; Yin, F.W.; Zhou, D.Y.; Xie, H.K.; Zhu, B.W.; Ma, X.C.; Tian, X.G.; Wang, C.; Shahidi, F. Stability of resveratrol esters with caprylic acid during simulated in vitro gastrointestinal digestion. Food Chem. 2019, 276, 675–679. [Google Scholar] [CrossRef]
- Liu, D.; Dhital, S.; Wu, P.; Chen, X.D.; Gidley, M.J. In Vitro Digestion of Apple Tissue Using a Dynamic Stomach Model: Grinding and Crushing Effects on Polyphenol Bioaccessibility. J. Agric. Food Chem. 2020, 68, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Lan, T.; Geng, T.; Ju, Y.; Cheng, G.; Que, Z.; Gao, G.; Fang, Y.; Sun, X. Nutritional properties and biological activities of kiwifruit (Actinidia) and kiwifruit products under simulated gastrointestinal in vitro digestion. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Remesy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, Y.; Zhao, Z.; Shimizu, M. Phenolic acids are absorbed from the rat stomach with different absorption rates. J. Agric. Food Chem. 2006, 54, 7539–7543. [Google Scholar] [CrossRef]
- Pforte, H.; Hempel, J.; Jacobasch, G. Distribution pattern of a flavonoid extract in the gastrointestinal lumen and wall of rats. Nahrung 1999, 43, 205–208. [Google Scholar] [CrossRef]
- Piskula, M.K.; Yamakoshi, J.; Iwai, Y. Daidzein and genistein but not their glucosides are absorbed from the rat stomach. FEBS Lett. 1999, 447, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Crespy, V.; Morand, C.; Besson, C.; Manach, C.; Demigne, C.; Remesy, C. Quercetin, but not its glycosides, is absorbed from the rat stomach. J. Agric. Food Chem. 2002, 50, 618–621. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Zafrilla, P.; Tomás-Barberán, F.A. An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Eur. Food Res. Technol. 2002, 214, 155–159. [Google Scholar] [CrossRef]
- Ortega, N.; Reguant, J.; Romero, M.P.; Macia, A.; Motilva, M.J. Effect of fat content on the digestibility and bioaccessibility of cocoa polyphenol by an in vitro digestion model. J. Agric. Food Chem. 2009, 57, 5743–5749. [Google Scholar] [CrossRef]
- Tsai, Y.M.; Chang-Liao, W.L.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Effects of polymer molecular weight on relative oral bioavailability of curcumin. Int. J. Nanomed. 2012, 7, 2957–2966. [Google Scholar] [CrossRef] [Green Version]
- Day, A.J.; Gee, J.M.; DuPont, M.S.; Johnson, I.T.; Williamson, G. Absorption of quercetin-3-glucoside and quercetin-4’-glucoside in the rat small intestine: The role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharmacol. 2003, 65, 1199–1206. [Google Scholar] [CrossRef]
- Henry-Vitrac, C.; Desmouliere, A.; Girard, D.; Merillon, J.M.; Krisa, S. Transport, deglycosylation, and metabolism of trans-piceid by small intestinal epithelial cells. Eur. J. Nutr. 2006, 45, 376–382. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murota, K.; Shimizu, S.; Chujo, H.; Moon, J.-H.; Terao, J. Efficiency of Absorption and Metabolic Conversion of Quercetin and Its Glucosides in Human Intestinal Cell Line Caco-2. Arch. Biochem. Biophys. 2000, 384, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; Raimondi, S.; Leonardi, A.; Quartieri, A.; Rossi, M. Hydrolysis of the rutinose-conjugates flavonoids rutin and hesperidin by the gut microbiota and bifidobacteria. Nutrients 2015, 7, 2788–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Q.; Li, D.; Zhou, Y.; Yang, J.; Yang, W.; Zhou, G.; Wen, J. Enhanced uptake and transport of (+)-catechin and (−)-epigallocatechin gallate in niosomal formulation by human intestinal Caco-2 cells. Int. J. Nanomed. 2014, 9, 2157–2165. [Google Scholar] [CrossRef] [Green Version]
- Borges, G.; Ottaviani, J.I.; van der Hooft, J.J.J.; Schroeter, H.; Crozier, A. Absorption, metabolism, distribution and excretion of (−)-epicatechin: A review of recent findings. Mol. Asp. Med. 2018, 61, 18–30. [Google Scholar] [CrossRef]
- Levy, E.; Yotov, W.; Seidman, E.G.; Garofalo, C.; Delvin, E.; Menard, D. Caco-2 cells and human fetal colon: A comparative analysis of their lipid transport. Biochim. Biophys. Acta 1999, 1439, 353–362. [Google Scholar] [CrossRef]
- Nauli, A.M.; Nauli, S.M. Intestinal transport as a potential determinant of drug bioavailability. Curr. Clin. Pharmacol. 2013, 8, 247–255. [Google Scholar] [CrossRef]
- Kern, S.M.; Bennett, R.N.; Needs, P.W.; Mellon, F.A.; Kroon, P.A.; Garcia-Conesa, M.T. Characterization of metabolites of hydroxycinnamates in the in vitro model of human small intestinal epithelium caco-2 cells. J. Agric. Food Chem. 2003, 51, 7884–7891. [Google Scholar] [CrossRef]
- Hu, J.N.; Zou, X.G.; He, Y.; Chen, F.; Deng, Z.Y. Esterification of Quercetin Increases Its Transport across Human Caco-2 Cells. J. Food Sci. 2016, 81, H1825–H1832. [Google Scholar] [CrossRef] [PubMed]
- Su, H.F.; Lin, Q.; Wang, X.Y.; Fu, Y.; Gong, T.; Sun, X.; Zhang, Z.R. Absorptive interactions of concurrent oral administration of (+)-catechin and puerarin in rats and the underlying mechanisms. Acta Pharmacol. Sin. 2016, 37, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudonne, S.; Dal-Pan, A.; Dube, P.; Varin, T.V.; Calon, F.; Desjardins, Y. Potentiation of the bioavailability of blueberry phenolic compounds by co-ingested grape phenolic compounds in mice, revealed by targeted metabolomic profiling in plasma and feces. Food Funct. 2016, 7, 3421–3430. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Dube, P.; Pilon, G.; Marette, A.; Jacques, H.; Weisnagel, J.; Desjardins, Y. Modulation of Strawberry/Cranberry Phenolic Compounds Glucuronidation by Co-Supplementation with Onion: Characterization of Phenolic Metabolites in Rat Plasma Using an Optimized muSPE-UHPLC-MS/MS Method. J. Agric. Food Chem. 2014, 62, 3244–3256. [Google Scholar] [CrossRef]
- Deglaire, A.; Moughan, P.J. Animal models for determining amino acid digestibility in humans—A review. Br. J. Nutr. 2012, 108 (Suppl. S2), S273–S281. [Google Scholar] [CrossRef] [Green Version]
- Vergauwen, H. The IPEC-J2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 125–134. [Google Scholar]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal Absorption and Antioxidant Activity of Grape Pomace Polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Tech. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordonez, M.; Llorach, R.; Farran-Codina, A.; Barupal, D.K.; Neveu, V.; Manach, C.; Andres-Lacueva, C.; Scalbert, A. Systematic analysis of the polyphenol metabolome using the Phenol-Explorer database. Mol. Nutr. Food Res. 2016, 60, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 113–124. [Google Scholar]
- Bergmann, H.; Rogoll, D.; Scheppach, W.; Melcher, R.; Richling, E. The Ussing type chamber model to study the intestinal transport and modulation of specific tight-junction genes using a colonic cell line. Mol. Nutr. Food Res. 2009, 53, 1211–1225. [Google Scholar] [CrossRef]
- Alsolmei, F.A.; Li, H.; Pereira, S.L.; Krishnan, P.; Johns, P.W.; Siddiqui, R.A. Polyphenol-Enriched Plum Extract Enhances Myotubule Formation and Anabolism while Attenuating Colon Cancer-induced Cellular Damage in C2C12 Cells. Nutrients 2019, 11, 1077. [Google Scholar] [CrossRef] [Green Version]
- Blanquer-Rossello, M.D.; Hernandez-Lopez, R.; Roca, P.; Oliver, J.; Valle, A. Resveratrol induces mitochondrial respiration and apoptosis in SW620 colon cancer cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 431–440. [Google Scholar] [CrossRef]
- Saunier, E.; Antonio, S.; Regazzetti, A.; Auzeil, N.; Laprevote, O.; Shay, J.W.; Coumoul, X.; Barouki, R.; Benelli, C.; Huc, L.; et al. Resveratrol reverses the Warburg effect by targeting the pyruvate dehydrogenase complex in colon cancer cells. Sci. Rep. 2017, 7, 6945. [Google Scholar] [CrossRef] [Green Version]
- Valdes, A.; Garcia-Canas, V.; Simo, C.; Ibanez, C.; Micol, V.; Ferragut, J.A.; Cifuentes, A. Comprehensive foodomics study on the mechanisms operating at various molecular levels in cancer cells in response to individual rosemary polyphenols. Anal. Chem. 2014, 86, 9807–9815. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef]
- Marin, L.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed. Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [Green Version]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patan, F.; Llorach, R.; Garrido, I.; Gomez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [Green Version]
- Neish, A.S. Microbes in gastrointestinal health and disease. Gastroenterology 2009, 136, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Arora, T.; Backhed, F. The gut microbiota and metabolic disease: Current understanding and future perspectives. J. Intern. Med. 2016, 280, 339–349. [Google Scholar] [CrossRef]
- Korpela, K. Diet, Microbiota, and Metabolic Health: Trade-Off between Saccharolytic and Proteolytic Fermentation. Annu. Rev. Food Sci. Technol. 2018, 9, 65–84. [Google Scholar] [CrossRef]
