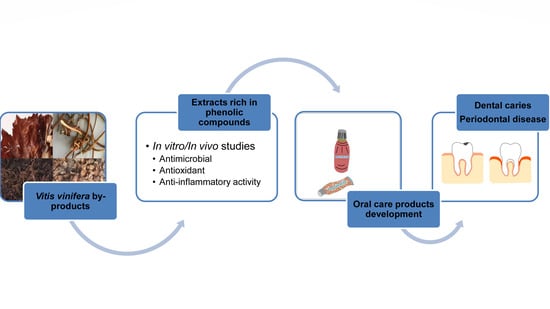
Research Advances in the Use of Bioactive Compounds from Vitis vinifera By-Products in Oral Care
Abstract
:1. Introduction
2. Active Compounds in V. vinifera By-Products
3. Bioactivity—Oral Cavity Conditions as Targets of Phenolic Compounds
3.1. Microbiota and Antimicrobial Activity of V. vinifera By-Products
3.1.1. Dental Caries
3.1.2. Antimicrobial Effects of V. vinifera Extracts in Dental Caries
3.1.3. Periodontal Disease
3.1.4. Antimicrobial Effects of V. vinifera Extracts in Periodontal Disease
3.2. Periodontal Inflammation
Antioxidant and Anti-Inflammatory Effects of V. vinifera Extracts in Periodontal Disease
3.3. Patent Review
3.4. Key-Points of the Oral Care Formulations based on V. vinifera By-Products
3.4.1. Selection of Ingredients for Oral Care
3.4.2. Formulation Design of Oral Care Products
4. Conclusions and Perspectives
- The use of emerging techniques with a low environmental impact and sustainable production costs for extract preparation.
- The use of GRAS solvents to obtain low toxicity and biocompatible extracts suitable for oral care products.
- The use of a systematic approach in cosmetic manufacturing that allows for the time-effective and cost-effective development of oral care products.
- Further studies should be conducted to assess the complex interaction between PhCs and inactive ingredients, and also between polyphenols and oral mucosa.
- The development of formulations that fulfill organoleptic characteristics required by consumers.
- The development of highly effective and cost-effective formulations that justify the recycling process.
Author Contributions
Funding
Conflicts of Interest
References
- Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 16 March 2020).
- Oral Infection/Inflammation as a Risk Factor for Systemic Diseases | FDI World Dental Federation. Available online: https://www.fdiworlddental.org/resources/policy-statements-and-resolutions/oral-infectioninflammation-as-a-risk-factor-for-systemic (accessed on 16 March 2020).
- Jin, L.J.; Lamster, I.B.; Greenspan, J.S.; Pitts, N.B.; Scully, C.; Warnakulasuriya, S. Global burden of oral diseases: Emerging concepts, management and interplay with systemic health. Oral Dis. 2016, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Lippert, F. An introduction to toothpaste - its purpose, history and ingredients. Monogr. Oral Sci. 2013, 23, 1–14. [Google Scholar] [PubMed]
- FAOSTAT, Food and Agriculture Organization of the United Nations. 2018. Available online: http://www.fao.org/faostat/en/?#data/QC (accessed on 28 May 2020).
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [Green Version]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H. Bin Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Zorraquín-Peña, I.; González de Llano, D.; Bartolomé, B.; Moreno-Arribas, M.V. The role of wine and food polyphenols in oral health. Trends Food Sci. Technol. 2017, 69, 118–130. [Google Scholar] [CrossRef]
- Pizzorno, J.E.; Murray, M.T.; Joiner-Bey, H.; Pizzorno, J.E.; Murray, M.T.; Joiner-Bey, H. Periodontal disease. In The Clinician’s Handbook of Natural Medicine; Churchill Livingstone: London, UK, 2016; pp. 787–795. [Google Scholar]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef] [Green Version]
- Soto, M.; Falqué, E.; Domínguez, H. Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todic, S.; Mutic, J.; Tesic, Z. Phenolic profiles of leaves, grapes and wine of grapevine variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraternale, D.; Ricci, D.; Verardo, G.; Gorassini, A.; Stocchia, V.; Sestili, P. Activity of Vitis vinifera tendrils extract against phytopathogenic fungi. Nat. Prod. Commun. 2015, 10, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- Singla, S.; Malhotra, R.; Shashikiran, N.D.; Saxena, S. Antibacterial efficacy of mouthwash prepared from pomegranate, grape seed and guava extracts against oral streptococci: An in vivo study. J. Clin. Pediatr. Dent. 2018, 42, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Bin, S.; Vallini, V.; Fava, F.; Michelini, E.; Roda, A.; Minnucci, G.; Bucchi, G.; Tassoni, A. Recovery of polyphenols from red grape pomace and assessment of their antioxidant and anti-cholesterol activities. New Biotechnol. 2016, 33, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Nieto, J.A.; Santoyo, S.; Prodanov, M.; Reglero, G.; Jaime, L. Valorisation of grape stems as a source of phenolic antioxidants by using a sustainable extraction methodology. Foods 2020, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, S.; Zhang, M.; Sun, B. Novel approach for extraction of grape skin antioxidants by accelerated solvent extraction: Box–Behnken design optimization. J. Food Sci. Technol. 2019, 56, 4879–4890. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Biasoto, A.C.T.; Schwember, A.R.; Granato, D.; Rasera, G.B.; Franchin, M.; Rosalen, P.L.; Alencar, S.M.; Shahidi, F. Should we ban total phenolics and antioxidant screening methods? The link between antioxidant potential and activation of NF-κB using phenolic compounds from grape by-products. Food Chem. 2019, 290, 229–238. [Google Scholar] [CrossRef]
