First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Samples and Extracts
2.3. High-Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (HPLC-ESI-QTOF-MS)
2.4. Total Antioxidant Capacity
2.5. In Vitro Digestion
2.6. Rat Everted Gut Sacs
2.7. Real-Time Monitoring of Phenolic First-Pass Metabolism
2.7.1. Spectrophotometry (Method 1)
2.7.2. Differential Pulse Voltammetry (DPV; Method 2)
2.7.3. HPLC-ESI-QTOF-MS (Method 3)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Profile of Berry Samples
3.2. Antioxidant Capacity of Berry Samples
3.3. Apparent Permeability of Berry Polyphenols
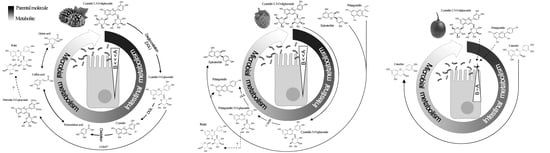
3.4. Ex Vivo Biotransformation of Berry Polyphenols
3.4.1. DPV
3.4.2. HPLC-ESI-QTOF-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, B.; Kortesniemi, M. Clinical evidence on potential health benefits of berries. Curr. Opin. Food Sci. 2015, 2, 36–42. [Google Scholar] [CrossRef]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morand, C.; De Roos, B.; Garcia-Conesa, M.T.; Gibney, E.R.; Landberg, R.; Manach, C.; Milenkovic, D.; Rodriguez-Mateos, A.; Van de Wiele, T.; Tomas-Barberan, F. Why interindividual variation in response to consumption of plant food bioactives matters for future personalized nutrition. Proc. Nutr. Soc. 2020, 1–11. [Google Scholar] [CrossRef]
- Domínguez-Ávila, J.A.; Wall-Medrano, A.; Velderrain-Rodríguez, G.R.; Chen, O.C.-Y.; Salazar-Lopez, N.J.; Robles-Sánchez, M.; Gonzalez-Aguilar, G. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF/MS characterization of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Olivas-Aguirre, F.J.; Gaytán-Martínez, M.; Mendoza-Díaz, S.O.; González-Aguilar, G.A.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Wall-Medrano, A. In vitro digestibility of phenolic compounds from edible fruits: Could it be explained by chemometrics? Int. J. Food Sci. Technol. 2017, 52, 2040–2048. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Zhang, W.; Ding, Y.; Zhao, T.; Zhang, M.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Bioaccessibility and biotransformation of anthocyanin monomers following in vitro simulated gastric-intestinal digestion and in vivo metabolism in rats. Food Funct. 2019, 10, 6052–6061. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Bento da Silva, A.; Abrankó, L.; Low, D.; Garcia Villalba, R.; Tomás Barberán, F.; Landberg, R.; Savolainen, O.; Alvarez-Acero, I.; Pascual-Teresa, D. Interlaboratory coverage test on plant food bioactive compounds and their metabolites by mass spectrometry-based untargeted metabolomics. Metabolites 2018, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Šeruga, M.; Novak, I.; Jakobek, L. Determination of polyphenols content and antioxidant activity of some red wines by differential pulse voltammetry, HPLC and spectrophotometric methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- Torres-Aguirre, G.A.; Muñoz-Bernal, Ó.A.; Álvarez-Parrilla, E.; Núñez-Gastélum, J.A.; Wall-Medrano, A.; Sáyago-Ayerdi, S.G.; de la Rosa, L.A. Optimización de la extracción e identificación de compuestos polifenólicos en anís (Pimpinella anisum), clavo (Syzygium aromaticum) y cilantro (Coriandrum sativum) mediante HPLC acoplado a espectrometría de masas. TIP Rev. Esp. Cie. Quim. Biol. 2018, 21, 103–115. [Google Scholar]