- Ma, N.; Tian, Y.; Wu, Y.; Ma, X. Contributions of the Interaction Between Dietary Protein and Gut Microbiota to Intestinal Health. Curr. Protein Pept. Sci. 2017, 18, 795–808. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.; Carbonero, F.; Zoetendal, E.G.; DeLany, J.P.; Wang, M.; Newton, K.; Gaskins, H.R.; O’Keefe, S.J. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr. 2013, 98, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [Green Version]
- Kabeerdoss, J.; Devi, R.S.; Mary, R.R.; Ramakrishna, B.S. Faecal microbiota composition in vegetarians: Comparison with omnivores in a cohort of young women in southern India. Br. J. Nutr. 2012, 108, 953–957. [Google Scholar] [CrossRef] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Deng, Y.; Wang, H.; Liu, W.; Zhuang, X.; Chu, W. Tea polyphenols as an antivirulence compound Disrupt Quorum-Sensing Regulated Pathogenicity of Pseudomonas aeruginosa. Sci. Rep. 2015, 5, 16158. [Google Scholar] [CrossRef] [Green Version]
- Stenvang, M.; Dueholm, M.S.; Vad, B.S.; Seviour, T.; Zeng, G.; Geifman-Shochat, S.; Søndergaard, M.T.; Christiansen, G.; Meyer, R.L.; Kjelleberg, S.; et al. Epigallocatechin Gallate Remodels Overexpressed Functional Amyloids in Pseudomonas aeruginosa and Increases Biofilm Susceptibility to Antibiotic Treatment. J. Biol. Chem. 2016, 291, 26540–26553. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, P.D.; Shah, S.; Ehlert, K.; Hara, Y.; Taylor, P.W. The beta-lactam-resistance modifier (−)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology 2007, 153, 2093–2103. [Google Scholar] [CrossRef] [Green Version]
- Hachimura, S.; Totsuka, M.; Hosono, A. Immunomodulation by food: Impact on gut immunity and immune cell function. Biosci. Biotechnol. Biochem. 2018, 82, 584–599. [Google Scholar] [CrossRef]
- Vitetta, L.; Vitetta, G.; Hall, S. Immunological Tolerance and Function: Associations Between Intestinal Bacteria, Probiotics, Prebiotics, and Phages. Front. Immunol. 2018, 9, 2240. [Google Scholar] [CrossRef] [Green Version]
- Stan, M.S.; Voicu, S.N.; Caruntu, S.; Nica, I.C.; Olah, N.K.; Burtescu, R.; Balta, C.; Rosu, M.; Herman, H.; Hermenean, A.; et al. Antioxidant and Anti-Inflammatory Properties of a Thuja occidentalis Mother Tincture for the Treatment of Ulcerative Colitis. Antioxidants 2019, 8, 416. [Google Scholar] [CrossRef] [Green Version]
- Mileo, A.M.; Nisticò, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Tokutake, S.; Kikuchi, M.; Kubota, Y.; Konishi, H.; Mitsuoka, T. Effect of Proanthocyanidin-Rich Extract from Grape Seeds on Human Fecal Flora and Fecal Odor. Microb. Ecol. Health Dis. 2001, 13, 25–31. [Google Scholar]
- Larrosa, M.; Luceri, C.; Vivoli, E.; Pagliuca, C.; Lodovici, M.; Moneti, G.; Dolara, P. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol. Nutr. Food Res. 2009, 53, 1044–1054. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [Green Version]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [Green Version]
- Cote, A.T.; Harris, K.C.; Panagiotopoulos, C.; Sandor, G.G.; Devlin, A.M. Childhood obesity and cardiovascular dysfunction. J. Am.Coll. Cardiol. 2013, 62, 1309–1319. [Google Scholar] [CrossRef] [Green Version]
- Levy, E.; Saenger, A.K.; Steffes, M.W.; Delvin, E. Pediatric Obesity and Cardiometabolic Disorders: Risk Factors and Biomarkers. Ejifcc 2017, 28, 6–24. [Google Scholar] [PubMed]
- Nehus, E.; Mitsnefes, M. Childhood Obesity and the Metabolic Syndrome. Pediatr. Clin. N. Am. 2019, 66, 31–43. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinehr, T.; Roth, C.L. Inflammation Markers in Type 2 Diabetes and the Metabolic Syndrome in the Pediatric Population. Curr. Diab. Rep. 2018, 18, 131. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.M.; Meigs, J.B.; Liu, S.; Saltzman, E.; Wilson, P.W.; Jacques, P.F. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabet. Care 2004, 27, 538–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spahis, S.; Alvarez, F.; Ahmed, N.; Dubois, J.; Jalbout, R.; Paganelli, M.; Grzywacz, K.; Delvin, E.; Peretti, N.; Levy, E. Non-alcoholic fatty liver disease severity and metabolic complications in obese children: Impact of omega-3 fatty acids. J. Nutr. Biochem. 2018, 58, 28–36. [Google Scholar] [CrossRef]
- Sabaté, J.; Wien, M. A perspective on vegetarian dietary patterns and risk of metabolic syndrome. Br. J. Nutr. 2015, 113 (Suppl. S2), S136–S143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaillzadeh, A.; Mirmiran, P.; Azizi, F. Whole-grain consumption and the metabolic syndrome: A favorable association in Tehranian adults. Eur. J. Clin. Nutr. 2005, 59, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Mallappa, R.H.; Rokana, N.; Duary, R.K.; Panwar, H.; Batish, V.K.; Grover, S. Management of metabolic syndrome through probiotic and prebiotic interventions. Indian J. Endocrinol. Metab. 2012, 16, 20–27. [Google Scholar]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit Polyphenols: A Review of Anti-inflammatory Effects in Humans. Crit. Rev. Food Sci. Nutr. 2016, 56, 419–444. [Google Scholar] [CrossRef]
- Gourineni, V.; Shay, N.F.; Chung, S.; Sandhu, A.K.; Gu, L. Muscadine grape (Vitis rotundifolia) and wine phytochemicals prevented obesity-associated metabolic complications in C57BL/6J mice. J. Agric. Food Chem. 2012, 60, 7674–7681. [Google Scholar] [CrossRef] [Green Version]
- Makino-Wakagi, Y.; Yoshimura, Y.; Uzawa, Y.; Zaima, N.; Moriyama, T.; Kawamura, Y. Ellagic acid in pomegranate suppresses resistin secretion by a novel regulatory mechanism involving the degradation of intracellular resistin protein in adipocytes. Biochem. Biophys. Res. Commun. 2012, 417, 880–885. [Google Scholar] [CrossRef]
- Lei, F.; Zhang, X.N.; Wang, W.; Xing, D.M.; Xie, W.D.; Su, H.; Du, L.J. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int. J. Obes. 2007, 31, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Monika, P.; Geetha, A. The modulating effect of Persea americana fruit extract on the level of expression of fatty acid synthase complex, lipoprotein lipase, fibroblast growth factor-21 and leptin—A biochemical study in rats subjected to experimental hyperlipidemia and obesity. Phytomedicine 2015, 22, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Espín, J.C.; Carr, T.P.; Tomás-Barberán, F.A.; Chung, S. Raspberry seed flour attenuates high-sucrose diet-mediated hepatic stress and adipose tissue inflammation. J. Nutr. Biochem. 2016, 32, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed]
- Gothai, S.; Ganesan, P.; Park, S.Y.; Fakurazi, S.; Choi, D.K.; Arulselvan, P. Natural Phyto-Bioactive Compounds for the Treatment of Type 2 Diabetes: Inflammation as a Target. Nutrients 2016, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-Atom versus Elecgron Transfer Mechanism. J. Phys. Chem. 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Spahis, S.; Borys, J.M.; Levy, E. Metabolic Syndrome as a Multifaceted Risk Factor for Oxidative Stress. Antioxid. Redox Signal. 2017, 26, 445–461. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhle, C.; Bock, K.W. Activation of coupled Ah receptor and Nrf2 gene batteries by dietary phytochemicals in relation to chemoprevention. Biochem. Pharmacol. 2006, 72, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qin, C.; Safe, S.H. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: Effects of structure and cell context. Environ. Health Perspect. 2003, 111, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.C.; McIntosh, M.K. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu. Rev. Nutr. 2011, 31, 155–176. [Google Scholar] [CrossRef]
- Diotallevi, C.; Fava, F.; Gobbetti, M.; Tuohy, K. Healthy dietary patterns to reduce obesity-related metabolic disease: Polyphenol: Microbiome interactions unifying health effects across geography. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 437–444. [Google Scholar] [CrossRef]
- Sanchez-Marzo, N.; Perez-Sanchez, A.; Barrajon-Catalan, E.; Castillo, J.; Herranz-Lopez, M.; Micol, V. Rosemary Diterpenes and Flavanone Aglycones Provide Improved Genoprotection against UV-Induced DNA Damage in a Human Skin Cell Model. Antioxidants 2020, 9, 255. [Google Scholar] [CrossRef] [Green Version]
- Matsuno, Y.; Atsumi, Y.; Alauddin, M.; Rana, M.M.; Fujimori, H.; Hyodo, M.; Shimizu, A.; Ikuta, T.; Tani, H.; Torigoe, H.; et al. Resveratrol and its Related Polyphenols Contribute to the Maintenance of Genome Stability. Sci. Rep. 2020, 10, 5388. [Google Scholar] [CrossRef]
- Huarte, E.; Cid, C.; Azqueta, A.; de Pena, M.P. DNA damage and DNA protection from digested raw and griddled green pepper (poly)phenols in human colorectal adenocarcinoma cells (HT-29). Eur. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Groh, I.A.M.; Bakuradze, T.; Pahlke, G.; Richling, E.; Marko, D. Consumption of anthocyanin-rich beverages affects Nrf2 and Nrf2-dependent gene transcription in peripheral lymphocytes and DNA integrity of healthy volunteers. BMC Chem. 2020, 14, 39. [Google Scholar] [CrossRef]