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Lavelli, V.; Kerr, W.L.; García-Lomillo, J.; González-SanJosé, M.L. Applications of Recovered Bioactive Compounds in Food Products. In Handbook of Grape Processing by-Products: Sustainable Solutions; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 230–267. [Google Scholar]
- Nunes, M.A.; Rodrigues, F.; Oliveira, M.B.P.P. Grape Processing By-Products as Active Ingredients for Cosmetic Proposes. In Handbook of Grape Processing By-Products: Sustainable Solutions; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 267–292. [Google Scholar]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine by-Products: Phenolic characterization and antioxidant activity evaluation of grapes and grape pomaces from six different French grape varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef] [Green Version]
- Moldovan, M.; Bogdan, C.; Iurian, S.; Roman, C.; Oniga, I.; Benedec, D. Phenolic content and antioxidant capacity of pomace and canes extracts of some Vitis vinifera varieties cultivated in Romania. Farmacia 2020, 68, 15–21. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phyther. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Farida, B.; Cadot, Y.; Djamai, R.; Djermoun, L. Determination of major anthocyanin pigments and flavonols in red grape skin of some table grape varieties (Vitis vinifera sp.) by high-performance liquid chromatography-photodiode array detection (HPLC-DAD). OENO One 2016, 50, 125–135. [Google Scholar]
- Gouvinhas, I.; Santos, R.A.; Queiroz, M.; Leal, C.; Saavedra, M.J.; Domínguez-Perles, R.; Rodrigues, M.; Barros, A. Monitoring the antioxidant and antimicrobial power of grape (Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crops Prod. 2018, 126, 83–91. [Google Scholar] [CrossRef]
- Leal, C.; Santos, R.A.; Pinto, R.; Queiroz, M.; Rodrigues, M.; José Saavedra, M.; Barros, A.; Gouvinhas, I. Recovery of bioactive compounds from white grape (Vitis vinifera L.) stems as potential antimicrobial agents for human health. Saudi J. Biol. Sci. 2020, 27, 1009–1015. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Grape stem extracts: Polyphenolic content and assessment of their in vitro antioxidant properties. Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Moldovan, M.L.; Carpa, R.; Fizeșan, I.; Vlase, L.; Bogdan, C.; Iurian, S.M.; Benedec, D.; Pop, A. Phytochemical profile and biological activities of tendrils and leaves extracts from a variety of Vitis vinifera L. Antioxidants 2020, 9, 373. [Google Scholar] [CrossRef]
- Fraternale, D.; Rudov, A.; Prattichizzo, F.; Olivieri, F.; Ricci, D.; Giacomini, E.; Carloni, S.; Azzolini, C.; Gordillo, B.; Jara-Palacios, M.J. Chemical composition and “in vitro” anti-inflammatory activity of Vitis vinifera L.(var. Sangiovese) tendrils extract. J. Funct. Foods 2016, 20, 291–302. [Google Scholar] [CrossRef]
- European Medicines Agency Assessment report on Vitis vinifera L., folium - Final. Available online: www.ema.europa.eu/contact (accessed on 27 April 2020).
- Dresch, R.; Dresch, M.; Guerreiro, A.; Biegelmeyer, R.; Holzschuh, M.; Rambo, D.; Henriques, A. Phenolic compounds from the leaves of Vitis labrusca and Vitis vinifera L. as a source of waste byproducts: Development and validation of LC Method and antichemotactic activity. Food Anal. Methods 2014, 7, 527–539. [Google Scholar] [CrossRef]
- Montero, L.; Sáez, V.; von Baer, D.; Cifuentes, A.; Herrero, M. Profiling of Vitis vinifera L. canes (poly)phenolic compounds using comprehensive two-dimensional liquid chromatography. J. Chromatogr. A 2018, 1536, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Göktürk Baydar, N. Chemical composition of grape canes. Ind. Crops Prod. 2011, 34, 994–998. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Vine-shoot waste aqueous extracts for re-use in agriculture obtained by different extraction techniques: Phenolic, volatile, and mineral compounds. J. Agric. Food Chem. 2014, 62, 10861–10872. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.; Iurian, S.; Bischin Pușcaș, C.; Silaghi-Dumitrescu, R.; Hanganu, D.; Bogdan, C.; Vlase, L.; Oniga, I.; Benedec, D. A Design of Experiments strategy to enhance the recovery of polyphenolic compounds from Vitis vinifera by-products through heat reflux extraction. Biomolecules 2019, 9, 529. [Google Scholar] [CrossRef] [Green Version]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Sun, S.; Yang, F.; Zhou, K. UHPLC/MS Identifying potent α-glucosidase inhibitors of grape pomace via Enzyme Immobilized Method. J. Food Sci. 2018, 83, 1131–1139. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J.L. Valorisation potential of Cabernet grape pomace for the recovery of polyphenols: Process intensification, optimisation and study of kinetics. Food Bioprod. Process. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Ferri, M.; Rondini, G.; Calabretta, M.M.; Michelini, E.; Vallini, V.; Fava, F.; Roda, A.; Minnucci, G.; Tassoni, A. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017, 39, 51–58. [Google Scholar] [CrossRef]
