Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders
Abstract
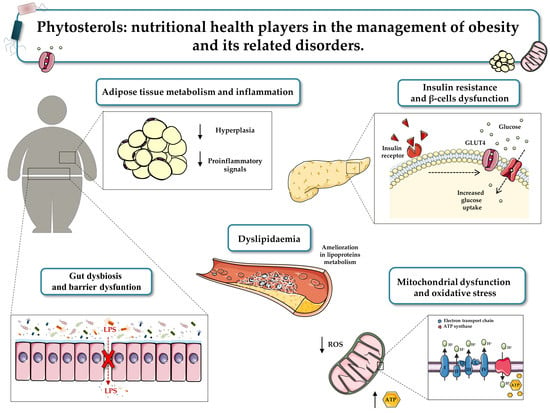
:1. Introduction
2. Phytosterols in Human Nutrition
2.1. Effects of Phytosterols on Adipose Tissue Metabolism
2.2. Effects of Phytosterols on Adipose Tissue Inflammation
2.3. Effects of Phytosterols on Oxidative Stress
2.4. Effects of Phytosterols on Blood Glucose and Insulin Resistance
2.5. Effects of Phytosterols on Obesity-Related Dyslipidaemia
2.6. Effects of Phytosterols on Gut Microbiota
3. Conclusions
4. Survey Methodology
Study Selection Criteria
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 September 2020).
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Das, U.N. Obesity: Genes, brain, gut, and environment. Nutrition 2010, 26, 459–473. [Google Scholar] [CrossRef]
- Ding, S.; Chi, M.M.; Scull, B.P.; Rigby, R.; Schwerbrock, N.M.; Magness, S.; Jobin, C.; Lund, P.K. High-fat diet: Bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 2010, 5, e12191. [Google Scholar] [CrossRef] [Green Version]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef]
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, T.F.; Collado, M.C.; Ferreira, C.L.; Bressan, J.; Peluzio Mdo, C. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr. Res. 2012, 32, 637–647. [Google Scholar] [CrossRef] [PubMed]
- West, D.S.; Coulon, S.M.; Monroe, C.M.; Wilson, D.K. Evidence-based lifestyle interventions for obesity and type 2 diabetes: The look AHEAD intensive lifestyle intervention as exemplar. Am. Psychol. 2016, 71, 614–627. [Google Scholar] [CrossRef]
- Goswami, G.; Shinkazh, N.; Davis, N. Optimal pharmacologic treatment strategies in obesity and type 2 diabetes. J. Clin. Med. 2014, 3, 595–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glandt, M.; Raz, I. Present and future: Pharmacologic treatment of obesity. J. Obes. 2011, 2011, 636181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalra, E.K. Nutraceutical—Definition and introduction. AAPS PharmSci 2003, 5, 27–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Sun, M.; Yao, H.; Liu, Y.; Gao, R. Herbal medicine for the treatment of obesity: An overview of scientific evidence from 2007 to 2017. Evid. Based Complement. Altern. Med. 2017, 2017, 8943059. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Gomez, C.I.; Rocha-Guzman, N.E.; Gallegos-Infante, J.A.; Moreno-Jimenez, M.R.; Vazquez-Cabral, B.D.; Gonzalez-Laredo, R.F. Plants with potential use on obesity and its complications. EXCLI J. 2015, 14, 809–831. [Google Scholar] [CrossRef] [PubMed]
- Pollak, O.J. Reduction of blood cholesterol in man. Circulation 1953, 7, 702–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srigley, C.T.; Haile, E.A. Quantification of plant sterols/stanols in foods and dietary supplements containing added phytosterols. J. Food Compos. Anal. 2015, 40, 163–176. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Demonty, I. Phytosterols: Natural compounds with established and emerging health benefits. Oléagineux Corps Gras Lipid. 2007, 14, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Xue, L.; Zhang, L.; Wang, X.; Qi, X.; Jiang, J.; Yu, L.; Wang, X.; Zhang, W.; Zhang, Q.; et al. Phytosterol contents of edible oils and their contributions to estimated phytosterol intake in the Chinese diet. Foods 2019, 8, 334. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Escrig, A.; Santos-Hidalgo, A.B.; Saura-Calixto, F. Common sources and estimated intake of plant sterols in the Spanish diet. J. Agric. Food Chem. 2006, 54, 3462–3471. [Google Scholar] [CrossRef] [Green Version]