- Abib, R.T.; Quincozes-Santos, A.; Zanotto, C.; Zeidán-Chuliá, F.; Lunardi, P.S.; Gonçalves, C.A.; Gottfried, C. Genoprotective effects of the green tea-derived polyphenol/epicatechin gallate in C6 astroglial cells. J. Med. Food 2010, 13, 1111–1115. [Google Scholar] [CrossRef]
- Quincozes-Santos, A.; Andreazza, A.C.; Nardin, P.; Funchal, C.; Gonçalves, C.A.; Gottfried, C. Resveratrol attenuates oxidative-induced DNA damage in C6 Glioma cells. Neurotoxicology 2007, 28, 886–891. [Google Scholar] [CrossRef]
- Han, K.C.; Wong, W.C.; Benzie, I.F. Genoprotective effects of green tea (Camellia sinensis) in human subjects: Results of a controlled supplementation trial. Br. J. Nutr. 2011, 105, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell. Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Young, M.R.; Bobe, G.; Colburn, N.H.; Milner, J.A. Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prev. Res. 2009, 2, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Tang, Y.; Hu, D.; Li, J. Inhibitory effect of genistein on mouse colon cancer MC-26 cells involved TGF-beta1/Smad pathway. Biochem. Biophys. Res. Commun. 2005, 333, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.A.; Cao, Y.; Chen, J.R.; Townsend, C.M., Jr.; Ko, T.C. Dietary fiber enhances a tumor suppressor signaling pathway in the gut. Ann. Surg. 2006, 243, 619–625. [Google Scholar] [CrossRef]
- Denis, M.C.; Furtos, A.; Dudonne, S.; Montoudis, A.; Garofalo, C.; Desjardins, Y.; Delvin, E.; Levy, E. Apple peel polyphenols and their beneficial actions on oxidative stress and inflammation. PLoS ONE 2013, 8, e53725. [Google Scholar] [CrossRef]
- Yeganeh, P.R.; Leahy, J.; Spahis, S.; Patey, N.; Desjardins, Y.; Roy, D.; Delvin, E.; Garofalo, C.; Leduc-Gaudet, J.P.; St-Pierre, D.; et al. Apple peel polyphenols reduce mitochondrial dysfunction in mice with DSS-induced ulcerative colitis. J. Nutr. Biochem. 2018, 57, 56–66. [Google Scholar] [CrossRef]
- Denis, M.C.; Roy, D.; Yeganeh, P.R.; Desjardins, Y.; Varin, T.; Haddad, N.; Amre, D.; Sane, A.T.; Garofalo, C.; Furtos, A.; et al. Apple peel polyphenols: A key player in the prevention and treatment of experimental inflammatory bowel disease. Clin. Sci. 2016, 130, 2217–2237. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Zheng, L.; Almeida, F.A. Epigenetic reprogramming in metabolic disorders: Nutritional factors and beyond. J. Nutr. Biochem. 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Lim, W.-J.; Kim, K.H.; Kim, J.-Y.; Jeong, S.; Kim, N. Identification of DNA-Methylated CpG Islands Associated With Gene Silencing in the Adult Body Tissues of the Ogye Chicken Using RNA-Seq and Reduced Representation Bisulfite Sequencing. Front. Genet. 2019, 10, 346. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, C.; Esteller, M. Chapter Seven—MicroRNAs and Epigenetics. In Advances in Cancer Research; Croce, C.M., Fisher, P.B., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 135, pp. 189–220. [Google Scholar]
- Bruce, K.D.; Cagampang, F.R. Epigenetic priming of the metabolic syndrome. Toxicol. Mech. Methods 2011, 21, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; You, M.; Toney, A.M.; Kim, J.; Giraud, D.; Xian, Y.; Ye, F.; Gu, L.; Ramer-Tait, A.E.; Chung, S. Red Raspberry Polyphenols Attenuate High-Fat Diet-Driven Activation of NLRP3 Inflammasome and its Paracrine Suppression of Adipogenesis via Histone Modifications. Mol. Nutr. Food Res. 2019, 64, e1900995. [Google Scholar] [CrossRef] [PubMed]
- Nettore, I.C.; Rocca, C.; Mancino, G.; Albano, L.; Amelio, D.; Grande, F.; Puoci, F.; Pasqua, T.; Desiderio, S.; Mazza, R.; et al. Quercetin and its derivative Q2 modulate chromatin dynamics in adipogenesis and Q2 prevents obesity and metabolic disorders in rats. J. Nutr. Biochem. 2019, 69, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Boqué, N.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; Olivares, M.; Bañuelos, O.; Soria, A.C.; Rodríguez-Sánchez, S.; Martínez, J.A.; Campión, J. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol. Nutr. Food Res. 2013, 57, 1473–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crescenti, A.; Solà, R.; Valls, R.M.; Caimari, A.; Del Bas, J.M.; Anguera, A.; Anglés, N.; Arola, L. Cocoa Consumption Alters the Global DNA Methylation of Peripheral Leukocytes in Humans with Cardiovascular Disease Risk Factors: A Randomized Controlled Trial. PLoS ONE 2013, 8, e65744. [Google Scholar] [CrossRef]
- Han, S.; Uludag, M.O.; Usanmaz, S.E.; Ayaloglu-Butun, F.; Akcali, K.C.; Demirel-Yilmaz, E. Resveratrol affects histone 3 lysine 27 methylation of vessels and blood biomarkers in DOCA salt-induced hypertension. Mol. Biol. Rep. 2015, 42, 35–42. [Google Scholar] [CrossRef]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Döring, F.; Wolffram, S.; Rimbach, G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Adair, B.; Alder, H.; Limagne, E.; Taccioli, C.; Ferracin, M.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis 2010, 31, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Rasheed, N.; Al-Shobaili, H.A. Epigallocatechin-3-O-gallate up-regulates microRNA-199a-3p expression by down-regulating the expression of cyclooxygenase-2 in stimulated human osteoarthritis chondrocytes. J. Cell. Mol. Med. 2016, 20, 2241–2248. [Google Scholar] [CrossRef] [Green Version]
- Carpi, S.; Scoditti, E.; Massaro, M.; Polini, B.; Manera, C.; Digiacomo, M.; Esposito Salsano, J.; Poli, G.; Tuccinardi, T.; Doccini, S.; et al. The Extra-Virgin Olive Oil Polyphenols Oleocanthal and Oleacein Counteract Inflammation-Related Gene and miRNA Expression in Adipocytes by Attenuating NF-κB Activation. Nutrients 2019, 11, 2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scoditti, E.; Carpi, S.; Massaro, M.; Pellegrino, M.; Polini, B.; Carluccio, M.A.; Wabitsch, M.; Verri, T.; Nieri, P.; De Caterina, R. Hydroxytyrosol Modulates Adipocyte Gene and miRNA Expression Under Inflammatory Condition. Nutrients 2019, 11, 2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaccaria, V.; Curti, V.; Di Lorenzo, A.; Baldi, A.; Maccario, C.; Sommatis, S.; Mocchi, R.; Daglia, M. Effect of Green and Brown Propolis Extracts on the Expression Levels of microRNAs, mRNAs and Proteins, Related to Oxidative Stress and Inflammation. Nutrients 2017, 9, 1090. [Google Scholar] [CrossRef]
- Howell, J.C.; Chun, E.; Farrell, A.N.; Hur, E.Y.; Caroti, C.M.; Iuvone, P.M.; Haque, R. Global microRNA expression profiling: Curcumin (diferuloylmethane) alters oxidative stress-responsive microRNAs in human ARPE-19 cells. Mol. Vis. 2013, 19, 544–560. [Google Scholar]
- Noratto, G.D.; Angel-Morales, G.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenolics from açaí (Euterpe oleracea Mart.) and red muscadine grape (Vitis rotundifolia) protect human umbilical vascular Endothelial cells (HUVEC) from glucose- and lipopolysaccharide (LPS)-induced inflammation and target microRNA-126. J. Agric. Food Chem. 2011, 59, 7999–8012. [Google Scholar] [CrossRef]
- Song, J.; Jun, M.; Ahn, M.R.; Kim, O.Y. Involvement of miR-Let7A in inflammatory response and cell survival/apoptosis regulated by resveratrol in THP-1 macrophage. Nutr. Res. Pract. 2016, 10, 377–384. [Google Scholar] [CrossRef]
- Boesch-Saadatmandi, C.; Wagner, A.E.; Wolffram, S.; Rimbach, G. Effect of quercetin on inflammatory gene expression in mice liver in vivo—Role of redox factor 1, miRNA-122 and miRNA-125b. Pharmacol. Res. 2012, 65, 523–530. [Google Scholar] [CrossRef]
- Mohamed, H.E.; Abo-ELmatty, D.M.; Salah, S.M.; Sakr, A.T. Ameliorative effect of grapre seed extract on metabolic disorders caused by high fat diet induced obesity in rats by reversing the indrease inhepatic miR-33a and miR-122. Afr. J. Pharm. Pharmacol. 2016, 10, 699–708. [Google Scholar]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Otton, R.; Bolin, A.P.; Ferreira, L.T.; Marinovic, M.P.; Rocha, A.L.S.; Mori, M.A. Polyphenol-rich green tea extract improves adipose tissue metabolism by down-regulating miR-335 expression and mitigating insulin resistance and inflammation. J. Nutr. Biochem. 2018, 57, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Gracia, A.; Miranda, J.; Fernández-Quintela, A.; Eseberri, I.; Garcia-Lacarte, M.; Milagro, F.I.; Martínez, J.A.; Aguirre, L.; Portillo, M.P. Involvement of miR-539-5p in the inhibition of de novo lipogenesis induced by resveratrol in white adipose tissue. Food Funct. 2016, 7, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Larrosa, M.; Yáñez-Gascón, M.J.; Dávalos, A.; Gil-Zamorano, J.; Gonzálvez, M.; García-Almagro, F.J.; Ruiz Ros, J.A.; Tomás-Barberán, F.A.; Espín, J.C.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef]
- Glässer, G.; Graefe, E.U.; Struck, F.; Veit, M.; Gebhardt, R. Comparison of antioxidative capacities and inhibitory effects on cholesterol biosynthesis of quercetin and potential metabolites. Phytomedicine 2002, 9, 33–40. [Google Scholar] [CrossRef]