- Meini, M.R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of phenolic antioxidants from Syrah grape pomace through the optimization of an enzymatic extraction process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef]
- Brianceau, S.; Turk, M.; Vitrac, X.; Vorobiev, E. Combined densification and pulsed electric field treatment for selective polyphenols recovery from fermented grape pomace. Innov. Food Sci. Emerg. Technol. 2015, 29, 2–8. [Google Scholar] [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of polyphenols and evaluation of antioxidant capacity in grape pomace of the cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef]
- Natolino, A.; Da Porto, C.; Rodríguez-Rojo, S.; Moreno, T.; Cocero, M.J. Supercritical antisolvent precipitation of polyphenols from grape marc extract. J. Supercrit. Fluids 2016, 118, 54–63. [Google Scholar] [CrossRef]
- Garrido, T.; Gizdavic-Nikolaidis, M.; Leceta, I.; Urdanpilleta, M.; Guerrero, P.; de la Caba, K.; Kilmartin, P.A. Optimizing the extraction process of natural antioxidants from chardonnay grape marc using microwave-assisted extraction. Waste Manag. 2019, 88, 110–117. [Google Scholar] [CrossRef]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of alternative physical treatments (Ultrasounds, Pulsed Electric Fields, and High-Voltage Electrical Discharges) on selective recovery of bio-compounds from fermented grape pomace. Food Bioprocess Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Rinaldi, A.; Iturmendi, N.; Jourdes, M.; Teissedre, P.L.; Moio, L. Transfer of tannin characteristics from grape skins or seeds to wine-like solutions and their impact on potential astringency. Food Sci. Technol. 2015, 63, 667–676. [Google Scholar] [CrossRef]
- Garcia-Jares, C.; Vazquez, A.; Lamas, J.P.; Pajaro, M.; Alvarez-Casas, M.; Lores, M.; Segura-Carretero, A.; Arrá Ez-Romá, D. Antioxidant white grape seed phenolics: Pressurized liquid extracts from different varieties. Antioxidants 2015, 4, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Chedea, V.S.; Echim, C.; Braicu, C.; Andjelkovic, M.; Verhe, R.; Socaciu, C. Composition in polyphenols and stability of aqueous grape seed extract from the Romanian variety ‘Merlot Recas’. J. Food Biochem. 2011, 35, 92–108. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Zawirska-Wojtasiak, R.; Gośliński, M. Phenolic compounds from winemaking waste and its antioxidant activity towards oxidation of rapeseed oil. Int. J. Food Sci. Technol. 2010, 45, 2272–2280. [Google Scholar] [CrossRef]
- Hernández-Jiménez, A.; Kennedy, J.A.; Bautista-Ortín, A.B.; Gómez-Plaza, E. Effect of ethanol on grape seed proanthocyanidin extraction. Am. J. Enol. Vitic. 2012, 63, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Kravchuk, O.; Skouroumounis, G.K.; Taylor, D.K. Response surface parallel optimization of extraction of total phenolics from separate white and red grape skin mixtures with microwave-assisted and conventional thermal methods. J. Clean. Prod. 2020, 251, 119563. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Jara-Palacios, M.J.; Hernández-Hierro, J.M.; Heredia, F.J. Evaluation of the influence of white grape seed extracts as copigment sources on the anthocyanin extraction from grape skins previously classified by near infrared hyperspectral tools. Food Chem. 2017, 221, 1685–1690. [Google Scholar] [CrossRef]
- Stavikova, L.; Polovka, M.; Hohnová, B.; Karásek, P.; Roth, M. Antioxidant activity of grape skin aqueous extracts from pressurized hot water extraction combined with electron paramagnetic resonance spectroscopy. Talanta 2011, 85, 2233–2240. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojcic Redovnikovic, I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Goutzourelas, N.; Stagos, D.; Spanidis, Y.; Liosi, M.; Apostolou, A.; Priftis, A.; Haroutounian, S.; Spandidos, D.A.; Tsatsakis, A.M.; Kouretas, D. Polyphenolic composition of grape stem extracts affects antioxidant activity in endothelial and muscle cells. Mol. Med. Rep. 2015, 12, 5846–5856. [Google Scholar] [CrossRef]