- Zarrouk, A.; Martine, L.; Gregoire, S.; Nury, T.; Meddeb, W.; Camus, E.; Badreddine, A.; Durand, P.; Namsi, A.; Yammine, A.; et al. Profile of fatty acids, tocopherols, phytosterols and polyphenols in Mediterranean oils (argan oils, olive oils, milk thistle seed oils and nigella seed oil) and evaluation of their antioxidant and cytoprotective activities. Curr. Pharm. Des. 2019, 25, 1791–1805. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Blanchard, C.L. Q-TOF LC/MS identification and UHPLC-online ABTS antioxidant activity guided mapping of barley polyphenols. Food Chem. 2018, 266, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.M.; Fonseca, F.A.; Ballus, C.A.; Figueiredo-Neto, A.M.; Meinhart, A.D.; de Godoy, H.T.; Izar, M.C. Common sources and composition of phytosterols and their estimated intake by the population in the city of Sao Paulo, Brazil. Nutrition 2013, 29, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.; Vilela, C.; Camacho, J.F.; Cordeiro, N.; Gouveia, M.; Freire, C.S.; Silvestre, A.J. Profiling of lipophilic and phenolic phytochemicals of four cultivars from cherimoya (Annona cherimola Mill.). Food Chem. 2016, 211, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.; Louvet, F.; Tarrade, S.; Meudec, E.; Grenier, K.; Landolt, C.; Ouk, T.S.; Bressollier, P. Enzyme-assisted extraction of bioactive compounds from Raspberry (Rubus idaeus L.) pomace. J. Food Sci. 2019, 84, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Segura, R.; Javierre, C.; Lizarraga, M.A.; Ros, E. Other relevant components of nuts: Phytosterols, folate and minerals. Br. J. Nutr. 2006, 96, S36–S44. [Google Scholar] [CrossRef] [Green Version]
- Ras, R.T.; Trautwein, E.A. Consumer purchase behaviour of foods with added phytosterols in six European countries: Data from a post-launch monitoring survey. Food Chem. Toxicol. 2017, 110, 42–48. [Google Scholar] [CrossRef]
- Lambert, C.; Cubedo, J.; Padro, T.; Sanchez-Hernandez, J.; Antonijoan, R.M.; Perez, A.; Badimon, L. Phytosterols and omega 3 supplementation exert novel regulatory effects on metabolic and inflammatory pathways: A proteomic study. Nutrients 2017, 9, 599. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.B. Plant sterols and stanols: Their role in health and disease. J. Clin. Lipidol. 2008, 2, S11–S19. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, I.; Perdicaro, D.J.; Brown, A.W.; Hammons, S.; Carden, T.J.; Carr, T.P.; Eskridge, K.M.; Walter, J. Diet-induced alterations of host cholesterol metabolism are likely to affect the gut microbiota composition in hamsters. Appl. Environ. Microbiol. 2013, 79, 516–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A. Sitosterol as an antioxidant in frying oils. Food Chem. 2013, 137, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of metabolic syndrome and dietary intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef] [Green Version]
- Ordovas, J.M.; Corella, D. Metabolic syndrome pathophysiology: The role of adipose tissue. Kidney Int. Suppl. 2008, 74, S10–S14. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, D.A.; Puglisi, M.J.; Hasty, A.H. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr. Diabetes Rep. 2009, 9, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Haczeyni, F.; Bell-Anderson, K.S.; Farrell, G.C. Causes and mechanisms of adipocyte enlargement and adipose expansion. Obes. Rev. 2018, 19, 406–420. [Google Scholar] [CrossRef]
- Rasouli, N. Adipose tissue hypoxia and insulin resistance. J. Investig. Med. 2016, 64, 830–832. [Google Scholar] [CrossRef]
- Boden, G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997, 46, 3–10. [Google Scholar] [CrossRef]
- Kelley, D.E.; Mokan, M.; Simoneau, J.A.; Mandarino, L.J. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J. Clin. Investig. 1993, 92, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Suganami, T.; Nishida, J.; Ogawa, Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: Role of free fatty acids and tumor necrosis factor alpha. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Charles, M.A.; Fontbonne, A.; Thibult, N.; Claude, J.R.; Warnet, J.M.; Rosselin, G.; Ducimetiere, P.; Eschwege, E. High plasma nonesterified fatty acids are predictive of cancer mortality but not of coronary heart disease mortality: Results from the Paris Prospective Study. Am. J. Epidemiol. 