- Merfort, I.; Heilmann, J.; Weiss, M.; Pietta, P.; Gardana, C. Radical scavenger activity of three flavonoid metabolites studied by inhibition of chemiluminescence in human PMNs. Planta Med. 1996, 62, 289–292. [Google Scholar] [CrossRef]
- Tang, Y.; Nakashima, S.; Saiki, S.; Myoi, Y.; Abe, N.; Kuwazuru, S.; Zhu, B.; Ashida, H.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides. Food Res. Int. 2016, 89, 716–723. [Google Scholar] [CrossRef]
- Liu, Y.; Kurita, A.; Nakashima, S.; Zhu, B.; Munemasa, S.; Nakamura, T.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic acid is a potential aldehyde dehydrogenase inducer in murine hepatoma Hepa1c1c7 cells. Biosci. Biotechnol. Biochem. 2017, 81, 1978–1983. [Google Scholar] [CrossRef] [Green Version]
- Monagas, M.; Khan, N.; Andres-Lacueva, C.; Urpi-Sarda, M.; Vazquez-Agell, M.; Lamuela-Raventos, R.M.; Estruch, R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br. J. Nutr. 2009, 102, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Rechner, A.R.; Kroner, C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 2005, 116, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cilleros, D.; Ramos, S.; Goya, L.; Martín, M.Á. Colonic metabolites from flavanols stimulate nitric oxide production in human endothelial cells and protect against oxidative stress-induced toxicity and endothelial dysfunction. Food Chem. Toxicol. 2018, 115, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Millan, E.; Ramos, S.; Alvarez, C.; Bravo, L.; Goya, L.; Martin, M.A. Microbial phenolic metabolites improve glucose-stimulated insulin secretion and protect pancreatic beta cells against tert-butyl hydroperoxide-induced toxicity via ERKs and PKC pathways. Food Chem. Toxicol. 2014, 66, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Gotteland, M.; Castillo, R.L.; Chen, C. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, protects against pancreatic beta-cells dysfunction induced by high cholesterol. Exp. Cell Res. 2015, 334, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Moore, L.H.; Dowell, V.R., Jr.; Bokkenheuser, V.D. C-ring cleavage of flavonoids by human intestinal bacteria. Appl. Environ. Microbiol. 1989, 55, 1203–1208. [Google Scholar] [CrossRef] [Green Version]
- Schneider, H.; Simmering, R.; Hartmann, L.; Pforte, H.; Blaut, M. Degradation of quercetin-3-glucoside in gnotobiotic rats associated with human intestinal bacteria. J. Appl. Microbiol. 2000, 89, 1027–1037. [Google Scholar] [CrossRef]
- Feng, X.; Li, Y.; Brobbey Oppong, M.; Qiu, F. Insights into the intestinal bacterial metabolism of flavonoids and the bioactivities of their microbe-derived ring cleavage metabolites. Drug Metab. Rev. 2018, 50, 343–356. [Google Scholar] [CrossRef]
- Hanske, L.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. The Bioavailability of Apigenin-7-Glucoside Is Influenced by Human Intestinal Microbiota in Rats. J. Nutr. 2009, 139, 1095–1102. [Google Scholar] [CrossRef] [Green Version]
- Labib, S.; Erb, A.; Kraus, M.; Wickert, T.; Richling, E. The pig caecum model: A suitable tool to study the intestinal metabolism of flavonoids. Mol. Nutr. Food Res. 2004, 48, 326–332. [Google Scholar] [CrossRef]
- Zou, W.; Luo, Y.; Liu, M.; Chen, S.; Wang, S.; Nie, Y.; Cheng, G.; Su, W.; Zhang, K. Human intestinal microbial metabolism of naringin. Eur. J. Drug Metab.Pharmacokinet. 2015, 40, 363–367. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Wang, J.-H.; Liu, X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora—Implications for health. Mol. Nutr. Food Res. 2007, 51, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Ihara, M.; Lopez, O.; Kakuta, C.; Lopresti, B.; Higashiyama, A.; Aizenstein, H.; Chang, Y.F.; Mathis, C.; Miyamoto, Y.; et al. Effect of S-equol and Soy Isoflavones on Heart and Brain. Curr. Cardiol. Rev. 2019, 15, 114–135. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.H. The antioxidant activity of daidzein metabolites, O-desmethylangolensin and equol, in HepG2 cells. Mol. Med. Rep. 2014, 9, 328–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, H.; Blaut, M. Anaerobic degradation of flavonoids by Eubacterium ramulus. Arch. Microbiol. 2000, 173, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.C.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.; Pereira Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.; et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Borges, G.; Momma, T.Y.; Spencer, J.P.E.; Keen, C.L.; Crozier, A.; Schroeter, H. The metabolome of [2-14C](−)-epicatechin in humans: Implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci. Rep. 2016, 6, 29034. [Google Scholar] [CrossRef] [Green Version]
- Mele, L.; Carobbio, S.; Brindani, N.; Curti, C.; Rodriguez-Cuenca, S.; Bidault, G.; Mena, P.; Zanotti, I.; Vacca, M.; Vidal-Puig, A.; et al. Phenyl-γ-valerolactones, flavan-3-ol colonic metabolites, protect brown adipocytes from oxidative stress without affecting their differentiation or function. Mol. Nutr. Food Res. 2017, 61, 1700074. [Google Scholar] [CrossRef]
- Lambert, J.D.; Rice, J.E.; Hong, J.; Hou, Z.; Yang, C.S. Synthesis and biological activity of the tea catechin metabolites, M4 and M6 and their methoxy-derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 873–876. [Google Scholar] [CrossRef]
- Uhlenhut, K.; Högger, P. Facilitated cellular uptake and suppression of inducible nitric oxide synthase by a metabolite of maritime pine bark extract (Pycnogenol). Free Radic. Biol. Med. 2012, 53, 305–313. [Google Scholar] [CrossRef]
- Lee, C.C.; Kim, J.H.; Kim, J.S.; Oh, Y.S.; Han, S.M.; Park, J.H.Y.; Lee, K.W.; Lee, C.Y. 5-(3’,4’-Dihydroxyphenyl-gamma-valerolactone), a Major Microbial Metabolite of Proanthocyanidin, Attenuates THP-1 Monocyte-Endothelial Adhesion. Int. J. Mol. Sci. 2017, 18, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagaki, A.; Nanjo, F. Effects of Metabolites Produced from (−)-Epigallocatechin Gallate by Rat Intestinal Bacteria on Angiotensin I-Converting Enzyme Activity and Blood Pressure in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2015, 63, 8262–8266. [Google Scholar] [CrossRef] [PubMed]
- Van Rymenant, E.; Grootaert, C.; Beerens, K.; Needs, P.W.; Kroon, P.A.; Kerimi, A.; Williamson, G.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.; et al. Vasorelaxant activity of twenty-one physiologically relevant (poly)phenolic metabolites on isolated mouse arteries. Food Funct. 2017, 8, 4331–4335. [Google Scholar] [CrossRef] [PubMed]
- Miladinović, B.; Kostić, M.; Šavikin, K.; Đorđević, B.; Mihajilov-Krstev, T.; Živanović, S.; Kitić, D. Chemical Profile and Antioxidative and Antimicrobial Activity of Juices and Extracts of 4 Black Currants Varieties (Ribes nigrum L.). J. Food Sci. 2014, 79, C301–C309. [Google Scholar] [CrossRef]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104 (Suppl. S3), S48–S66. [Google Scholar] [CrossRef] [Green Version]
- Gowd, V.; Bao, T.; Wang, L.; Huang, Y.; Chen, S.; Zheng, X.; Cui, S.; Chen, W. Antioxidant and antidiabetic activity of blackberry after gastrointestinal digestion and human gut microbiota fermentation. Food Chem. 2018, 269, 618–627. [Google Scholar] [CrossRef]
- Ho, G.T.; Wangensteen, H.; Barsett, H. Elderberry and Elderflower Extracts, Phenolic Compounds, and Metabolites and Their Effect on Complement, RAW 264.7 Macrophages and Dendritic Cells. Int. J. Mol. Sci. 2017, 18, 584. [Google Scholar] [CrossRef] [Green Version]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.T. Esterase activity able to hydrolyze dietary antioxidant hydroxycinnamates is distributed along the intestine of mammals. J. Agric. Food Chem. 2001, 49, 5679–5684. [Google Scholar] [CrossRef]
- Couteau, D.; McCartney, A.L.; Gibson, G.R.; Williamson, G.; Faulds, C.B. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001, 90, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Periago, P.; Espín, J.C.; Tomás-Barberán, F.A. Identification of Urolithin A as a Metabolite Produced by Human Colon Microflora from Ellagic Acid and Related Compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, R.; Borges, G.; Mullen, W.; Crozier, A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J. Agric. Food Chem. 2010, 58, 3933–3939. [Google Scholar] [CrossRef]
- Iino, T.; Tashima, K.; Umeda, M.; Ogawa, Y.; Takeeda, M.; Takata, K.; Takeuchi, K. Effect of ellagic acid on gastric damage induced in ischemic rat stomachs following ammonia or reperfusion. Life Sci. 2002, 70, 1139–1150. [Google Scholar] [CrossRef]
- Yu, Y.M.; Wang, Z.H.; Liu, C.H.; Chen, C.S. Ellagic acid inhibits IL-1beta-induced cell adhesion molecule expression in human umbilical vein endothelial cells. Br. J. Nutr. 2007, 97, 692–698. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.C.; Yu, Y.M.; Chiang, S.Y.; Tseng, C.Y. Ellagic acid suppresses oxidised low-density lipoprotein-induced aortic smooth muscle cell proliferation: Studies on the activation of extracellular signal-regulated kinase 1/2 and proliferating cell nuclear antigen expression. Br. J. Nutr. 2008, 99, 709–714. [Google Scholar] [CrossRef] [Green Version]