- Sun, H.; Lin, Q.; Wei, W.; Qin, G. Ultrasound-assisted extraction of resveratrol from grape leaves and its purification on mesoporous carbon. Food Sci. Biotechnol. 2018, 27, 1353–1359. [Google Scholar] [CrossRef]
- Aguilar, T.; Loyola, C.; de Bruijn, J.; Bustamante, L.; Vergara, C.; von Baer, D.; Mardones, C.; Serra, I. Effect of thermomaceration and enzymatic maceration on phenolic compounds of grape must enriched by grape pomace, vine leaves and canes. Eur. Food Res. Technol. 2016, 242, 1149–1158. [Google Scholar] [CrossRef]
- Jesus, M.S.; Ballesteros, L.F.; Pereira, R.N.; Genisheva, Z.; Carvalho, A.C.; Pereira-Wilson, C.; Teixeira, J.A.; Domingues, L. Ohmic heating polyphenolic extracts from vine pruning residue with enhanced biological activity. Food Chem. 2020, 316, 126298. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Plants and other natural products used in the management of oral infections and improvement of oral health. Acta Trop. 2016, 154, 6–18. [Google Scholar] [CrossRef]
- Digel, I.; Kern, I.; Geenen, E.M.; Akimbekov, N. Dental plaque removal by ultrasonic toothbrushes. Dent. J. 2020, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, M.C.; Ribeiro-Vidal, H.; Esteban-Fernández, A.; Bartolomé, B.; Figuero, E.; Moreno-Arribas, M.V.; Sanz, M.; Herrera, D. Antimicrobial activity of red wine and oenological extracts against periodontal pathogens in a validated oral biofilm model. BMC Complement. Altern. Med. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Abdul, W.M.; Hussain, M.M.; Razvi, S.S.I. Oral health and herbal medicine. In SpringerBriefs in Public Health; Springer International Publishing: New York, NY, USA, 2019; pp. 1–46. [Google Scholar]
- Levine, R.S. Advancing the scientific basis of oral health education. Community Dent. Health 2015, 32, 66–67. [Google Scholar]
- Milovanova-Palmer, J.; Pendry, B. Is there a role for herbal medicine in the treatment and management of periodontal disease? J. Herb. Med. 2018, 12, 33–48. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Wu, C.D. Grape Products and Oral Health. J. Nutr. 2009, 139, 1818S–1823S. [Google Scholar] [CrossRef] [Green Version]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus mutans, Candida albicans, and the human mouth: A sticky situation. PLoS Pathog. 2013, 9, e1003616. [Google Scholar] [CrossRef] [Green Version]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Chen, X.; Jiang, W.; Wang, S.; Xu, L.; Tu, Y.; Zheng, P.; Wang, Y.; Lin, X.; et al. Profiling of oral microbiota in early childhood caries using single-molecule real-time sequencing. Front. Microbiol. 2017, 8, 2244. [Google Scholar] [CrossRef]
- Kouidhi, B.; Al Qurashi, Y.M.A.; Chaieb, K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microb. Pathog. 2015, 80, 39–49. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Salvador, A.A.; Smânia, A.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef]
- Van Loveren, C.; Broukal, Z.; Oganessian, E. Functional foods/ingredients and dental caries. Eur. J. Nutr. 2012, 51, S15–S25. [Google Scholar] [CrossRef]
- Yadav, D.; Kumar, A.; Kumar, P.; Mishra, D. Antimicrobial properties of black grape (Vitis vinifera L.) peel extracts against antibiotic-resistant pathogenic bacteria and toxin producing molds. Ind. J. Pharmacol. 2015, 47, 663. [Google Scholar] [CrossRef] [Green Version]
- Rivero-Cruz, J.F.; Zhu, M.; Kinghorn, A.D.; Wu, C.D. Antimicrobial constituents of Thompson seedless raisins (Vitis vinifera) against selected oral pathogens. Phytochem. Lett. 2008, 1, 151–154. [Google Scholar] [CrossRef]
- Zhao, W.; Xie, Q.; Bedran-Russo, A.K.; Pan, S.; Ling, J.; Wu, C.D. The preventive effect of grape seed extract on artificial enamel caries progression in a microbial biofilm-induced caries model. J. Dent. 2014, 42, 1010–1018. [Google Scholar] [CrossRef]
- Furiga, A.; Lonvaud-Funel, A.; Dorignac, G.; Badet, C. In vitro anti-bacterial and anti-adherence effects of natural polyphenolic compounds on oral bacteria. J. Appl. Microbiol. 2008, 105, 1470–1476. [Google Scholar] [CrossRef]
- Thimothe, J.; Bonsi, I.A.; Padilla-Zakour, O.I.; Koo, H. Chemical characterization of red wine grape (Vitis vinifera and Vitis interspecific hybrids) and pomace phenolic extracts and their biological activity against Streptococcus mutans. J. Agric. Food Chem. 2007, 55, 10200–10207. [Google Scholar] [CrossRef]
- Furiga, A.; Lonvaud-Funel, A.; Badet, C. In vitro study of antioxidant capacity and antibacterial activity on oral anaerobes of a grape seed extract. Food Chem. 2009, 113, 1037–1040. [Google Scholar] [CrossRef]