2001, 153, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Maison, P.; Halsall, D.; Martensz, N.; Hales, C.N.; Wareham, N.J. Cross-sectional but not longitudinal associations between non-esterified fatty acid levels and glucose intolerance and other features of the metabolic syndrome. Diabet. Med. 1999, 16, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Daquinag, A.C.; Zhang, Y.; Kolonin, M.G. Vascular targeting of adipose tissue as an anti-obesity approach. Trends Pharm. Sci. 2011, 32, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Phelan, S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 2005, 82, 222S–225S. [Google Scholar] [CrossRef] [PubMed]
- Thornton, S.J.; Wong, I.T.; Neumann, R.; Kozlowski, P.; Wasan, K.M. Dietary supplementation with phytosterol and ascorbic acid reduces body mass accumulation and alters food transit time in a diet-induced obesity mouse model. Lipids Health Dis. 2011, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Rideout, T.C.; Harding, S.V.; Jones, P.J. Consumption of plant sterols reduces plasma and hepatic triglycerides and modulates the expression of lipid regulatory genes and de novo lipogenesis in C57BL/6J mice. Mol. Nutr. Food Res. 2010, 54, S7–S13. [Google Scholar] [CrossRef]
- Bhaskaragoud, G.; Rajath, S.; Mahendra, V.P.; Kumar, G.S.; Gopala Krishna, A.G.; Kumar, G.S. Hypolipidemic mechanism of oryzanol components- ferulic acid and phytosterols. Biochem. Biophys. Res. Commun. 2016, 476, 82–89. [Google Scholar] [CrossRef]
- Lee, J.A.; Cho, Y.R.; Hong, S.S.; Ahn, E.K. Anti-obesity activity of saringosterol isolated from Sargassum muticum (Yendo) fensholt extract in 3T3-L1 cells. Phytother. Res. 2017, 31, 1694–1701. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, A.B.; Begdache, L.A.; Fink, C.S. Effect of sterols and fatty acids on growth and triglyceride accumulation in 3T3-L1 cells. J. Nutr. Biochem. 2000, 11, 153–158. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.A.; Kang, M.J.; Choi, J.S.; Kim, G.D. Fucosterol, isolated from Ecklonia stolonifera, inhibits adipogenesis through modulation of FoxO1 pathway in 3T3-L1 adipocytes. J. Pharm. Pharmacol. 2017, 69, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Oh, G.H.; Kim, M.B.; Hwang, J.K. Fucosterol inhibits adipogenesis through the activation of AMPK and Wnt/β-catenin signaling pathways. Food Sci. Biotechnol. 2017, 26, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Tomono, Y.; Ito, K.; Furutani, N.; Yoshida, H.; Tada, N. Diacylglycerol oil for the metabolic syndrome. Nutr. J. 2007, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Li, C.L.; Li, R.; Chen, Y.; Zhang, M.; Guo, P.P.; Shi, D.; Ji, X.N.; Feng, R.N.; Sun, C.H. Associations of dietary phytosterols with blood lipid profiles and prevalence of obesity in Chinese adults, a cross-sectional study. Lipids Health Dis. 2018, 17, 54. [Google Scholar] [CrossRef] [Green Version]
- Ghaedi, E.; Varkaneh, H.K.; Rahmani, J.; Mousavi, S.M.; Mohammadi, H.; Fatahi, S.; Pantovic, A.; Darooghegi Mofrad, M.; Zhang, Y. Possible anti-obesity effects of phytosterols and phytostanols supplementation in humans: A systematic review and dose-response meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1246–1257. [Google Scholar] [CrossRef]
- Amiot, M.J.; Knol, D.; Cardinault, N.; Nowicki, M.; Bott, R.; Antona, C.; Borel, P.; Bernard, J.P.; Duchateau, G.; Lairon, D. Phytosterol ester processing in the small intestine: Impact on cholesterol availability for absorption and chylomicron cholesterol incorporation in healthy humans. J. Lipid Res. 2011, 52, 1256–1264. [Google Scholar] [CrossRef] [Green Version]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Unamuno, X.; Gomez-Ambrosi, J.; Rodriguez, A.; Becerril, S.; Fruhbeck, G.; Catalan, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef] [Green Version]