- Velderrain-Rodríguez, G.; Torres-Moreno, H.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; Robles-Zepeda, R.E.; Wall-Medrano, A.; González-Aguilar, G.A. Gallic acid content and antioxidant mechanism are responsible for the antiproliferative activity of ‘Ataulfo’mango peel on LS180 cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos-Vega, R.; Vázquez-Sánchez, K.; López-Barrera, D.; Loarca-Piña, G.; Mendoza-Díaz, S.; Oomah, B.D. Simulated gastrointestinal digestion and in vitro colonic fermentation of spent coffee (Coffea arabica L.): Bioaccessibility and intestinal permeability. Food Res. Int. 2015, 77, 156–161. [Google Scholar] [CrossRef]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-mohizea, A.M. Everted gut sac model as a tool in pharmaceutical research: Limitations and applications. J. Pharm. Pharmacol. 2012, 64, 326–336. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Iuhas, C.I.; Ayvaz, H.; Rugină, D.; Stanilă, A.; Dulf, F.; Bunea, A.; Socaci, S.A.; Socaciu, C.; Pintea, A. Phytochemical characterization of commercial processed blueberry, blackberry, blackcurrant, cranberry, and raspberry and their antioxidant activity. Antioxidants 2019, 8, 540. [Google Scholar] [CrossRef] [Green Version]
- Colombo, F.; Di Lorenzo, C.; Regazzoni, L.; Fumagalli, M.; Sangiovanni, E.; de Sousa, L.P.; Bavaresco, L.; Tomasi, D.; Bosso, A.; Aldini, G.; et al. Phenolic profiles and anti-inflammatory activities of sixteen table grape (Vitis vinifera L.) varieties. Food Funct. 2019, 10, 1797–1807. [Google Scholar] [CrossRef]
- He, F.; He, J.-J.; Pan, Q.-H.; Duan, C.-Q. Mass-spectrometry evidence confirming the presence of pelargonidin-3-O-glucoside in the berry skins of Cabernet Sauvignon and Pinot Noir (Vitis vinifera L.). Aust. J. Grape Wine Res. 2010, 16, 464–468. [Google Scholar] [CrossRef]
- Aguirre, M.J.; Chen, Y.Y.; Isaacs, M.; Matsuhiro, B.; Mendoza, L.; Torres, S. Electrochemical behaviour and antioxidant capacity of anthocyanins from Chilean red wine, grape and raspberry. Food Chem. 2010, 121, 44–48. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; Gonzalez-Aguilar, G.; De la Rosa, L.A.; Ramos-Jimenez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Feng, Y.; Diao, T.; Zeng, W.; Zuo, Y. Experimental and theoretical study on antioxidant activity of the four anthocyanins. J. Mol. Struct. 2020, 1204, 127509. [Google Scholar] [CrossRef]
- Janeiro, P.; Brett, A.M.O. Redox behavior of anthocyanins present in Vitis vinifera L. Electroanalysis 2017, 19, 1779–1786. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Stintzing, A.S.; Carle, R.; Frei, B.; Wrolstad, R.E. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J. Agric. Food Chem. 2002, 50, 6172–6181. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Kosińska-Cagnazzo, A.; Diering, S.; Prim, D.; Andlauer, W. Identification of bioaccessible and uptaken phenolic compounds from strawberry fruits in in vitro digestion/Caco-2 absorption model. Food Chem. 2015, 170, 288–294. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Navarro-Coves, S.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion. Ind. Crops Prod. 2016, 94, 774–782. [Google Scholar] [CrossRef]
- Jiao, X.; Li, B.; Zhang, Q.; Gao, N.; Zhang, X.; Meng, X. Effect of in vitro-simulated gastrointestinal digestion on the stability and antioxidant activity of blueberry polyphenols and their cellular antioxidant activity towards HepG2 cells. Int. J. Food Sci. Technol. 2018, 53, 61–71. [Google Scholar] [CrossRef]
- Dixit, P.; Jain, D.K.; Dumbwani, J. Standardization of an ex vivo method for determination of intestinal permeability of drugs using everted rat intestine apparatus. J. Pharmacol. Toxicol. Methods 2012, 65, 13–17. [Google Scholar] [CrossRef]
- Rastogi, H.; Jana, S. Evaluation of physicochemical properties and intestinal permeability of six dietary polyphenols in human intestinal colon adenocarcinoma Caco-2 cells. Eur. J. Drug Metab. Pharm. 2016, 41, 33–43. [Google Scholar] [CrossRef]