- Ishimoto, H.; Tai, A.; Yoshimura, M.; Amakura, Y.; Yoshida, T.; Hatano, T.; Ito, H. Antioxidative properties of functional polyphenols and their metabolites assessed by an ORAC assay. Biosci. Biotechnol. Biochem. 2012, 76, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Bialonska, D.; Kasimsetty, S.G.; Khan, S.I.; Ferreira, D. Urolithins, intestinal microbial metabolites of Pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J. Agric. Food Chem. 2009, 57, 10181–10186. [Google Scholar] [CrossRef]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011, 55 (Suppl. S1), S35–S43. [Google Scholar] [CrossRef]
- Komatsu, W.; Kishi, H.; Yagasaki, K.; Ohhira, S. Urolithin A attenuates pro-inflammatory mediator production by suppressing PI3-K/Akt/NF-κB and JNK/AP-1 signaling pathways in lipopolysaccharide-stimulated RAW264 macrophages: Possible involvement of NADPH oxidase-derived reactive oxygen species. Eur. J. Pharmacol. 2018, 833, 411–424. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Mo, Y.; Li, Y.; Zhong, Y.; He, S.; Zhang, Y.; Tang, Y.; Fu, S.; Wang, X.; Chen, A. Urolithin A alleviates myocardial ischemia/reperfusion injury via PI3K/Akt pathway. Biochem. Biophys. Res. Commun. 2017, 486, 774–780. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Larrosa, M.; Tomás-Barberán, F.A.; Dolara, P.; Espín, J.C. NF-kappaB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br. J. Nutr. 2010, 104, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Larrosa, M.; Tomás-Barberán, F.; Espín, J.C.; García-Conesa, M.T. Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-α-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol. Nutr. Food Res. 2012, 56, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Dell’Agli, M.; Facchi, A.; De Fabiani, E.; Lucchi, L.; Boraso, M.S.; Marinovich, M.; Galli, C.L. Enterodiol and enterolactone modulate the immune response by acting on nuclear factor-kappaB (NF-kappaB) signaling. J. Agric. Food Chem. 2010, 58, 6678–6684. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Antioxidant Activity of Secoisolariciresinol Diglucoside-derived Metabolites, Secoisolariciresinol, Enterodiol, and Enterolactone. Int. J. Angiol. 2000, 9, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Thompson, L.U. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol. Cell. Biochem. 1999, 202, 91–100. [Google Scholar] [CrossRef]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wähälä, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In Vitro Metabolism of Plant Lignans: New Precursors of Mammalian Lignans Enterolactone and Enterodiol. J. Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites-Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.L.; Ding, D.J.; Yan, W.J.; Li, R.R.; Dai, F.; Wang, Q.; Yu, S.S.; Li, Y.; Jin, X.L.; Zhou, B. Influence of glucuronidation and reduction modifications of resveratrol on its biological activities. Chembiochem 2013, 14, 1094–1104. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Heiss, C.; Borges, G.; Crozier, A. Berry (poly)phenols and cardiovascular health. J. Agric. Food Chem. 2014, 62, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-Y.; Yu, C.Y.; Chen, Y.-P.; Hua, K.-F.; Chen, Y.-L.S. 3,4-Dihydroxytoluene, a metabolite of rutin, inhibits inflammatory responses in lipopolysaccharide-activated macrophages by reducing the activation of NF-κB signaling. BMC Complement Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Calvo, J.M.; Rodríguez-Iglesias, M.A.; Molinillo, J.M.G.; Macías, F.A. Soy isoflavones and their relationship with microflora: Beneficial effects on human health in equol producers. Phytochem. Rev. 2013, 12, 979–1000. [Google Scholar] [CrossRef]
- Tousen, Y.; Uehara, M.; Abe, F.; Kimira, Y.; Ishimi, Y. Effects of short-term fructooligosaccharide intake on equol production in Japanese postmenopausal women consuming soy isoflavone supplements: A pilot study. Nutr. J. 2013, 12, 127. [Google Scholar] [CrossRef] [Green Version]
- Setchell, K.D.; Brown, N.M.; Summer, S.; King, E.C.; Heubi, J.E.; Cole, S.; Guy, T.; Hokin, B. Dietary factors influence production of the soy isoflavone metabolite s-(−)equol in healthy adults. J. Nutr. 2013, 143, 1950–1958. [Google Scholar] [CrossRef] [Green Version]
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A.; Shimatsu, A.; Inaguma, A.; Ueno, T.; Uchiyama, S.; et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. 2013, 78, 365–372. [Google Scholar] [CrossRef]
- Yoshikata, R.; Myint, K.Z.Y.; Ohta, H. Effects of Equol Supplement on Bone and Cardiovascular Parameters in Middle-Aged Japanese Women: A Prospective Observational Study. J. Altern. Complement. Med. 2018, 24, 701–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birru, R.L.; Ahuja, V.; Vishnu, A.; Evans, R.W.; Miyamoto, Y.; Miura, K.; Usui, T.; Sekikawa, A. The impact of equol-producing status in modifying the effect of soya isoflavones on risk factors for CHD: A systematic review of randomised controlled trials. J. Nutr. Sci. 2016, 5, e30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Newton, K.M.; Bowles, E.J.; Yong, M.; Lampe, J.W. Demographic, anthropometric, and lifestyle factors and dietary intakes in relation to daidzein-metabolizing phenotypes among premenopausal women in the United States. Am. J. Clin. Nutr. 2008, 87, 679–687. [Google Scholar] [CrossRef]
- Liu, Z.M.; Ho, S.C.; Chen, Y.M.; Liu, J.; Woo, J. Cardiovascular risks in relation to daidzein metabolizing phenotypes among Chinese postmenopausal women. PLoS ONE 2014, 9, e87861. [Google Scholar] [CrossRef]
- Guo, K.; Zhang, B.; Chen, C.; Uchiyama, S.; Ueno, T.; Chen, Y.; Su, Y. Daidzein-metabolising phenotypes in relation to serum lipids and uric acid in adults in Guangzhou, China. Br. J. Nutr. 2010, 104, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Ryan, H.H.; Jones, E.; Simas, T.A.M.; Lichtenstein, A.H.; Sun, Q.; Hayman, L.L. Urinary isoflavone concentrations are inversely associated with cardiometabolic risk markers in pregnant U.S. women. J. Nutr. 2014, 144, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Frankenfeld, C.L.; Atkinson, C.; Wähälä, K.; Lampe, J.W. Obesity prevalence in relation to gut microbial environments capable of producing equol or O-desmethylangolensin from the isoflavone daidzein. Eur. J. Clin. Nutr. 2014, 68, 526–530. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary intake of 337 polyphenols in French adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Sansone, R.; Rodriguez-Mateos, A.; Heuel, J.; Falk, D.; Schuler, D.; Wagstaff, R.; Kuhnle, G.G.C.; Spencer, J.P.E.; Schroeter, H.; Merx, M.W.; et al. Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: A randomised, controlled, double-masked trial: The Flaviola Health Study. Br. J. Nutr. 2015, 114, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Stote, K.S.; Clevidence, B.A.; Novotny, J.A.; Henderson, T.; Radecki, S.V.; Baer, D.J. Effect of cocoa and green tea on biomarkers of glucose regulation, oxidative stress, inflammation and hemostasis in obese adults at risk for insulin resistance. Eur. J. Clin. Nutr. 2012, 66, 1153–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castello, F.; Costabile, G.; Bresciani, L.; Tassotti, M.; Naviglio, D.; Luongo, D.; Ciciola, P.; Vitale, M.; Vetrani, C.; Galaverna, G.; et al. Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Arch. Biochem. Biophys. 2018, 646, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Boeres, A.; Weber, T.; Dos Santos, C.N.; Ventura, M.R.; Heiss, C. Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study. Mol. Nutr. Food Res. 2016, 60, 2130–2140. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, G.Y.; Li, J.; Cichon, M.J.; Riedl, K.M.; Kopec, R.E.; Bruno, R.S. Green Tea Extract Treatment in Obese Mice with Nonalcoholic Steatohepatitis Restores the Hepatic Metabolome in Association with Limiting Endotoxemia-TLR4-NFκB-Mediated Inflammation. Mol. Nutr. Food Res. 2019, 63, e1900811. [Google Scholar] [CrossRef]
- Panchal, S.K.; Ward, L.; Brown, L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2013, 52, 559–568. [Google Scholar] [CrossRef]
- Rani, U.P.; Kesavan, R.; Ganugula, R.; Avaneesh, T.; Kumar, U.P.; Reddy, G.B.; Dixit, M. Ellagic acid inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and prevents atheroma formation in streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2013, 24, 1830–1839. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef]