- Haase, J.; Weyer, U.; Immig, K.; Kloting, N.; Bluher, M.; Eilers, J.; Bechmann, I.; Gericke, M. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia 2014, 57, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Kintscher, U.; Hartge, M.; Hess, K.; Foryst-Ludwig, A.; Clemenz, M.; Wabitsch, M.; Fischer-Posovszky, P.; Barth, T.F.; Dragun, D.; Skurk, T.; et al. T-lymphocyte infiltration in visceral adipose tissue: A primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1304–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, T.; Craig, C.; Liu, L.F.; Perelman, D.; Allister, C.; Spielman, D.; Cushman, S.W. Adipose cell size and regional fat deposition as predictors of metabolic response to overfeeding in insulin-resistant and insulin-sensitive humans. Diabetes 2016, 65, 1245–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005, 87, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.C.; Lai, M.H.; Hsu, K.P.; Kuo, Y.H.; Chen, J.; Tsai, M.C.; Li, C.X.; Yin, X.J.; Jeyashoke, N.; Chao, L.K. Identification of beta-Sitosterol as in vitro anti-inflammatory constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Eigenbrod, T.; Munoz-Planillo, R.; Nunez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.S.; West, A.P.; Ghosh, S. NF-κB and the immune response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef] [Green Version]
- Catrysse, L.; van Loo, G. Inflammation and the metabolic syndrome: The tissue-specific functions of NF-κB. Trends Cell Biol. 2017, 27, 417–429. [Google Scholar] [CrossRef]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Feng, J.; Lu, S.; Ou, B.; Liu, Q.; Dai, J.; Ji, C.; Zhou, H.; Huang, H.; Ma, Y. The role of JNk signaling pathway in obesity-driven insulin resistance. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; Rondinone, C.M. Pharmacological inhibition of p38 MAP kinase results in improved glucose uptake in insulin-resistant 3T3-L1 adipocytes. Metabolism Clin. Exp. 2005, 54, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Lee, I.A.; Gu, W.; Hyam, S.R.; Kim, D.H. β-Sitosterol attenuates high-fat diet-induced intestinal inflammation in mice by inhibiting the binding of lipopolysaccharide to toll-like receptor 4 in the NF-κB pathway. Mol. Nutr. Food Res. 2014, 58, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.S.; Shin, J.S.; Choi, H.E.; Cho, Y.W.; Bang, M.H.; Baek, N.I.; Lee, K.T. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-κB and p38 mitogen-activated protein kinase in RAW264.7 macrophages. Food Chem. 2012, 135, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.W.; Kuo, Y.H.; Lin, B.F. Anti-inflammatory activity of new compounds from Andrographis paniculata by NF-κB transactivation inhibition. J. Agric. Food Chem. 2010, 58, 2505–2512. [Google Scholar] [CrossRef]
- Valerio, M.; Awad, A.B. β-Sitosterol down-regulates some pro-inflammatory signal transduction pathways by increasing the activity of tyrosine phosphatase SHP-1 in J774A.1 murine macrophages. Int. Immunopharmacol. 2011, 11, 1012–1017. [Google Scholar] [CrossRef]
- Kurano, M.; Hasegawa, K.; Kunimi, M.; Hara, M.; Yatomi, Y.; Teramoto, T.; Tsukamoto, K. Sitosterol prevents obesity-related chronic inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 191–198. [Google Scholar] [CrossRef]
- Satheesh, G.; Ramachandran, S.; Jaleel, A. Metabolomics-based prospective studies and prediction of type 2 diabetes mellitus risks. Metab. Syndr. Relat. Disord. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Turner, N. Mitochondrial dysfunction and insulin resistance: An update. Endocr. Connect. 2015, 4, R1–R15. [Google Scholar] [CrossRef] [Green Version]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid. Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Grune, T.; Speckmann, B. The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol. Chem. 2016, 397, 709–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, G.; Lindroos-Christensen, J.; Einwallner, E.; Husa, J.; Zapf, T.C.; Lipp, K.; Rauscher, S.; Groger, M.; Spittler, A.; Loewe, R.; et al. HO-1 inhibits preadipocyte proliferation and differentiation at the onset of obesity via ROS dependent activation of Akt2. Sci. Rep. 2017, 7, 40881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Hartigh, L.J.; Omer, M.; Goodspeed, L.; Wang, S.; Wietecha, T.; O’Brien, K.D.; Han, C.Y. Adipocyte-specific deficiency of NADPH oxidase 4 delays the onset of insulin resistance and attenuates adipose tissue inflammation in obesity. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 466–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefranc, C.; Friederich-Persson, M.; Palacios-Ramirez, R.; Nguyen Dinh Cat, A. Mitochondrial oxidative stress in obesity: Role of the mineralocorticoid receptor. J. Endocrinol. 2018, 238, R143–R159. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar]
- Pallavi, M.; Suchitra, M.; Alok Sachan, L.A.; Srinivasa Rao, P. Role of adipokines, oxidative stress, and endotoxins in the pathogenesis of non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. Int. J. Res. Med. Sci. 2019, 7, 1644. [Google Scholar]
- Han, C.Y. Roles of reactive oxygen species on insulin resistance in adipose tissue. Diabetes Metab. J. 2016, 40, 272–279. [Google Scholar] [CrossRef]
- Cruz, C.M.; Rinna, A.; Forman, H.J.; Ventura, A.L.; Persechini, P.M.; Ojcius, D.M. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 2007, 282, 2871–2879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, M.; Apostolova, N.; Diaz-Rua, R.; Muntane, J.; Victor, V.M. Mitochondria and T2D: Role of autophagy, ER stress, and inflammasome. Trends Endocrinol. Metab. 2020, 31, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.B.M.; Konings, M.; Schaart, G.; Groen, A.K.; Lutjohann, D.; van Marken Lichtenbelt, W.D.; Schrauwen, P.; Plat, J. In vitro effects of sitosterol and sitostanol on mitochondrial respiration in human brown adipocytes, myotubes and hepatocytes. Eur. J. Nutr. 2020, 59, 2039–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, H.S.; Chen, N.; Leong, P.K.; Ko, K.M. β-Sitosterol enhances cellular glutathione redox cycling by reactive oxygen species generated from mitochondrial respiration: Protection against oxidant injury in H9c2 cells and rat hearts. Phytother. Res. 2014, 28, 999–1006. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, A.K.; Dobhal, M.P.; Sharma, M.C.; Gupta, R.S. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, M.; Moreno, J.J. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic. Biol. Med. 2005, 39, 91–97. [Google Scholar] [CrossRef]
- Ward, M.G.; Li, G.; Barbosa-Lorenzi, V.C.; Hao, M. Stigmasterol prevents glucolipotoxicity induced defects in glucose-stimulated insulin secretion. Sci. Rep. 2017, 7, 9536. [Google Scholar] [CrossRef] [Green Version]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- Reaven, G.; Abbasi, F.; McLaughlin, T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog. Horm. Res. 2004, 59, 207–223. [Google Scholar] [CrossRef]
- Polsky, S.; Ellis, S.L. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 277–282. [Google Scholar] [CrossRef]
- Leguisamo, N.M.; Lehnen, A.M.; Machado, U.F.; Okamoto, M.M.; Markoski, M.M.; Pinto, G.H.; Schaan, B.D. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc. Diabetol. 2012, 11, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eid, H.M.; Haddad, P.S. The antidiabetic potential of quercetin: Underlying mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghaalipour, F.; Khalilpourfarshbafi, M.; Arya, A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. Int. J. Biol. Sci. 2015, 11, 508–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Huang, M.; Yang, J.; Ma, X.; Zheng, S.; Deng, S.; Huang, Y.; Yang, X.; Zhao, P. Anti-diabetic activity of stigmasterol from soybean oil by targeting the GLUT4 glucose transporter. Food Nutr. Res. 2017, 61, 1364117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivorra, M.; D’Ocon, M.; Paya, M.; Villar, A. Antihyperglycemic and insulin-releasing effects of β-sitosterol 3-β-D-glucoside and its aglycone, β-sitosterol. Arch. Int. Pharmacodyn. Thér. 1988, 296, 224. [Google Scholar] [PubMed]