- Furiga, A.; Roques, C.; Badet, C. Preventive effects of an original combination of grape seed polyphenols with amine fluoride on dental biofilm formation and oxidative damage by oral bacteria. J. Appl. Microbiol. 2014, 116, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-González, I.; Thurnheer, T.; Bartolomé, B.; Moreno-Arribas, M.V. Red wine and oenological extracts display antimicrobial effects in an oral bacteria biofilm model. J. Agric. Food Chem. 2014, 62, 4731–4737. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Sorg, J.; Spitzmüller, B.; Hannig, M.; Al-Ahmad, A. Polyphenolic beverages reduce initial bacterial adherence to enamel in situ. J. Dent. 2009, 37, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; Xie, Q.; Hara, A.T.; Bedran-Russo, A.K. Biomimetic approach for root caries prevention using a proanthocyanidin- rich agent. Caries Res. 2011, 45, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epasinghe, D.J.; Yiu, C.K.Y.; Burrow, M.F.; Hiraishi, N.; Tay, F.R. The inhibitory effect of proanthocyanidin on soluble and collagen-bound proteases. J. Dent. 2013, 41, 832–839. [Google Scholar] [CrossRef]
- Firouzmandi, M.; Shafiei, F.; Jowkar, Z.; Nazemi, F. Effect of silver diamine fluoride and proanthocyanidin on mechanical properties of caries-affected dentin. Eur. J. Dent. 2019, 13, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Bedran-Russo, A.K.B.; Pereira, P.N.R.; Duarte, W.R.; Drummond, J.L.; Yamauchi, M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80B, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Bedran-Russo, A.K.B.; Pashley, D.H.; Agee, K.; Drummond, J.L.; Miescke, K.J. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 330–334. [Google Scholar] [CrossRef] [PubMed]
- MacEdo, G.V.; Yamauchi, M.; Bedran-Russo, A.K. Effects of chemical cross-linkers on caries-affected dentin bonding. J. Dent. Res. 2009, 88, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Mu, C.; Shi, J.; Zhang, Q.; Shi, B.; Lin, W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Fang, M.; Xiao, Y.; Li, F.; Yu, L.; Zhao, S.; Shen, L.; Chen, J. The effect of transient proanthocyanidins preconditioning on the cross-linking and mechanical properties of demineralized dentin. J. Mater. Sci. Mater. Med. 2011, 22, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Al-Ammar, A.; Drummond, J.L.; Bedran-Russo, A.K. The use of collagen cross-linking agents to enhance dentin bond strength. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91B, 419–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellan, C.S.; Pereira, P.N.; Grande, R.H.M.; Bedran-Russo, A.K. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent. Mater. 2010, 26, 968–973. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.; Liu, R.; Xiao, Y.; Li, F.; Wang, D.; Hou, R.; Chen, J. Biomodification to dentin by a natural crosslinker improved the resin-dentin bonds. J. Dent. 2012, 40, 458–466. [Google Scholar] [CrossRef]
- Green, B.; Yao, X.; Ganguly, A.; Xu, C.; Dusevich, V.; Walker, M.P.; Wang, Y. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. J. Dent. 2010, 38, 908–915. [Google Scholar] [CrossRef] [Green Version]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral Biofilms: Development, control, and analysis. High-Throughput 2018, 7, E24. [Google Scholar]
- Subbarao, K.; Nattuthurai, G.; Sundararajan, S.; Sujith, I.; Joseph, J.; Syedshah, Y. Gingival crevicular fluid: An overview. J. Pharm. Bioallied Sci. 2019, 11, S135–S139. [Google Scholar] [CrossRef]
- Khurshid, Z.; Mali, M.; Naseem, M.; Najeeb, S.; Zafar, M. Human gingival crevicular fluids (GCF) proteomics: An overview. Dent. J. 2017, 5, 12. [Google Scholar] [CrossRef]
- Dahiya, P.; Kamal, R.; Gupta, R.; Bhardwaj, R.; Chaudhary, K.; Kaur, S. Reactive oxygen species in periodontitis. J. Indian Soc. Periodontol. 2013, 17, 411–416. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández, A.; Zorraquín-PenÌa, I.; Ferrer, M.D.; Mira, A.; Bartolomé, B.; González De Llano, D.; Victoria Moreno-Arribas, M. Inhibition of oral pathogens adhesion to human gingival fibroblasts by wine polyphenols alone and in combination with an oral probiotic. J. Agric. Food Chem. 2018, 66, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara-Hirata, C.; Yamashiro, K.; Yamamoto, T.; Aoyagi, H.; Ideguchi, H.; Kawamura, M.; Suzuki, R.; Ono, M.; Wake, H.; Nishibori, M.; et al. Anti-HMGB1 neutralizing antibody attenuates periodontal inflammation and bone resorption in a murine periodontitis model. Infect. Immun. 2018, 86, e00111-18. [Google Scholar] [CrossRef] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. IJBS 2008, 4, 89. [Google Scholar]
- Chen, S.; Meng, X.F.; Zhang, C. Role of NADPH oxidase-mediated reactive oxygen species in podocyte injury. Biomed Res. Int. 2013, 2013, 839761. [Google Scholar] [CrossRef] [Green Version]