- Ishimoto, H.; Shibata, M.; Myojin, Y.; Ito, H.; Sugimoto, Y.; Tai, A.; Hatano, T. In vivo anti-inflammatory and antioxidant properties of ellagitannin metabolite urolithin A. Bioorg. Med. Chem. Lett. 2011, 21, 5901–5904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, Y. Immunochemical detection of food-derived polyphenols in the aorta: Macrophages as a major target underlying the anti-atherosclerotic activity of polyphenols. Biosci. Biotechnol. Biochem. 2011, 75, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; González-Sarrías, A.; Salas-Salvadó, J.; Andrés-Lacueva, C.; Alasalvar, C.; Örem, A.; Tomás-Barberán, F.A.; Espín, J.C. The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: Comparison between normoweight, overweight-obesity and metabolic syndrome. Clin. Nutr. 2018, 37, 897–905. [Google Scholar] [CrossRef]
- Gutiérrez-Díaz, I.; Fernández-Navarro, T.; Salazar, N.; Bartolomé, B.; Moreno-Arribas, M.V.; López, P.; Suárez, A.; de Los Reyes-Gavilán, C.G.; Gueimonde, M.; González, S. Could Fecal Phenylacetic and Phenylpropionic Acids Be Used as Indicators of Health Status? J. Agric. Food Chem. 2018, 66, 10438–10446. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, Y.; Henning, S.M.; Chan, B.; Long, J.; Zhong, J.; Acin-Perez, R.; Petcherski, A.; Shirihai, O.; Heber, D.; et al. Ellagic Acid and Its Microbial Metabolite Urolithin A Alleviate Diet-Induced Insulin Resistance in Mice. Mol. Nutr. Food Res. 2020, 64, e2000091. [Google Scholar] [CrossRef]
- Xia, B.; Shi, X.C.; Xie, B.C.; Zhu, M.Q.; Chen, Y.; Chu, X.Y.; Cai, G.H.; Liu, M.; Yang, S.Z.; Mitchell, G.A.; et al. Urolithin A exerts antiobesity effects through enhancing adipose tissue thermogenesis in mice. PLoS Biol. 2020, 18, e3000688. [Google Scholar] [CrossRef] [Green Version]
- Adolphe, J.L.; Whiting, S.J.; Juurlink, B.H.; Thorpe, L.U.; Alcorn, J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br. J. Nutr. 2010, 103, 929–938. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Ahmad, N.; Anjum, F.M.; Khan, M.K.; Mushtaq, Z.; Nadeem, M.; Hussain, S. Potential protective properties of flax lignan secoisolariciresinol diglucoside. Nutr. J. 2015, 14, 71. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, X.; Liu, Y.; Tian, H.; Flickinger, B.; Empie, M.W.; Sun, S.Z. Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br. J. Nutr. 2008, 99, 1301–1309. [Google Scholar] [CrossRef]
- Prasad, K. Secoisolariciresinol diglucoside from flaxseed delays the development of type 2 diabetes in Zucker rat. J. Lab. Clin. Med. 2001, 138, 32–39. [Google Scholar] [CrossRef]
- Kottke, T.E.; Ogwang, Z.; Smith, J.C. Reasons for not meeting coronary artery disease targets of care in ambulatory practice. Perm. J. 2010, 14, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary Lignans: Definition, Description and Research Trends in Databases Development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [Green Version]
- Corona, G.; Kreimes, A.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Costabile, A. Impact of lignans in oilseed mix on gut microbiome composition and enterolignan production in younger healthy and premenopausal women: An in vitro pilot study. Microb. Cell Fact. 2020, 19, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, A.F.; Zang, Y. A Review of Lignan Metabolism, Milk Enterolactone Concentration, and Antioxidant Status of Dairy Cows Fed Flaxseed. Molecules 2018, 24, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rienks, J.; Barbaresko, J.; Nöthlings, U. Association of Polyphenol Biomarkers with Cardiovascular Disease and Mortality Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2017, 9, 415. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Wedick, N.M.; Pan, A.; Townsend, M.K.; Cassidy, A.; Franke, A.A.; Rimm, E.B.; Hu, F.B.; van Dam, R.M. Gut microbiota metabolites of dietary lignans and risk of type 2 diabetes: A prospective investigation in two cohorts of U.S. women. Diabet. Care 2014, 37, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- Struja, T.; Richard, A.; Linseisen, J.; Eichholzer, M.; Rohrmann, S. The association between urinary phytoestrogen excretion and components of the metabolic syndrome in NHANES. Eur. J. Nutr. 2014, 53, 1371–1381. [Google Scholar] [CrossRef] [Green Version]
- Peñalvo, J.L.; López-Romero, P. Urinary enterolignan concentrations are positively associated with serum HDL cholesterol and negatively associated with serum triglycerides in U.S. adults. J. Nutr. 2012, 142, 751–756. [Google Scholar] [CrossRef] [Green Version]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Alberdi, G.; Rodríguez, V.M.; Miranda, J.; Macarulla, M.T.; Churruca, I.; Portillo, M.P. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 2013, 141, 1530–1535. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez-del Villar, M.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Lizárraga-Valdez, R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javkhedkar, A.A.; Quiroz, Y.; Rodriguez-Iturbe, B.; Vaziri, N.D.; Lokhandwala, M.F.; Banday, A.A. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R840–R846. [Google Scholar] [CrossRef] [Green Version]
- Dolinsky, V.W.; Chakrabarti, S.; Pereira, T.J.; Oka, T.; Levasseur, J.; Beker, D.; Zordoky, B.N.; Morton, J.S.; Nagendran, J.; Lopaschuk, G.D.; et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta 2013, 1832, 1723–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.M.; Eckardt, P.; Aleman, J.O.; da Rosa, J.C.; Liang, Y.; Iizumi, T.; Etheve, S.; Blaser, M.J.; Breslow, J.L.; Holt, P.R. The effects of trans-resveratrol on insulin resistance, inflammation, and microbiota in men with the metabolic syndrome: A pilot randomized, placebo-controlled clinical trial. J. Clin. Transl. Res. 2019, 4, 122–135. [Google Scholar] [PubMed]
- Wenzel, E.; Soldo, T.; Erbersdobler, H.; Somoza, V. Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats. Mol. Nutr. Food Res. 2005, 49, 482–494. [Google Scholar] [CrossRef]
- Boocock, D.J.; Patel, K.R.; Faust, G.E.; Normolle, D.P.; Marczylo, T.H.; Crowell, J.A.; Brenner, D.E.; Booth, T.D.; Gescher, A.; Steward, W.P. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 848, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug. Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Azorín-Ortuño, M.; Yáñez-Gascón, M.J.; Vallejo, F.; Pallarés, F.J.; Larrosa, M.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Metabolites and tissue distribution of resveratrol in the pig. Mol. Nutr. Food Res. 2011, 55, 1154–1168. [Google Scholar] [CrossRef] [Green Version]


| Substrates | Sources | Experimental Conditions and Model | End Products | Main Observations | Conclusions | Ref |
|---|---|---|---|---|---|---|
| Procyanidins polymers (2–6 mers) | Cocoa | Incubation in simulated gastric juice (pH 2.0) at 37 °C for up to 3.5 h | Catechin/epicatechin Monomer and dimer | Time-dependant hydrolysis of oligomers. | Role of stomach in the processing of phenolic compounds. | [64] |
| Dimeric catechin/ epicatechin | Cocoa | Incubation in simulated gastric juice (pH 1.8) at 37 °C for up to 60 min | Catechin/epicatechin Dimers isomerization | Time-dependant hydrolysis of dimers. | Role of stomach in the processing of phenolic compounds. | [65] |
| Free and conjugated Hydroxytyrosol and Tyrosol | Olive oil | Incubation in simulated gastric juice (pH 2.0) at 37 °C for up to 4 h | Free hydroxytyrosol and tyrosol | Time-dependent hydrolysis of hydroxytyrosol and tyrosol conjugates. | Stomach hydrolyzes phenolic compounds conjugates. | [66] |
| Monomeric/ Polymeric Catechin/ epicatechin | Grape seed extract | Incubation in simulated gastric juice (pH 2.0) + pepsin at 37 °C for 2 h | Catechin/epicatechin Oligomers | Stability of catechin/epicat. | Stability of phenolic compounds at the gastric level. | [67] |
| Hydroxycinnamic acid derivatives, Flavonols, dihydrochlcones monomeric flavans-3-ols, procyanidin B2 | Apple juice | Incubation in simulated gastric juice (pH 2.0) + pepsin at 37 °C for up to 4 h | Hydroxycinnamic acid derivatives, flavonols, dihydrochlcones monomeric, Epicatechin monomer | Stability of Hydroxycinnamic acid derivatives, flavonols, dihydrochalcones monomeric. Hydrolysis of procyanidin B2. | Stability of phenolic compounds in the stomach dependent on structure. | [68] |
| Purified Hesperidin 2S | Citrus sinsensis peel extract | Digestion in the simulator of human intestinal microbial ecosystem (pH 2.0). | Intact hesperidin 2S | No degradation of Hesperidin 2S. | Hesperidin is resistant to the degradation in the stomach. | [69] |
| Resveratrol caprylic esters | Synthesis product | Incubation in simulated gastric juice (pH 1.2) + pepsin at 37 °C for up to 2 h. | Intact resveratrol caprylic esters | No hydrolysis of resveratrol caprylic esters. | Resveratrol caprylic esters are not metabolized in the gastric phase. | [70] |
| Polyphenols | Simulated oral digestion of peeled apple tissue | Incubation in simulated gastric juice (pH 1.6) + pepsin at 37 °C for up to 1 h in a dynamic rat stomach wall model | Released from initial material in deceasing order: chlorogenic acid, epicatechin, catechin, procyanidin B2 flavan-3-ols, hydoxycinnamic acids, dihydrochalcones flavonols. | All polyphenols were stable except for procyanidin B2 that was hydrolyzed to epicatechin. | Polyphenol resistance to degradation dependent on structure. | [71] |
