Antioxidant Properties of Embelin in Cell Culture. Electrochemistry and Theoretical Mechanism of Scavenging. Potential Scavenging of Superoxide Radical through the Cell Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Equipment
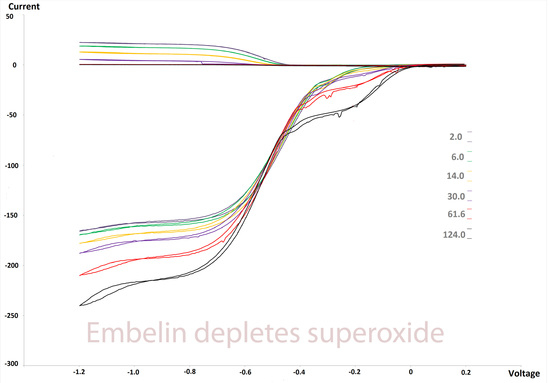
2.3. RRDE Study
2.4. Computational Study
2.5. Cells in Culture
2.6. Intracellular ROS Determination
2.7. Proliferation Studies
2.8. MTT Assay
2.9. Statistical Analysis
3. Results
3.1. Electrochemistry
3.2. Density Functional Theory
3.3. Modeling Cell Membrane Interaction of Embelin
3.4. Antioxidant and Biological Activity of Embelin in Cells in Culture
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atlabachew, M.; Mehari, B.; Combrinck, S.; McCrindle, R. Single-step isolation of embelin using high performance countercurrent chromatography and determination of the fatty acid composition of seeds of Embelia schimperi. Biomed. Chromatogr. 2017, 31. [Google Scholar] [CrossRef]
- Kundap, U.P.; Bhuvanendran, S.; Kumari, Y.; Othman, I.; Shaikh, M.F. Plant derived phytocompound, embelin in CNS disorders: A systematic review. Front. Pharmacol. 2017, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Siegelin, M.D.; Gaiser, T.; Siegelin, Y. The XIAP inhibitor embelin enhances TRAIL-mediated apoptosis in malignant glioma cells by down-regulation of the short isoform of FLIP. Neurochem. Int. 2009, 55, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Lee, S.-G.; Yang, W.M.; Um, J.-Y.; Sethi, G.; Mishra, S.; Shanmugam, M.K.; Ahn, K.S. The application of embelin for cancer prevention and therapy. Molecules 2018, 23, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumalatha, K.; Gowda, M.; Meenakshisundaram, S. ROS-mediated induction of apoptosis by benzoquinone embelin in human colon adenocarcinoma cells HT-29. J. Complement. Integr. Med. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, R.; Zhu, K.; Li, Y.; Li, J.; Miao, M.; Wang, H.; Yao, K.; Liu, Z. Involvement of oxidative stress in embelin-induced cell death in leukemia HL-60 cells. Zhonghuaxue ye xuezazhi=Zhonghuaxueyexuezazhi 2015, 36, 465–468. [Google Scholar] [CrossRef]
- Naik, S.R.; Niture, N.T.; Ansari, A.A.; Shah, P.D. Anti-diabetic activity of embelin: Involvement of cellular inflammatory mediators, oxidative stress and other biomarkers. Phytomedicine 2013, 20, 797–804. [Google Scholar] [CrossRef]
- Caruso, F.; Paumier, S.; Rossi, M. X-Ray crystal structure of embelin and its DFT scavenging of superoxide radical. J. Comput. Chem. 2018, 39, 1143–1148. [Google Scholar] [CrossRef]
- Chen, X.; Gao, M.; Jian, R.; Hong, W.D.; Tang, X.; Li, Y.; Zhao, D.; Zhang, K.; Chen, W.; Zheng, X.; et al. Design, synthesis and α-glucosidase inhibition study of novel embelin derivatives. J. Enzyme Inhib. Med. Chem. 2020, 35, 565–573. [Google Scholar] [CrossRef]
- SreeHarsha, N. Embelin impact on paraquat-induced lung injury through suppressing oxidative stress, inflammatory cascade, and MAPK/NF-κB signaling pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22456. [Google Scholar] [CrossRef]