- Farrell, T.L.; Poquet, L.; Dew, T.P.; Barber, S.; Williamson, G. Predicting phenolic acid absorption in Caco-2 cells: A theoretical permeability model and mechanistic study. Drug Metab. Disp. 2012, 40, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Estudante, M.; Morais, J.G.; Soveral, G.; Benet, L.Z. Intestinal drug transporters: An overview. Adv. Drug Deliv. Rev. 2013, 65, 1340–1356. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Cazares, L.A.; Hernández-Navarro, F.; Ramírez-Jiménez, A.K.; Campos-Vega, R.; de la Luz Reyes-Vega, M.; Loarca-Piña, G.; Morales-Sanchez, E.; Wall-Medrano, A.; Gaytan-Martinez, M. Mango-bagasse functional-confectionery: Vehicle for enhancing bioaccessibility and permeability of phenolic compounds. Food Funct. 2017, 8, 3906–3916. [Google Scholar] [CrossRef] [PubMed]
- Luzardo-Ocampo, I.; Campos-Vega, R.; Gaytán-Martínez, M.; Preciado-Ortiz, R.; Mendoza, S.; Loarca-Piña, G. Bioaccessibility and antioxidant activity of free phenolic compounds and oligosaccharides from corn (Zea mays L.) and common bean (Phaseolus vulgaris L.) chips during in vitro gastrointestinal digestion and simulated colonic fermentation. Food Res. Int. 2017, 100, 304–311. [Google Scholar] [CrossRef]
- Gil, E.S.; Couto, R.O. Flavonoid electrochemistry: A review on the electroanalytical applications. Rev. Bras. Farmacog. 2013, 23, 542–558. [Google Scholar] [CrossRef] [Green Version]
- Alcalde, B.; Granados, M.; Saurina, J. Exploring the antioxidant features of polyphenols by spectroscopic and electrochemical methods. Antioxidants 2019, 8, 523. [Google Scholar] [CrossRef] [Green Version]
- Blasco, A.J.; González, M.C.; Escarpa, A. Electrochemical approach for discriminating and measuring predominant flavonoids and phenolic acids using differential pulse voltammetry: Towards an electrochemical index of natural antioxidants. Anal. Chim. Acta 2004, 511, 71–81. [Google Scholar] [CrossRef]
- Guiberteau-Cabanillas, A.; Godoy-Cancho, B.; Bernalte, E.; Tena-Villares, M.; Guiberteau Cabanillas, C.; Martinez-Canas, M.A. Electroanalytical behavior of gallic and ellagic acid using graphene modified screen-printed electrodes. Method for the determination of total low oxidation potential phenolic compounds content in cork boiling waters. Electroanalysis 2015, 27, 177–184. [Google Scholar] [CrossRef]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Rompp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Brit. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [Green Version]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin absorption and metabolism by human intestinal Caco-2 cells—A review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef] [Green Version]
- Berto, S.; Carena, L.; Chiavazza, E.; Marletti, M.; Fin, A.; Giacomino, A.; Malandrino, M.; Barolo, C.; Prenesti, E.; Vione, D. Off-line and real-time monitoring of acetaminophen photodegradation by an electrochemical sensor. Chemosphere 2018, 204, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Bounab, L.; Iglesias, O.; Pazos, M.; Sanromán, M.Á.; González-Romero, E. Effective monitoring of the electro-Fenton degradation of phenolic derivatives by differential pulse voltammetry on multi-walled-carbon nanotubes modified screen-printed carbon electrodes. Appl. Catal. B Environ. 2016, 180, 544–550. [Google Scholar] [CrossRef]
- Barik, S.K.; Russell, W.R.; Moar, K.M.; Cruickshank, M.; Scobbie, L.; Duncan, G.; Hoggard, N. The anthocyanins in black currants regulate postprandial hyperglycaemia primarily by inhibiting α-glucosidase while other phenolics modulate salivary α-amylase, glucose uptake and sugar transporters. J. Nutr. Biochem. 2020, 78, 108325. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W. Anthocyanins and their C6-C3-C6 metabolites in humans and animals. Molecules 2019, 24, 4024. [Google Scholar] [CrossRef] [Green Version]
- Steinert, R.E.; Ditscheid, B.; Netzel, M.; Jahreis, G. Absorption of black currant anthocyanins by monolayers of human intestinal epithelial Caco-2 cells mounted in ussing type chambers. J. Agric. Food Chem. 2008, 56, 4995–5001. [Google Scholar] [CrossRef]