- Ivorra, M.; Paya, M.; Villar, A. Effect of β-sitosterol-3-β-D-glucoside on insulin secretion in vivo in diabetic rats and in vitro in isolated rat islets of Langerhans. Die Pharm. 1990, 45, 271. [Google Scholar]
- Chai, J.-W.; Lim, S.-L.; Kanthimathi, M.; Kuppusamy, U.R. Gene regulation in β-sitosterol-mediated stimulation of adipogenesis, glucose uptake, and lipid mobilization in rat primary adipocytes. Genes Nutr. 2011, 6, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.L.; Kim, H.N.; Jung, H.H.; Kim, J.E.; Choi, D.K.; Hur, J.M.; Lee, J.Y.; Song, H.; Song, K.S.; Huh, T.L. Beneficial effects of β-sitosterol on glucose and lipid metabolism in L6 myotube cells are mediated by AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2008, 377, 1253–1258. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [Green Version]
- Helkin, A.; Stein, J.J.; Lin, S.; Siddiqui, S.; Maier, K.G.; Gahtan, V. Dyslipidemia part 1—Review of lipid metabolism and vascular cell physiology. Vasc. Endovasc. Surg. 2016, 50, 107–118. [Google Scholar] [CrossRef]
- Schofield, J.D.; Liu, Y.; Rao-Balakrishna, P.; Malik, R.A.; Soran, H. Diabetes dyslipidemia. Diabetes Ther. 2016, 7, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metab. Clin. Exp. 2019, 92, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Esteve, E.; Ricart, W.; Fernandez-Real, J.M. Dyslipidemia and inflammation: An evolutionary conserved mechanism. Clin. Nutr. 2005, 24, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Alphonse, P.A.; Ramprasath, V.; Jones, P.J. Effect of dietary cholesterol and plant sterol consumption on plasma lipid responsiveness and cholesterol trafficking in healthy individuals. Br. J. Nutr. 2017, 117, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinowski, J.M.; Gehret, M.M. Phytosterols for dyslipidemia. Am. J. Health Syst. Pharm. 2010, 67, 1165–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas, S.A.; Sichieri, R.; Moreira, A.S.B.; Souza, D.O.; Cabral, C.T.F.; Gianinni, D.T.; Cunha, D.B. Phytosterol-enriched milk lowers LDL-cholesterol levels in Brazilian children and adolescents: Double-blind, cross-over trial. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.A.; Puska, P.; Gylling, H.; Vanhanen, H.; Vartiainen, E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N. Engl. J. Med. 1995, 333, 1308–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellegard, L.H.; Andersson, S.W.; Normen, A.L.; Andersson, H.A. Dietary plant sterols and cholesterol metabolism. Nutr. Rev. 2007, 65, 39–45. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Duchateau, G.S.; Lin, Y.; Mel’nikov, S.M.; Molhuizen, H.O.; Ntanios, F.Y. Proposed mechanisms of cholesterol-lowering action of plant sterols. Eur. J. Lipid Sci. Technol. 2003, 105, 171–185. [Google Scholar] [CrossRef]
- De Smet, E.; Mensink, R.P.; Plat, J. Effects of plant sterols and stanols on intestinal cholesterol metabolism: Suggested mechanisms from past to present. Mol. Nutr. Food Res. 2012, 56, 1058–1072. [Google Scholar] [CrossRef]
- Cox, A.; West, N. Cripps AWJTLD. Endocrinology obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federico, A.; Dallio, M.; Di Sarno, R.; Giorgio, V.; Miele, L. Gut microbiota, obesity and metabolic disorders. Minerva Gastroenterol. Dietol. 2017, 63, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [Green Version]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [Green Version]
- Castaner, O.; Goday, A.; Park, Y.M.; Lee, S.H.; Magkos, F.; Shiow, S.T.E.; Schroder, H. The gut microbiome profile in obesity: A systematic review. Int. J. Endocrinol. 2018, 2018, 4095789. [Google Scholar] [CrossRef]