- Lin, Z.; Jensen, J.K.; Hong, Z.; Shi, X.; Hu, L.; Andreasen, P.A.; Huang, M. Structural insight into inactivation of plasminogen activator inhibitor-1 by a small-molecule antagonist. Chem. Biol. 2013, 20, 253–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.P.; Sharma, N.; Kalivendi, S.V. Embelin averts MPTP-induced dysfunction in mitochondrial bioenergetics and biogenesis via activation of SIRT1. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, K.; Strmcnik, D.; Blizanac, B.; Stamenkovic, V.; Arenz, M.; Markovic, N. Measurement of oxygen reduction activities via the rotating disc electrode method: From Pt model surfaces to carbon-supported high surface area catalysts. Electrochim. Acta 2008, 53, 3181–3188. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Lombardo, E.; Sabellico, C.; Hájek, J.; Staňková, V.; Filipský, T.; Balducci, V.; De Vito, P.; Leone, S.; Bavavea, E.I.; Proietti Silvestri, I.; et al. Protection of cells against oxidative stress by nanomolar levels of hydroxyflavones indicates a new type of intracellular antioxidant mechanism. PLoS ONE 2013, 8, e60796. [Google Scholar] [CrossRef]
- Rossi, M.; Caruso, F.; Kwok, L.; Lee, G.; Caruso, A.; Gionfra, F.; Candelotti, E.; Belli, S.L.; Molasky, N.; Raley-Susman, K.M.; et al. Protection by extra virgin olive oil against oxidative stress in vitro and in vivo. Chemical and biological studies on the health benefits due to a major component of the Mediterranean diet. PLoS ONE 2017, 12, e0189341. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Tao, X.; Cheng, H. Ammonia-containing dimethyl sulfoxide: An improved solvent for the dissolution of formazan crystals in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Anal. Biochem. 2012, 421, 324–326. [Google Scholar] [CrossRef]
- Belli, S.; Rossi, M.; Molasky, N.; Middleton, L.; Caldwell, C.; Bartow-McKenney, C.; Duong, M.; Chiu, J.; Gibbs, E.; Caldwell, A.; et al. Effective and novel application of superoxide radical scavenging by natural phenolic antioxidants. Antioxidants 2019, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.; Wen, K.; Caruso, F.; Belli, S. Emodin scavenging of superoxide radical includes π–π interaction. X-Ray crystal structure, hydrodynamic voltammetry and theoretical studies. Antioxidants 2020, 9, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, G.W.; Ingold, K.U. Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 1981, 103, 6472–6477. [Google Scholar] [CrossRef]
- Simonart, P.; Verachtert, H. Hydroxylation of diphenols by aspergillus fumigatus Fresenius. Bull. Soc. Chim. Biol. 1969, 51, 919–924. [Google Scholar]
- Seyoum, A.; Asres, K.; El-Fiky, F.K. Structure-radical scavenging activity relationships of flavonoids. Phytochemistry 2006, 67, 2058–2070. [Google Scholar] [CrossRef]
- Chaudhari, H.S.; Bhandari, U.; Khanna, G. Preventive effect of embelin from embelia ribes on lipid metabolism and oxidative stress in high-fat diet-induced obesity in rats. Planta Med. 2012, 78, 651–657. [Google Scholar] [CrossRef]
- Chaudhari, H.S.; Bhandari, U.; Khanna, G. Embelia ribes extract reduces high fat diet and low dose streptozotocin-induced diabetic nephrotoxicity in rats. EXCLI J. 2013, 12, 858–871. [Google Scholar]
- Caioli, S.; Candelotti, E.; Pedersen, J.Z.; Saba, L.; Antonini, A.; Incerpi, S.; Zona, C. Baicalein reverts L-valine-induced persistent sodium current up-modulation in primary cortical neurons. Biochim. Biophys. Acta 2016, 1862, 566–575. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Gao, Y.; Du, Z.; Wang, Y.; Cheng, P.; Chen, A.; Huang, H. The fascinating effects of baicalein on cancer: A review. Int. J. Mol. Sci. 2016, 17, 1681. [Google Scholar] [CrossRef] [Green Version]