- Wirth, R.; Bódi, N.; Maróti, G.; Bagyánszki, M.; Talapka, P.; Fekete, É.; Bagi, Z.; Kovacs, K. Regionally distinct alterations in the composition of the gut microbiota in rats with streptozotocin-induced diabetes. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Yin, R.; Kuo, H.C.; Hudlikar, R.; Sargsyan, D.; Li, S.; Wang, L.; Wu, R.; Kong, A.N. Gut microbiota, dietary phytochemicals, and benefits to human health. Curr. Pharmacol. Rep. 2019, 5, 332–344. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Verny, M.A.; Besson, C.; Rémésy, C.; Scalbert, A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef] [Green Version]




| Compound | rt | m/z | Grape | Raspberry | Blackberry | TPSA | LogP |
|---|---|---|---|---|---|---|---|
| Catechin | 1.5 | 289.1 | 86 ± 10 | -- | -- | 110 | 1.37 |
| Epicatechin | 2.7 | 289.1 | 10 ± 5 c | 451 ± 6 b | 1121 ± 95 a | 110 | 1.37 |
| Cyanidin-3-O-β-glucoside | 4.2 | 450.1 | -- | -- | 2762 ± 31 | 181 | 0.34 |
| Cyanidin-3-O-arabinoside | 5.6 | 420.2 | -- | -- | 21 ± 0 | 161 | −2.37 |
| Pelargonidin | 6.6 | 272.1 | 67 ± 4 a | 47 ± 1 b | 65 ± 2 a | 82 | −0.26 |
| Pelargonidin-3-O-glucoside | 7.1 | 433.2 | -- | 20 ± 3 a | 15 ± 0 b | 171.2 | −2.30 |
| Cyanidin-3,5-O-diglucoside | 7.9 | 612.4 | 134 ± 8 a | 58 ± 4 c | 111 ± 3 b | 270.6 | −4.61 |
| Total polyphenols | 297 ± 27 c | 576 ± 14 b | 4095 ± 131 a |
| Parameter | Red Globe Grape | Raspberry | Blackberry |
|---|---|---|---|
| At0 (TPFC) | 2 ± 0.0 c | 3.6 ± 0.1 b | 4.2 ± 0.1 a |
| At120 | 1.3 ± 0.3 b | 1.5 ± 0.1 b | 2.4 ± 0.1 a |
| Bt120 | 0.10 ± 0.0 b | 0.09 ± 0.0 b | 0.13 ± 0.0 a |
| Absorptive Papp (At120→Bt120) | 1.20 | 0.06 | 0.07 |
| Secretory Papp (Bt120→At120) | 1.55 | 0.98 | 1.38 |
| Efflux ratio (B→A)*(A→B)−1 | 1.29 | 16.12 | 19.12 |
| Uptake ratio (A→B)*(B→A)−1 | 0.78 | 0.06 | 0.05 |
| p (ER vs. UR) | 0.02 | 0.002 | <0.0001 |
| Sample | Phenolic | rt | m/z | Ion Abundance (IA) | Δ (%) | |
|---|---|---|---|---|---|---|
| t0 | t120 | |||||
| Raspberry | Quinic acid | 0.6 | 191.1 | 104,000 ± 1061 | 45,600 ± 636 | −56 ± 0 |
| Epicatechin | 3.0 | 289.1 | 9500 ± 707 | 3100 ± 141 | −67 ± 1 | |
| Cy3G | 4.2 | 450.1 | 16,500 ± 707 | 4750 ± 354 | −71 ± 1 | |
| Blackberry | Quinic acid | 0.6 | 191.0 | 8950 ± 212 | 4600 ± 566 | −47 ± 9 |
| Chlorogenic acid | 1.6 | 353.1 | 71,300 ± 1768 | 47,300 ± 354 | −34 ± 1 | |
| Caffeic acid | 2.3 | 179.0 | 3750 ± 354 | 23,500 ± 707 | 530 ± 78 | |
| Ma3G | 4.9 | 494.1 | 950 ± 71 | 8450 ± 354 | 1006 ± 8 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivas-Aguirre, F.J.; Mendoza, S.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G.A.; Villegas-Ochoa, M.A.; Quintero-Vargas, J.T.J.; Wall-Medrano, A. First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach. Antioxidants 2020, 9, 311. https://doi.org/10.3390/antiox9040311
Olivas-Aguirre FJ, Mendoza S, Alvarez-Parrilla E, Gonzalez-Aguilar GA, Villegas-Ochoa MA, Quintero-Vargas JTJ, Wall-Medrano A. First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach. Antioxidants. 2020; 9(4):311. https://doi.org/10.3390/antiox9040311
Chicago/Turabian StyleOlivas-Aguirre, Francisco J., Sandra Mendoza, Emilio Alvarez-Parrilla, Gustavo A. Gonzalez-Aguilar, Monica A. Villegas-Ochoa, Jael T.J. Quintero-Vargas, and Abraham Wall-Medrano. 2020. "First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach" Antioxidants 9, no. 4: 311. https://doi.org/10.3390/antiox9040311
APA StyleOlivas-Aguirre, F. J., Mendoza, S., Alvarez-Parrilla, E., Gonzalez-Aguilar, G. A., Villegas-Ochoa, M. A., Quintero-Vargas, J. T. J., & Wall-Medrano, A. (2020). First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach. Antioxidants, 9(4), 311. https://doi.org/10.3390/antiox9040311