| Polyphenols | Simulated oral digestion of Kiwifruit tissue | Incubation in simulated gastric juice (pH 1.2) + pepsin at 37 °C for 2 h | Release of the 16 identified polyphenols from initial material during stomach digestion: catechin, epicatechin, quercetin, rutin, chlorogenic acid, caffeic acid, ferulic acid, p-coumaric acid, gallic acid, salicylic acid, vanillic acid. | All polyphenols were stable. | Polyphenol resistance to degradation. | [72] |
| Procyanidins oligomers and flavonol monomers | Cocoa | Oral administration of procyanidin oligomers and flavonol monomers to healthy subjects. Gastric contents collected and analyzed at 20 min. | Intact procyanidins oligomers and flavonol monomers | Stomach Procyanidins oligomer and flavonol monomer profiles similar to original product. | Procyanidins oligomers and flavonol monomers are stable in the gastric environment. | [73] |
| Caffeic acid, gallic acid, chlorogenic acid, ferulic acid, coumaric acid | Purchased purified phenolic acids | In vivo rat ligated pylorus model for in situ gastric digestion at 37 °C for 25 min. Portal vein and abdominal aorta blood collected. Plasma analyzed with and without sulfatase and β-glucuronidase treatment. | Intact and conjugated coumaric acid, ferulic acid, caffeic acid, gallic acid, chlorogenic acid | Rapid appearance of coumaric acid > ferulic acid > caffeic acid > gallic acid > chlorogenic acid in portal vein and abdominal artery. Rapid appearance mainly of coumaric and ferulic acid conjugates in portal vein and abdominal artery. | Differential absorption efficiency of phenolic acids and differential affinity of monocarboxylic acid transporters. | [74] |
| Flavone glycosides (apigenin, luteolin, chrysoeriol) and flavonoid glycosides (kaempferol, quercetin, isorhamnetin) | Parsley | Oral administration of glycoside extracts to rats. Animals sacrificed at 1, 1.5, 2, 4, or 12 h post administration. GI tract segmented (stomach, small intestine, colon, cecum). Stomach wall and lumen content analyzed at 2 h. | At 2 h: flavonoid glycosides in the stomach lumen and wall. Quercetin aglycone in the stomach wall. | At 2 h: flavonoid composition of stomach wall similar to stomach lumen but concentration lower. One aglycone present. | Stomach absorbs intact flavonoid glycosides. | [75] |
| Isoflavones (Daidzein, daidzin, genistein, and genistin) | Commercial source | In vivo rat ligated pylorus model for in situ gastric digestion at 37 °C for 25 min. Jugular vein blood analyzed for daidzein, daidzin, genistein, and genistin up to 30 min. | Daidzein and genetein (isoflavone aglycones) | Time-dependent absorption and transport of Daidzein and genetein, but not their respective glycosides daidzin and genistin. | Selective absorption and transport of isoflavone aglycones by the stomach. | [76] |
| Quercetin, rutin, and isoquercetin | Commercial source | In vivo rat ligated pylorus model for in situ gastric digestion at 37 °C for 30 min. Biliary duct cannulation and content analyzed. Aortic blood collected and analyzed. | Biliary quercetin and 3′-O-methyl-quercetin | Querecetin absorbed by the stomach and secreted in bile. No absorption of rutin or isoquercetin. | Limited role of the stomach in flavonoid glycosides. Selective absorption and transport of aglycones. | [77] |
| Substrates | Sources | Experimental Conditions | Main Observations | Conclusions | Ref |
|---|---|---|---|---|---|
| hydroxytyrosol, tyrosol, and oleuropein | Olive oil | Polyphenols added to the apical chamber and incubation for 2 h. Apical and basolateral compartments collected and analyzed. | ↓ hydroxytyrosol and tyrosol in the apical and ↑ in the basolateral media. Appearance of 3-O-methyl-tyrosol and glutathionyl-hydroxytyrosol in the apical and basolateral compartments. Φ transport of oleuropein from the apical to the basolateral compartment. | Hydroxytyrosol transported through the enterocyte apical membrane to the basolateral compartment with formation of conjugates. | [66] |
| Trans-piceid (Resveratrol 3-β-mono-d-glucoside) | V. Vinifera | Incubation with trans-piceid up to 360 min. | Bidirectional (apical to basolateral and inverse) transport of trans-piceid. Detectable trans-resveratrol in both chambers. | Trans-piceid and its aglycone are transported across the apical side and effluxed by the basolateral membrane. | [82] |
| Trans-piceid (Resveratrol 3-β-mono-d-glucoside) | V. Vinifera | Pre-incubated with ± chrysin (5,7-Dihydroxyflavone) and ± d-saccharic lactone in 6-well plates. Incubated with trans-piceid or trans- resveratrol 24 h. | Appearance of trans- Resveratrol after incubation with trans-piceid. ↑ trans- resveratrol-glucuronides production in chrysin (UDP-glucuronosyl transferase inducer) treated cells. | Trans-piceid undergoes hydrolysis to its aglycone and trans-resveratrol undergoes phase II metabolism within the enterocyte. | [82] |
| Quercetin, Quercetin-4-O-β-d-glucoside, Quercetin-3-O-β-d-glucoside, Quercetin-3,4-di-O-β-d-glucoside | Purified polyphenols | Polyphenols added to the apical chamber and incubation up to 2 h. Apical, cellular, and basolateral compartments collected. | Time-dependent ↓ quercetin in apical chamber. Stability of quercetin glycoside in apical chamber. Time-dependent appearance of quercetin glycosides in apical chamber and cellular compartment when quercetin is added. Time-dependent ↑ quercetin glycosides in basolateral compartment and stable low quercetin. | Quercetin aglycone is preferentially transported through the enterocyte apical membrane to the basolateral compartment after intracellular conjugation. | [84] |
| Catechin, epigallocatechin gallate encapsulated or not in non-ionic surfactant-based vesicles (niosomes) | Purified polyphenols | Polyphenols added to the apical chamber and incubation up to 6 h at 37 °C or 4 °C. Cell and basolateral compartments collected and analyzed. | Time-, concentration-, and temperature-dependent uptake of polyphenols. ↑ uptake when inserted into niosomes. Time-, concentration-, and temperature-dependent transport of polyphenol to the basolateral compartment, ↑ with niosomes. ↓ transport with ATP inhibitor, ↑transport with EDTA and P-glycoproteins and multidrug resistance proteins inhibitors. | Temperature dependence of uptake suggested energy-driven process. Deactivation of efflux pumps resulted in increased uptake by apical membrane and efflux in basolateral compartment. | [86] |
| Free and methyl esters of Hydroxycinnamic acids (ferulic, sinapic, p-coumaric and caffeic methyl esters), Ethyl esters of 5,5-diferulate, 8-O-4-diferulate, 8,5-benzofuran | Purified polyphenols | Medium collected after 24 h incubation and analyzed. | Glucuronides of ferulic, sinapic, p-coumaric and caffeic methyl esters, sulfates of ferulic, sinapic, p-coumaric and caffeic methyl esters, ferulic, sinapic, p-coumaric sulfates. | Metabolites produced either intra-cellularly and excreted in medium or produced in the medium by secreted phase I and phase II enzymes. | [90] |
| Quercetin, Quercetin-3,7,3,4-O-tetra-ethylacetate | Purified polyphenols | Polyphenols added to the apical (A) or basolateral (B) chamber and incubate up to 2 h. Apical, and basolateral compartments collected. | Time- and temperature-dependent bidirectional but preferential B-A transport of quercetin and ethylacetate derivative. Transport more efficient for the ethylacetate derivative. P-glycoproteins and multidrug resistance proteins inhibitors. ↑ quercetin permeation coefficient from A-B and ↓ from B-A. Φ on ethylacetate derivative. | Quercetin might be a substrate of P-glycoproteins and multidrug resistance proteins, causing the ↓ bioavailability of quercetin. Quercetin ethylacetate derivative exhibited better membrane permeation than parent compound. | [91] |
| Catechin, puerarin (Daidzein-8-C-glucoside) | Purified polyphenols | Incubation up to 2 h. | Time- and concentration-dependent uptake. Catechin enhanced uptake and transcellular transport of puerarin but puerarin inhibited that of catechin. P-glycoproteins and multidrug resistance proteins inhibitors. ↑ polyphenol uptake and transport. | Deactivation of efflux pumps resulted in increased uptake by apical membrane and efflux in basolateral compartment. | [92] |
| Polyphenols | Experimental Model | Tx Duration (Week) | Polyphenol Dosage | Epigenetic Modifications | Outcomes in Response to Polyphenols | Ref |
|---|---|---|---|---|---|---|
| Obesity and Insulin Resistance | ||||||
| Raspberry extract | HFD-fed mice | 16 | 120 mg/kg/d | ↑AT Histone methylation and acetylation | ↓Obesity ↓IR ↓Inflammation ↓Liver steatosis | [188] |
| Quercetin and Q2 derivative | HFD-fed rats | 12 | 0.26 mg/kg/d | ↑AT Histone methylation | ↓Obesity ↓IR ↓Dyslipidemia ↓Liver steatosis | [189] |
| Apples | HFHSD-fed rats | 8 | 700 mg/kg/d | ↑Methylation Aqp7 ↑PGC genes ↑Methylation leptin gene | ↓Obesity ↓IR ↑AT lipolysis | [190] |