- Davis, C.D. The gut microbiome and its role in obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.H.; Wu, C.Y. The gut microbiome in obesity. J. Formos. Med. Assoc. Taiwan Yi Zhi 2019, 118, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, B.; Gewirtz, A.T. Has provoking microbiota aggression driven the obesity epidemic? BioEssays 2016, 38, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Andriamiarina, R.; Laraki, L.; Pelletier, X.; Debry, G. Effects of stigmasterol-supplemented diets on fecal neutral sterols and bile acid excretion in rats. Ann. Nutr. Metab. 1989, 33, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Quilliot, D.; Boman, F.; Creton, C.; Pelletier, X.; Floquet, J.; Debry, G. Phytosterols have an unfavourable effect on bacterial activity and no evident protective effect on colon carcinogenesis. Eur. J. Cancer Prev. 2001, 10, 237–243. [Google Scholar] [CrossRef] [PubMed]

| Source | Phytosterols Content (mg/100 g Dry Product) | Reference | |
|---|---|---|---|
| Oils | |||
| Refined olive oil | 235.9 | [21] | |
| Virgin olive oil | 259.7 | [21] | |
| Argan oil | 188.1 | [22] | |
| Sunflower oil | 492.5 | [21] | |
| Vegetables | |||
| Artichoke | 48.5 | [21] | |
| Green asparagus | 10.6 | [21] | |
| Green beans | 18.8 | [21] | |
| Broccoli | 18.3 | [21] | |
| Cabbage | 27.4 | [21] | |
| Carrot | 18.6 | [21] | |
| Cauliflower | 44.3 | [21] | |
| Celery | 7.8 | [21] | |
| Chard | 16.6 | [21] | |
| Cucumber | 7 | [21] | |
| Eggplant | 5.9 | [21] | |
| Endive | 16.9 | [21] | |
| Escarole | 18.1 | [21] | |
| Garlic | 18.2 | [21] | |
| Leek | 11.7 | [21] | |
| Lettuce | 13.5 | [21] | |
| Marrow | 2.4 | [21] | |
| Onion | 7.2 | [21] | |
| Parsley | 7.4 | [21] | |
| Potato | 4.3 | [21] | |
| Green pepper | 9.4 | [21] | |
| Red pepper | 7.4 | [21] | |
| Spinach | 16.3 | [21] | |
| Tomato | 9.9 | [21] | |
| Cereals | |||
| Rice | 29 | [21] | |
| White wheat | 41.9 | [21] | |
| Wheat grain | 315.7 | [21] | |
| Wheat bran | 459 | [21] | |
| Wheat flour | 140 | [21] | |
| Barley | 130.8 | [23] | |
| Rice bran | 450 | [21] | |
| Corn bran | 300 | [21] | |
| Oat bran | 150 | [21] | |
| Legumes | |||
| Chickpea | 121.1 | [21] | |
| Lentil | 117.3 | [21] | |
| White bean | 108.1 | [21] | |
| Peanuts | 406 | [24] | |
| Fruit | |||
| Apple | 16 | [21] | |
| Apricot | 15.2 | [21] | |
| Banana | 20.1 | [21] | |
| Cherry | 20.1 | [21] | |
| White grape | 32.6 | [21] | |
| Kiwi | 7.1 | [21] | |
| Melon | 3.3 | [21] | |
| Olive | 37.7 | [21] | |
| Orange | 30.4 | [21] | |
| Peach | 14.6 | [21] | |
| Pear | 11 | [21] | |
| Plum | 18.9 | [21] | |
| Strawberry | 11.3 | [21] | |
| Watermelon | 4.5 | [21] | |
| Avocado | 25.5 | [25] | |
| Pineapple | 4.9 | [25] | |
| Apple | 5.31 | [25] | |
| Custard apple | 62.3 | [26] | |
| Raspberry | 25 | [27] | |
| Nuts | |||
| Almond | 148.6 | [21] | |
| Hazelnut | 128.1 | [21] | |
| Peanut | 143.6 | [21] | |
| Pistachio | 242.7 | [21] | |
| Sunflower seed | 226.9 | [21] | |
| Walnut | 131.3 | [21] | |
| Brazil nuts | 95 | [28] | |
| Cashews | 150 | [28] | |
| Macadamia nuts | 187 | [28] | |
| Pecans | 157 | [28] | |
| Pine nuts | 236 | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vezza, T.; Canet, F.; de Marañón, A.M.; Bañuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants 2020, 9, 1266. https://doi.org/10.3390/antiox9121266
Vezza T, Canet F, de Marañón AM, Bañuls C, Rocha M, Víctor VM. Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants. 2020; 9(12):1266. https://doi.org/10.3390/antiox9121266
Chicago/Turabian StyleVezza, Teresa, Francisco Canet, Aranzazu M. de Marañón, Celia Bañuls, Milagros Rocha, and Víctor Manuel Víctor. 2020. "Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders" Antioxidants 9, no. 12: 1266. https://doi.org/10.3390/antiox9121266
APA StyleVezza, T., Canet, F., de Marañón, A. M., Bañuls, C., Rocha, M., & Víctor, V. M. (2020). Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants, 9(12), 1266. https://doi.org/10.3390/antiox9121266