Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
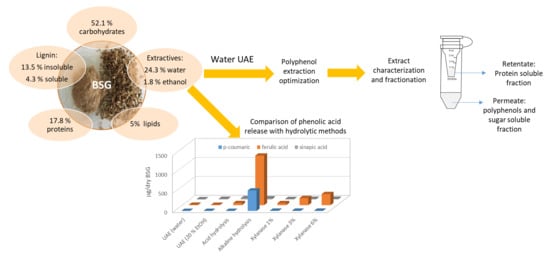
2.2. Biomass Characterization
2.3. Mechanical Stirring and Ultrasound Assisted Extraction
2.4. Hydrolysis Treatments
2.5. Total Polyphenol Content (TPC) and Antioxidant Capacity
2.6. Identification and Quantification of Extracts Components
2.7. Solvent Extraction Kinetics
2.7.1. Power Law Model
2.7.2. Weibull’s Model
2.8. Centrifuge Ultrafiltration
2.9. Statistical Analysis
3. Results and Discussion
3.1. Biomass Characterization
3.2. Kinetics of Extraction of Phenolic Compounds from BSG
3.2.1. Comparison of UAE and Mechanical Stirring Extraction
3.2.2. Effect of Solvent to Mass Ratio by UAE
3.2.3. Effect of Temperature on Polyphenol Extraction Kinetics by UAE
3.2.4. Effect of Ethanol Concentration on Polyphenol Extraction Kinetics by UAE
3.2.5. Kinetic Modelling
3.3. Determination of Extract Components
3.4. Partial Concentration by Centrifuge Ultrafiltration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Socaci, S.A.; Fărcaş, A.C.; Diaconeasa, Z.M.; Vodnar, D.C.; Rusu, B.; Tofană, M. Influence of the extraction solvent on phenolic content, antioxidant, antimicrobial and antimutagenic activities of brewers’ spent grain. J. Cereal Sci. 2018, 80, 180–187. [Google Scholar] [CrossRef]
- The Brewer of Europe Beer statistics. 2018. Available online: https://brewersofeurope.org/uploads/mycms-files/documents/publications/2018/EU-beer-statistics-2018-web.pdf (accessed on 18 February 2020).
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Paz, A.; Outeiriño, D.; Pérez Guerra, N.; Domínguez, J.M. Enzymatic hydrolysis of brewer’s spent grain to obtain fermentable sugars. Bioresour. Technol. 2019, 275, 402–409. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; Neugart, S.; Piggott, C.O.; Connolly, A.; Jansen, M.A.K.; Krumbein, A.; Schreiner, M.; FitzGerald, R.J.; O’Brien, N.M. The hydroxycinnamic acid content of barley and brewers’ spent grain (BSG) and the potential to incorporate phenolic extracts of BSG as antioxidants into fruit beverages. Food Chem. 2013, 141, 2567–2574. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Kitanović, S.; Milenović, D.; Veljković, V.B. Empirical kinetic models for the resinoid extraction from aerial parts of St. John’s wort (Hypericum perforatum L.). Biochem. Eng. J. 2008, 41, 1–11. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef]
- Arranz, S.; Saura Calixto, F. Analysis of polyphenols in cereals may be improved performing acidic hydrolysis: A study in wheat flour and wheat bran and cereals of the diet. J. Cereal Sci. 2010, 51, 313–318. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Alvarez, V.H.; Alonso, E.; Cocero, M.J.; Saldaña, M.D.A. Pressurized aqueous ethanol extraction of β-glucans and phenolic compounds from waxy barley. Food Res. Int. 2015, 75, 252–259. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.S.; Yin, G.M.; He, Y.Z.; Hu, S.Q.; Li, B.; Li, L.; Liang, H.L.; Borthakur, D. Recovery of protein from brewer’s spent grain by ultrafiltration. Biochem. Eng. J. 2009, 48, 1–5. [Google Scholar] [CrossRef]