- Sumino, M.; Sekine, T.; Ruangrungsi, N.; Igarashi, K.; Ikegami, F. Ardisiphenols and other antioxidant principles from the fruits of ardisia colorata. Chem. Pharm. Bull. 2002, 50, 1484–1497. [Google Scholar] [CrossRef] [Green Version]
- Gaman, L.; Dragos, D.; Vlad, A.; Robu, G.C.; Radoi, M.P.; Stroica, L.; Badea, M.; Gilca, M. Phytoceuticals in acute pancreatitis: Targeting the balance between apoptosis and necrosis. Evid. Based Complement. Altern. Med. 2018, 2018, 5264592. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Tokizane, K.; Konishi, H.; Yu, H.-R.; Kiyama, H. Agonists for G-protein-coupled receptor 84 (GPR84) alter cellular morphology and motility but do not induce pro-inflammatory responses in microglia. J. Neuroinflamm. 2017, 14, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, B.D.; Anubolu, H.; Koneru, M.; Kumar, J.M.; Kuncha, M.; Rachamalla, S.S.; Sistla, R. Cardioprotective effect of embelin on isoproterenol-induced myocardial injury in rats: Possible involvement of mitochondrial dysfunction and apoptosis. Life Sci. 2014, 107, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, S.; Badami, S.; Ravi, S.; Thippeswamy, B.S.; Veerapur, V.P. Synthesis and evaluation of analgesic and anti-inflammatory activities of most active free radical scavenging derivatives of embelin—A structure-activity relationship. Chem. Pharm. Bull. 2011, 59, 913–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]























| Compound | Toluene | BHT | DMBQ | Embelin |
|---|---|---|---|---|
| Slope | 0 | −1.6 × 103 | −1.6 × 104 | −6.0 × 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, F.; Rossi, M.; Kaur, S.; Garcia-Villar, E.; Molasky, N.; Belli, S.; Sitek, J.D.; Gionfra, F.; Pedersen, J.Z.; Incerpi, S. Antioxidant Properties of Embelin in Cell Culture. Electrochemistry and Theoretical Mechanism of Scavenging. Potential Scavenging of Superoxide Radical through the Cell Membrane. Antioxidants 2020, 9, 382. https://doi.org/10.3390/antiox9050382
Caruso F, Rossi M, Kaur S, Garcia-Villar E, Molasky N, Belli S, Sitek JD, Gionfra F, Pedersen JZ, Incerpi S. Antioxidant Properties of Embelin in Cell Culture. Electrochemistry and Theoretical Mechanism of Scavenging. Potential Scavenging of Superoxide Radical through the Cell Membrane. Antioxidants. 2020; 9(5):382. https://doi.org/10.3390/antiox9050382
Chicago/Turabian StyleCaruso, Francesco, Miriam Rossi, Sarjit Kaur, Emmanuel Garcia-Villar, Nora Molasky, Stuart Belli, Joanna D. Sitek, Fabio Gionfra, Jens Z. Pedersen, and Sandra Incerpi. 2020. "Antioxidant Properties of Embelin in Cell Culture. Electrochemistry and Theoretical Mechanism of Scavenging. Potential Scavenging of Superoxide Radical through the Cell Membrane" Antioxidants 9, no. 5: 382. https://doi.org/10.3390/antiox9050382
APA StyleCaruso, F., Rossi, M., Kaur, S., Garcia-Villar, E., Molasky, N., Belli, S., Sitek, J. D., Gionfra, F., Pedersen, J. Z., & Incerpi, S. (2020). Antioxidant Properties of Embelin in Cell Culture. Electrochemistry and Theoretical Mechanism of Scavenging. Potential Scavenging of Superoxide Radical through the Cell Membrane. Antioxidants, 9(5), 382. https://doi.org/10.3390/antiox9050382