- Rubiolo, J.A.; Mithieux, G.; Vega, F.V. Resveratrol protects primary rat hepatocytes against oxidative stress damage: Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008, 591, 66–72. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nichols, T.C.; Fischer, T.H.; Deliargyris, E.N.; Baldwin, A.S. Role of nuclear factor-kappa B (NF-kappa B) in inflammation, periodontitis, and atherogenesis. Ann. Periodontol. 2001, 6, 20–29. [Google Scholar] [CrossRef]
- Bondm, C. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase -1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc. Res. 2001, 50, 556. [Google Scholar] [CrossRef]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef]
- Badisa, R.B.; Tzakou, O.; Couladis, M.; Pilarinou, E. Cytotoxic activities of Salvia plants of the Labiatae family. Pharm. Biol. 2004, 42, 640–645. [Google Scholar] [CrossRef]
- Yucel-Lindberg, T.; Båge, T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 2013, 15, e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krayer, J.W.; Leite, R.S.; Kirkwood, K.L. Non-surgical chemotherapeutic treatment strategies for the management of periodontal diseases. Dent. Clin. 2010, 54, 13–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabholkar, C.S.; Shah, M.; Kathariya, R.; Bajaj, M.; Doshi, Y. Comparative evaluation of antimicrobial activity of pomegranate-containing mouthwash against oral-biofilm forming organisms: An in vitro microbial study. J. Clin. Diagn. Res. 2016, 10, ZC65. [Google Scholar] [CrossRef]
- Palaska, I.; Papathanasiou, E.; Theoharides, T.C. Use of polyphenols in periodontal inflammation. Eur. J. Pharmacol. 2013, 720, 77–83. [Google Scholar] [CrossRef]
- Forester, S.C.; Lambert, J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Tsao, R.; Li, H. Antioxidant properties in vitro and in vivo: Realistic assessments of efficacy of plant extracts. Plant Sci. Rev. 2012, 7, 11–13. [Google Scholar]
- Goutzourelas, N.; Stagos, D.; Housmekeridou, A.; Karapouliou, C.; Kerasioti, E.; Aligiannis, N.; Skaltsounis, A.L.; Spandidos, D.A.; Tsatsakis, A.M.; Kouretas, D. Grape pomace extract exerts antioxidant effects through an increase in GCS levels and GST activity in muscle and endothelial cells. Int. J. Mol. Med. 2015, 36, 433–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, C.-C.; McIntosh, M.K. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu. Rev. Nutr. 2011, 31, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Köhle, C.; Bock, K.W. Activation of coupled Ah receptor and Nrf2 gene batteries by dietary phytochemicals in relation to chemoprevention. Biochem. Pharmacol. 2006, 72, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, D.K. Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targets 2007, 6, 168–173. [Google Scholar] [CrossRef]
- Rizzo, A.; Bevilacqua, N.; Guida, L.; Annunziata, M.; Romano Carratelli, C.; Paolillo, R. Effect of resveratrol and modulation of cytokine production on human periodontal ligament cells. Cytokine 2012, 60, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, M.G.; Pires, P.R.; Ribeiro, F.V.; Pimentel, S.Z.; Casarin, R.C.V.; Cirano, F.R.; Tenenbaum, H.T.; Casati, M.Z. Systemic treatment with resveratrol and/or curcumin reduces the progression of experimental periodontitis in rats. J. Periodontal Res. 2017, 52, 201–209. [Google Scholar] [CrossRef]
- Zare Javid, A.; Hormoznejad, R.; Allah Yousefimanesh, H.; Zakerkish, M.; Haghighi-zadeh, M.H.; Dehghan, P.; Ravanbakhsh, M. The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phyther. Res. 2017, 31, 108–114. [Google Scholar] [CrossRef]
- Houde, V.; Grenier, D.; Chandad, F. Protective effects of grape seed proanthocyanidins against oxidative stress induced by lipopolysaccharides of periodontopathogens. J. Periodontol. 2006, 77, 1371–1379. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.-L.; Haroutounian, S.A. Bioactive non-coloured polyphenols content of grapes, wines and vinification by-products: Evaluation of the antioxidant activities of their extracts. Food Res. Int. 2010, 43, 805–813. [Google Scholar] [CrossRef]
- La, V.D.; Bergeron, C.; Gafner, S.; Grenier, D. Grape seed extract suppresses lipopolysaccharide-induced matrix metalloproteinase (MMP) secretion by macrophages and inhibits Human MMP-1 and− 9 activities. J. Periodontol. 2009, 80, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Zhang, X.H.; Seo, J.S. Suppression of oxidative stress by grape seed supplementation in rats. Nutr. Res. Pract. 2012, 6, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.G.; Zhang, X.Y.; Wu, Y.J.; Tian, X. Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta Pharmacol. Sin. 2001, 12, 1117–1120. [Google Scholar]
- Özden, F.O.; Sakallioğlu, E.E.; Sakallioğlu, U.; Ayas, B.; Erişgin, Z. Effects of grape seed extract on periodontal disease: An experimental study in rats. J. Appl. Oral Sci. 2017, 25, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Bonifait, L.; Grenier, D. Cranberry polyphenols: Potential benefits for dental caries and periodontal disease. J. Can. Dent. Assoc. (Tor.) 2010, 76, a130. [Google Scholar]