- Robertson, J.A.; I’Anson, K.J.A.; Treimo, J.; Faulds, C.B.; Brocklehurst, T.F.; Eijsink, V.G.H.; Waldron, K.W. Profiling brewers’ spent grain for composition and microbial ecology at the site of production. LWT–Food Sci. Technol. 2010, 43, 890–896. [Google Scholar] [CrossRef]
- Illera, A.E.; Sanz, M.T.; Benito-Román, O.; Varona, S.; Beltrán, S.; Melgosa, R.; Solaesa, A.G. Effect of thermosonication batch treatment on enzyme inactivation kinetics and other quality parameters of cloudy apple juice. Innov. Food Sci. Emerg. Technol. 2018, 47, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Galvan D’Alessandro, L.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Sologubik, C.A.; Fernández, M.B.; Manrique, G.D.; D’Alessandro, L.G. Extraction of antioxidant phenolic compounds from brewer’s spent grain: Optimization and kinetics modeling. Antioxidants 2018, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Dent, M.; Dragović-Uzelac, V.; Penić, M.; Brñić, M.; Bosiljkov, T.; Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Birsan, R.I.; Wilde, P.; Waldron, K.W.; Rai, D.K. Recovery of polyphenols from brewer’s spent grains. Antioxidants 2019, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Bartolomé, B.; Gómez-Cordovés, C. Barley spent grain: Release of hydroxycinnamic acids (ferulic and p-coumaric acids) by commercial enzyme preparations. J. Sci. Food Agric. 1999, 79, 435–439. [Google Scholar] [CrossRef]
- Moreira, M.M.; Morais, S.; Carvalho, D.O.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. Brewer’s spent grain from different types of malt: Evaluation of the antioxidant activity and identification of the major phenolic compounds. Food Res. Int. 2013, 54, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Reis, S.F.; Coelho, E.; Coimbra, M.A.; Abu-Ghannam, N. Improved efficiency of brewer’s spent grain arabinoxylans by ultrasound-assisted extraction. Ultrason. Sonochem. 2015, 24, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.S.; Tian, Y.J.; He, Y.Z.; Li, L.; Hu, S.Q.; Li, B. Optimisation of ultrasonic-assisted protein extraction from brewer’s spent grain. Czech J. Food Sci. 2010, 28, 9–17. [Google Scholar] [CrossRef] [Green Version]








| Original BSG | Ground BSG | ||
|---|---|---|---|
| Size, mm | Mass Percentage, % | Size, mm | Mass Percentage, % |
| >4 | 4.4 | >1 | 2.9 |
| 2–4 | 84.5 | 0.5–1 | 31.3 |
| 1–2 | 10.3 | 0.25–0.50 | 34.8 |
| 0.5–1 | 0.71 | 0.125–0.25 | 24.6 |
| 0.25–0.50 | 0.06 | <0.125 | 6.5 |
| Component | g/100 gBSG,dry |
|---|---|
| Extractives in water | 24.3 ± 0.6 |
| Extractives in ethanol | 1.8 ± 0.2 |
| Starch | 7.9 ± 0.2 |
| Cellulose | 18.2 ± 1.6 |
| Hemicellulose | 26.1 ± 1.8 |
| Insoluble Lignin | 13.5 ± 0.5 |
| Soluble lignin | 4.3 ± 0.1 |
| Proteins | 17.8 ± 0.1 |
| Lipids | 5.90 ± 0.01 |
| Ash | 2.92 ± 0.02 |
| Extraction Mode | Pretreatment | T, °C | Solvent | mL: gBSG,dry | Power Law Model | Weibull Model | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | n | RMS | A | k | n | RMS | |||||
| MS | - | 47 | Water | 21.7:1 | 0.208 | 0.264 | 5.74 | 5.2450 | 0.027 | 0.341 | 4.88 |
| UAE | - | 47 | Water | 21.7:1 | 0.431 | 0.241 | 7.32 | 4.1643 | 0.088 | 0.319 | 6.56 |
| MS | ground | 47 | Water | 21.7:1 | 1.197 | 0.097 | 5.23 | 6.133 | 0.197 | 0.127 | 6.37 |
| UAE | ground | 47 | Water | 21.7:1 | 2.066 | 0.057 | 6.47 | 5.895 | 0.415 | 0.084 | 6.80 |