| Hypertension | ||||||
| Cocoa | Humans Pre-hypertension or hypercholesterolemia | 2 | 6 g/d | ↓Leuk DNA methylation ↓ DNA Mtases methylation | ND | [191] |
| Resveratrol | Salt-sensitive hypertensive rats | 0–12 | 50 g/L drinking water | ↑histone H3K27me3 in renal aorta | Prevention of hypertension ↑Antioxidant defence | [192] |
| Polyphenols | Experimental Model/Conditions | Regulated miRNAs | Expression Pattern and Function | Ref |
|---|---|---|---|---|
| Cellular models | ||||
| Quercetin and Isorhamnetin | Pre LPS Tx stimulation of murine RAW 264.7 macrophages. Polyphenols (0, 10–100 µmol/L) | ↓miR-155 | ↓TNFα, ↓ iNOS, ↓IL-1β, ↓IL-6, ↓MIP1α, ↓NF-kB ↑Nrf2 and ↑ARE | [193] |
| Resveratrol | Human THP-1 cell line HPBMC | ↑miR-663 ↓miR-155 | ↓basal AP-1 and ↓LPS-induced AP-1 ↓ JunB/D mRNA | [194] |
| EGCG | IL-1β-stimulated human OA chondrocytes | ↑hsa-miR-199a-3p | ↓COX-2 mRNA/protein expression ↓PGE2 production | [195] |
| EVOO oleocanthal (OC) and oleacein (OA) secoiridoids | SGBS adipocytes pretreated with OC or OA before stimulation by TNFα | ↓miR-155-5p, ↓miR-34a-5p ↑let-7c-5p | ↓IL-1β, ↓COX-2, ↓MMP-2, ↓NF-kB, ↓NADPH oxidase ↑SOD and ↑GPx, ↑PPARγ ↓MCP-1, ↓CXCL-10, ↓M-CSF | [196] |
| Olive oil hydroxytyrosol (HT) | SGBS adipocytes pretreated with HT before stimulation by TNFα | ↓miR-155-5p, ↓miR-34a-5p ↑let-7c-5p | ↓MCP-1, ↓CXCL-10, ↓IL-1β, ↓IL-6, ↓vEGF, ↓COX-2, ↓M-CSF, ↓MMP-2, ↓NF-B and ↓ROS production ↑GPX ↑eNOS, ↑PGC-1α | [197] |
| Propolis extracts | HaCat cell line treated for 24 h with propolis extracts (3.125, 1.56, and 0.78 mg/mL) | ↑miR-19a-3p ↑ miR-203a-3p ↑miR-27a-3p ↓miR-17-3p | ↓TNFα mRNA ↓NFE2L2 mRNA ↑GPX2, ↑MnSOD and ↑TRXR2 mRNAs | [198] |
| Curcumin polyphenolic compound | ARPE-19 cells treated with 20 μΜ curcumin and 200 μΜ H2O2 | ↑miR-146a ↑miR-155 ↓miR-23b ↓miR-27b ↓miR-26b ↓miR-15b ↓miR-9 ↓miR-30b, miR-30e | ↓NF-κB ↑CAT, ↑GPx | [199] |
| Açai and red muscadine grape polyphenols | HUVEC ROS induction by 25 mM glucose for 30 min | ↑miR-126 ↑MiR146a | ↓IL-6, ↓IL-8, ↓NF-kB, ↓PXR, ↓VCAM-1 ↑CYP1A1, ↑MDRP1, ↑CAT, ↑GST activity | [200] |
| Resveratrol | LPS-stimulated THP-1 macrophages pretreated with resveratrol | ↑miR-Let7A | ↓TNFα, ↓IL-6, ↓IL-10, ↓IL-4, ↓SIRT1 mRNAs | [201] |
| Animal models | ||||
| Quercetin | Ctrl or HFD C57BL/6 J fed mice 0.2 or 2.0 mg/g diet | ↑miR-125b | ↓IL-6, ↓CRP, ↓MCP-1, ↓AOAH, ↓HO-1, ↓Ref-1, ↓TLR-2 mRNAs | [202] |
| Grape seed extract | HFD-fed obese Rats 30 mg/kg/d | ↓miR-33a, ↓miR-122 | ↓TC, ↓TAG, ↓LDL-C, ↓TNFα, ↓liver MDA ↑SOD, CAT; ↑liver GSH | [203] |
| Polydatin (3,4’,5-trihydroxy-stilbene-3-β-d-glucoside) | Crtl, or HFrD, HFrD+Polydatin (7.5, 15, 30 mg/kg), HFrD + PG (4 mg/kg) fed SD rats IG saline, polydatin or PG 7 week | ↓ miR200-a | ↓ TXNIP, ↓NLRP3, ↓ASC, ↓Casp-1, ↓ SREBP-1 and ↓SCD-1 ↑ PPAR-α and ↑CPT-1 | [204] |
| Tea extract | HFD-fed mice for 12 weeks 500 mg/kg/d | ↓miR-335 ↓ miR-155 in AT | ↓Obesity, ↓IR, ↓Inflammation, ↑Energy expenditure | [205] |
| Resveratrol | HFHS-fed rats for 6 weeks 30 mg/kg/d | ↑miR-211-3p ↑miR-1224 ↑miR-539-5p ↓AT miR-511-3p | ↓Obesity, ↓AT lipogenesis | [206] |
| Human studies | ||||
| Resveratrol | T2D HT patients. 1-year daily intake of grape extract (8.1 mg/d for first 6 months and 16.2 mg/d for last 6 months) | HPBMC ↑miR-21 ↑miR-181b ↑miR-663 ↑miR-30c2 | ↓IL-6, CCL-3, IL-1β, TNFα, ↑LRRFIP-1 | [207] |
| Polyphenols | Subclasses | Metabolites | Bacterial Catabolism | Metabolites Functions | Ref |
|---|---|---|---|---|---|
Flavonols  | Quercetin | 3,4-DHPAA 3-HPAA 4-HPAA | Clostridium orbiscidens Eubacterium oxidoreducens Eubacterium ramulus Enterococcus casseliflavus | Oxygen radical scavenging (all the metabolites), SOD- like activities (3,4 DHPAA), ↑glutathione S-transferase (3,4 DHPAA), ↑Nrf2-AhR (3,4 DHPAA) ↓Proinflammatory cytokines (3,4 DHPAA) ↑Glucose induced-insulin secretion (3,4 DHPAA) ↑Function and survival of pancreatic β-cells (3,4 DHPAA) Protective effect against OxS induced-endothelial dysfunction (3,4 DHPAA) | [132,208,209,210,211,212,213,214,215,216,217,218,219,220] |
Flavones | Apigenin | Phloretin 3-HPPA 4-HPPA 4-HCA | Clostridium orbiscindens | ↓Oxygen radical scavenging (3-HPPA) ↓Proinflammatory cytokines (3-HPPA) ↑Glucose induced-insulin secretion (3-HPPA) ↑Function and survival of pancreatic β-cells (3-HPPA) Protective effect against OxS induced-endothelial dysfunction (3-HPPA) | [132,208,209,212,213,214,215,221] |
Flavanones  | Naringenin | 3,4-DHPPA 3-HPPA 4-HPPA | Clostridium strains Eubacterium ramulus | ↓Oxygen radical scavenging (3-HPPA) ↓Proinflammatory; 3,4 DHPPA) ↑Glucose induced-insulin secretion (3-HPPA) Protective effect against OxS induced-endothelial dysfunction (3-HPPA) | [34,60,209,212,213,214,215,217,222,223] |
Isoflavones  | Daidzein | (S)-Equol O-DMA | Bacteroides ovatus, Streptococcus intermedius, Ruminococcus productus, Eggerthella sp.Julong 732, Enterococcus faecium EPI1, Lactobacillus mucosae EPI2, Finegoldia magna EPI3 Clostridium spp. HGHA136 | Stimulation of cellular antioxidant systems ↑Catalase and SOD activity Anti-atherogenic effect | [224,225,226] |
Flavan-3-ols  | Monomers (catechins, epicatechins) and proanthocyanidins | 3-HPPA 3,4-DHPPA 3′,4′-DHPVL 3,4-DHPVA 3′-HPVL 3′,4′,5′-THPVL 3′,5′-DHPVL | Clostridium coccoides, Bifidobacterium spp. Eggerthella lenta Flavonifractor plautii | ↓Oxygen radical scavenging (3-HPPA) ↓ROS generation (3′-HPVL, 3′,4′-DHPVL) ↓NF-κB transcriptional activity ↓NO synthesis (3′,4′,5′-THPVL; 3′,4′-DHPVL) ↓iNOS expression (3′,4′-DHPVL) Maintenance of endothelial homeostasis and functions (3′,4′-DHPVL): ↓Endothelial adhesion (3′,4′-DHPVL) ↓VCAM1 and MCP1 (3′,4′-DHPVL) ↓Systolic blood pressure (3′,4′,5′-THPVL; 3′,5′-DHPVL) | [87,92,109,131,227,228,229,230,231,232,233,234,235,236] |
Anthocyanins  | Cyanidin Peonidin Pelargonidin Malvidin Delphinidin | Protocatechuic acid Vanillic acid 4-Hydroxybenzoic acid Syringic acid Gallic acid | Lactobacillus plantarum, Lactobacillus casei Lactobacillus acidophilus LA-5 Bifidobacterium lactis BB-12 | Antidiabetic activities due to their antioxidant capacity ↓DNA damages, ↓ROS production ↑Cellular glutathione level, ↑glucose uptake by HepG2 and human skeletal cells, ↑glycogen production by HepG2 cells, ↑Mitochondria homeostasis | [237,238,239,240,241,242] |
Hydroxycinnamic acids  | Chlorogenic acids | 3-HPPA 3,4-DHPPA Caffeic acid | Escherichia coli, Bifidobacterium lactis, Lactobacillus gasseri | ↓Oxygen radical scavenging(3-HPPA) ↓Proinflammatory cytokines (3-HPPA; 3,4 DHPPA) Antidiabetic activities due to its antioxidant capacity (caffeic acid): ↑Cellular glutathione level ↓DNA damages ↓Cytotoxicity, ↓ROS production ↑Glucose consumption ↑Glycogen production | [209,212,214,215,243,244] |
Hydrolyzables tannins | Ellagitannins | Ellagic acid Urolithin A Urolithin B | Butyrivibrio spp. | ↓Intracellular ROS accumulation (Urolithin A) ↓Cellular injury by ROS ↓Proinflammatory mediators (Ellagic acid and Urolithin A) ↓NADPH oxidase activation (Urolithin A) ↓PGE2 production (Urolithin A and B) ↓mPGES-1 and COX-2 expression (Urolithin A and B) ↓Proteins glycation (Urolithin A and B) ↓Triglycerides accumulation (Ellagic acid and Urolithin A) ↓Expression of adipogenic protein and gene (Urolithin A) ↑Fatty acid β-oxidation (Urolithin A) Alleviation of myocardial ischemia/reperfusion injury (Urolithin A) | [245,246,247,248,249,250,251,252,253,254,255,256,257] |
Lignans  | Secoisolariciresinol | Enterodiol Enterolactone | Bacteroides distasonis, Bacteroides fragilis, Bacteroides ovatus, Clostridium cocleatum, Butyribacterium methylotrophicum, Eubacterium callanderi, Eubacterium limosum, Peptostreptococcus productus, Clostridium scindens, Eggerthella lenta | Antioxidant capacity OH-scavenging activity Immunomodulatory effects in human cells ↓NF-κB transcriptional activity ↓Proinflammatory cytokines expression | [258,259,260,261] |
Stilbenes  | Trans-resveratrol | DHR 3,4′-dihydroxy-trans-stilbene 3,4′-dihydroxybibenzyl (lunularin) | Slackia equolifaciens Adlercreutzia equolifaciens | Antioxidant activity Free radical scavenging (DHR) ↓NO production (DHR) | [262,263,264] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants 2020, 9, 982. https://doi.org/10.3390/antiox9100982
Koudoufio M, Desjardins Y, Feldman F, Spahis S, Delvin E, Levy E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants. 2020; 9(10):982. https://doi.org/10.3390/antiox9100982
Chicago/Turabian StyleKoudoufio, Mireille, Yves Desjardins, Francis Feldman, Schohraya Spahis, Edgard Delvin, and Emile Levy. 2020. "Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders?" Antioxidants 9, no. 10: 982. https://doi.org/10.3390/antiox9100982
APA StyleKoudoufio, M., Desjardins, Y., Feldman, F., Spahis, S., Delvin, E., & Levy, E. (2020). Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants, 9(10), 982. https://doi.org/10.3390/antiox9100982