- Llobera, A. Study on the antioxidant activity of grape stems (Vitis vinifera). A preliminary assessment of crude extracts. Food Nutr. Sci. 2012, 3, 500–504. [Google Scholar]
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Hayes, A.W.; Tsatsakis, A.M.; Kouretas, D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef]
- Fraternale, D.; De Bellis, R.; Calcabrini, C.; Potenza, L.; Cucchiarini, L.; Mancini, U.; Dachà, M.; Ricci, D. Aqueous extract from Vitis vinifera tendrils is able to enrich keratinocyte antioxidant defences. Nat. Prod. Commun. 2011, 6, 1315–1319. [Google Scholar] [CrossRef] [Green Version]
- Esatbeyoglu, T.; Ewald, P.; Yasui, Y.; Yokokawa, H.; Wagner, A.E.; Matsugo, S.; Winterhalter, P.; Rimbach, G. Chemical characterization, free radical scavenging, and cellular antioxidant and anti-inflammatory properties of a stilbenoid-rich root extract of Vitis vinifera. Oxid. Med. Cell. Longev. 2016, 2016, 8591286. [Google Scholar] [CrossRef] [Green Version]
- Katalinić, V.; Generalić Mekinić, I.; Skroza, D.; Ljubenkov, I.; Teskera, A.; Konta, I.; Boban, M. Insight in the phenolic composition and antioxidative properties of Vitis vinifera leaves extracts. Croat. J. Food Sci. Technol. 2009, 1, 7–15. [Google Scholar]
- Sangiovanni, E.; Di Lorenzo, C.; Colombo, E.; Colombo, F.; Fumagalli, M.; Frigerio, G.; Restani, P.; Dell’Agli, M. The effect of in vitro gastrointestinal digestion on the anti-inflammatory activity of Vitis vinifera L. leaves. Food Funct. 2015, 6, 2453–2463. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Di Lorenzo, C.; Piazza, S.; Manzoni, Y.; Brunelli, C.; Fumagalli, M.; Magnavacca, A.; Martinelli, G.; Colombo, F.; Casiraghi, A.; et al. Vitis vinifera L. Leaf extract inhibits in vitro mediators of inflammation and oxidative stress involved in inflammatory-based skin diseases. Antioxidants 2019, 8, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marabini, L.; Melzi, G.; Lolli, F.; Dell’Agli, M.; Piazza, S.; Sangiovanni, E.; Marinovich, M. Effects of Vitis vinifera L. leaves extract on UV radiation damage in human keratinocytes (HaCaT). J. Photochem. Photobiol. B Biol. 2020, 204, 111810. [Google Scholar] [CrossRef] [PubMed]
- Aouey, B.; Samet, A.; Fetoui, H.; Simmonds, M.; Bouaziz, M. Anti-oxidant, anti-inflammatory, analgesic and antipyretic activities of grapevine leaf extract (Vitis vinifera) in mice and identification of its active constituents by LC–MS/MS analyses. Biomed. Pharmacother. 2016, 84, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Chidambara Murthy, K.N.; Singh, R.P.; Jayaprakasha, G.K. Antioxidant activities of grape (Vitis vinifera) pomace extracts. J. Agric. Food Chem. 2002, 50, 5909–5914. [Google Scholar] [CrossRef]
- Maluf, D.F.; Gonçalves, M.M.; D’Angelo, R.W.O.; Girassol, A.B.; Tulio, A.P.; Pupo, Y.M.; Farago, P.V. Cytoprotection of antioxidant biocompounds from grape pomace: Further exfoliant phytoactive ingredients for cosmetic products. Cosmetics 2018, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Torre, E.; Iviglia, G.; Cassinelli, C.; Morra, M.; Russo, N. Polyphenols from grape pomace induce osteogenic differentiation in mesenchymal stem cells. Int. J. Mol. Med. 2020, 45, 1721. [Google Scholar] [CrossRef] [Green Version]
- Russo, N.; Cassinelli, C.; Torre, E.; Morra, M.; Iviglia, G. Improvement of the physical properties of guided bone regeneration membrane from porcine pericardium by polyphenols-rich pomace extract. Materials 2019, 12, 2564. [Google Scholar] [CrossRef] [Green Version]
- Silveira, N.; Sandjo, L.P.; Biavatti, M.W. Spilanthol-containing products: A patent review (1996–2016). Trends Food Sci. Technol. 2018, 74, 107–111. [Google Scholar] [CrossRef]
- Lawlor, T.M. Oral Care Compositions. US6706256B2, 16 March 2004. Available online: https://patents.google.com/patent/US6706256B2/en (accessed on 28 April 2020).
- Patricia, M.; LOPESIO. Multi-Purpose Dental Appliance Cleaner. WO2012/112337Al, 23 August 2020. Available online: https://patents.google.com/patent/WO2012112337A1/en (accessed on 28 April 2020).
- Jean-Claude, V. Use of an Aqueous Grape Seed Extract Combined with at Least One Fluorine Salt to Combat the Formation or Accumulation of Dental Biofilm and Compositions Comprising Said Combination. US20100129297A1, 27 May 2010. Available online: https://patents.google.com/patent/US20100129297A1/en?oq=US20100129297A1 (accessed on 28 April 2020).
- Lawrence, J.S. Oral Rinse Composition and Method. US8273385B, 25 September 2012. Available online: https://patents.google.com/patent/US8273385B1/en?oq=US8273385B1 (accessed on 28 April 2020).