| UAE | ground | 47 | Water | 35.3:1 | 2.027 | 0.057 | 8.89 | 5.699 | 0.429 | 0.080 | 10.10 |
| UAE | ground | 47 | Water | 18.1:1 | 1.744 | 0.065 | 5.97 | 5.120 | 0.4070 | 0.090 | 6.52 |
| UAE | ground | 47 | Water | 13.6:1 | 1.297 | 0.097 | 8.94 | 5.796 | 0.244 | 0.125 | 9.40 |
| UAE | ground | 47 | Water | 10.9:1 | 1.438 | 0.084 | 9.22 | 4.643 | 0.391 | 0.096 | 5.92 |
| UAE | ground | 39 | Water | 21.7:1 | 1.837 | 0.057 | 20.68 | 6.664 | 0.316 | 0.075 | 21.82 |
| UAE | ground | 58 | Water | 21.7:1 | 2.159 | 0.058 | 9.66 | 6.418 | 0.403 | 0.079 | 9.93 |
| UAE | ground | 47 | 20% EtOH | 21.7:1 | 2.229 | 0.062 | 4.92 | 8.144 | 0.314 | 0.080 | 4.97 |
| UAE | ground | 47 | 50% EtOH | 21.7:1 | 2.110 | 0.046 | 4.37 | 4.830 | 0.559 | 0.072 | 4.59 |
| UAE | ground | 47 | 80% EtOH | 21.7:1 | 1.130 | 0.074 | 1.59 | 3.458 | 0.376 | 0.107 | 1.52 |
| UAE | ground | 47 | 100% EtOH | 21.7:1 | 0.073 | 0.342 | 5.35 | 3.491 | 0.019 | 0.372 | 5.68 |
| Compounds | Formula | UAE-W | UAE-20EtOH | Acid-Hydrolysis | Alkaline-Hydrolysis | Xylanase 1% | Xylanase 3% | Xylanase 6% |
|---|---|---|---|---|---|---|---|---|
| p-hydroxybenzoic acid |  | 10.0 ± 0.5 a | 10.0 ± 0.7 a | n.d. | 59.3 ± 2.2 b | n.d. | n.d. | n.d. |
| Vanillic acid |  | n.d. | n.d. | n.d. | 48.8 ± 1.5 c | 17.9 ± 1.1 a | 42.6 ± 2.3 b | 61.2 ± 3.3 d |
| Syringic acid |  | n.d. | n.d. | n.d. | 106.1 ± 5.7 | n.d. | n.d. | n.d. |
| p-Coumaric acid |  | n.d. | n.d. | n.d. | 538.2 ± 4.4 b | 5.9 ± 1.4 a | 5.3 ± 1.7 a | 5.3 ± 0.4 a |
| Vanillin |  | n.d. | n.d. | n.d. | 217.2 ± 1.4 c | 110.5 ± 2.6 a | 191.2 ± 0.1 b | 203.5 ± 10 b,c |
| Ferulic acid |  | 10.7 ± 0.3 a | 9.5 ± 0.3 a | 54.4 ± 0.3 b | 1305.7 ± 0.5 e | 52.4 ± 0.9 b | 185.8 ± 4.5 c | 292.4 ± 2.6 d |
| Sinapic acid |  | 2.8 ± 0.2 a | 13.5 ± 0.3 c,d | 31.1 ± 0.5 f | 27.2 ± 1.2 e | 7.5 ± 0.2 b | 12.9 ± 0.6 c | 14.9 ± 1.2 d |
| TPC (Folin-Cioculteau) mg GAE/gBSG,dry·min | 3.28 ± 0.12 a0.5h | 3.55 ± 0.07 a0.5h | 30 ± 5 e24h | 16.2 ± 0.2 c4h | 7.2 ± 0.2 b24h | 25.2 ± 0.1 d24h | 42.0 ± 0.4 f24h | |
| Productivity, mg GAE/gBSG,dry·min* | 0.109 ± 0.002 a,b | 0.118 ± 0.004 b | non-determined | 0.55 ± 0.04c | 0.050 ± 0.004 a | 0.065 ± 0.002 a,b | 0.087 ± 0.001 a,b | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Riaño, P.; Sanz Diez, M.T.; Blanco, B.; Beltrán, S.; Trigueros, E.; Benito-Román, O. Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration. Antioxidants 2020, 9, 265. https://doi.org/10.3390/antiox9030265
Alonso-Riaño P, Sanz Diez MT, Blanco B, Beltrán S, Trigueros E, Benito-Román O. Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration. Antioxidants. 2020; 9(3):265. https://doi.org/10.3390/antiox9030265
Chicago/Turabian StyleAlonso-Riaño, Patricia, María Teresa Sanz Diez, Beatriz Blanco, Sagrario Beltrán, Ester Trigueros, and Oscar Benito-Román. 2020. "Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration" Antioxidants 9, no. 3: 265. https://doi.org/10.3390/antiox9030265
APA StyleAlonso-Riaño, P., Sanz Diez, M. T., Blanco, B., Beltrán, S., Trigueros, E., & Benito-Román, O. (2020). Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration. Antioxidants, 9(3), 265. https://doi.org/10.3390/antiox9030265