- Kamath, S.; Nair, R. Red Herbal Dentifrice. US7736629B2, 15 June 2010. Available online: https://patents.google.com/patent/US7736629B2/en?oq=US7736629B2 (accessed on 28 April 2020).
- Hurwitz, M.M. Oral Hygiene Tablets and Capsules for Direct Oral Delivery of Active Ingredients. US8728446B2, 20 May 2014. Available online: https://patents.google.com/patent/US8728446B2/en?oq=US8728446B2 (accessed on 28 April 2020).
- Mumoli, J.A. Single-Dose Quick-Dissolving Cleansing Agent with Medicinal Properties. US6664225B2, 16 December 2003. Available online: https://patents.google.com/patent/US6664225B2/en?oq=US6664225B2 (accessed on 28 April 2020).
- Mason, S. Dental Hygiene. In Poucher’s Perfumes, Cosmetics and Soaps; Butler, H., Ed.; Springer: Heidelberg, Germany, 2000; pp. 217–253. [Google Scholar]
- Requena, T.; Monagas, M.; Pozo-Bayón, M.A.; Martín-Álvarez, P.J.; Bartolomé, B.; del Campo, R.; Ávila, M.; Martínez-Cuesta, M.C.; Peláez, C.; Moreno-Arribas, M.V. Perspectives of the potential implications of wine polyphenols on human oral and gut microbiota. Trends Food Sci. Technol. 2010, 21, 332–344. [Google Scholar] [CrossRef]
- Braga, A.S.; Girotti, L.D.; de Melo Simas, L.L.; Pires, J.G.; Pelá, V.T.; Buzalaf, M.A.R.; Magalhães, A.C. Effect of commercial herbal toothpastes and mouth rinses on the prevention of enamel demineralization using a microcosm biofilm model. Biofouling 2019, 35, 796–804. [Google Scholar] [CrossRef]
- Iqbal, K.; Asmat, M.; Jawed, S.; Mushtaque, A.; Mohsin, F.; Hanif, S.; Sheikh, N. Role of different ingredients of tooth pastes and mouthwashes in oral health. JPDA 2011, 20, 163–170. [Google Scholar]
- Toothpastes. Available online: https://www.ada.org/en/member-center/oral-health-topics/toothpastes (accessed on 21 April 2020).
- Shim, Y.J.; Choi, J.H.; Ahn, H.J.; Kwon, J.S. Effect of sodium lauryl sulfate on recurrent aphthous stomatitis: A randomized controlled clinical trial. Oral Dis. 2012, 18, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Crichard, S.; Ling-Mountford, N.; Milward, M.; Hubber, N.; Platten, S.; Gupta, A.K.; Chapple, I.L.C. A randomised clinical study comparing the effect of Steareth 30 and SLS containing toothpastes on oral epithelial integrity (desquamation). J. Dent. 2019, 80, S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Patel, K. ‘Natural’ toothpastes. Br. Dent. J. 2019, 226, 912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolando, P.; Xiaojing, L.; Yuyan, Z.; Chengkang, T. Toothpaste Composition. WO2014059678A1, 24 April 2014. Available online: https://patents.google.com/patent/WO2014059678A1/en?oq=WO2014059678A1 (accessed on 14 May 2020).
- Perry, D.A.; Takei, H.H.; Do, J.H. Plaque biofilm control for the periodontal patient. In Newman and Carranza’s Clinical Periodontology; Elsevier—Health Sciences Division: Philadelphia, PA, USA, 2019; pp. 510–520. [Google Scholar]
- Nayudu, A.; Lam, T.; Ho, J.; Forghany, A.; Vu, T.; Ngo, W.; Ajdaharian, J.; Wilder-Smith, P. Plaque removal and gingival health after use of a novel dental gel: A clinical study. Dentistry 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vranić, E.; Lacević, A.; Mehmedagić, A.; Uzunović, A. Formulation ingredients for toothpastes and mouthwashes. Bosn. J. Basic Med. Sci. 2004, 4, 51–58. [Google Scholar] [CrossRef]
- Takenaka, S.; Ohsumi, T.; Noiri, Y. Evidence-based strategy for dental biofilms: Current evidence of mouthwashes on dental biofilm and gingivitis. Jpn. Dent. Sci. Rev. 2019, 55, 33–40. [Google Scholar] [CrossRef]
- Werner, C.W.D.A.; Seymour, R.A. Are alcohol containing mouthwashes safe? Br. Dent. J. 2009, 207, E19. [Google Scholar] [CrossRef] [Green Version]
- Mouthwash (Mouthrinse). Available online: https://www.ada.org/en/member-center/oral-health-topics/mouthrinse (accessed on 22 April 2020).
- Marchetti, E.; Tecco, S.; Caterini, E.; Casalena, F.; Quinzi, V.; Mattei, A.; Marzo, G. Alcohol-free essential oils containing mouthrinse efficacy on three-day supragingival plaque regrowth: A randomized crossover clinical trial. Trials 2017, 18, 154. [Google Scholar] [CrossRef] [Green Version]
- Gandini, S.; Negri, E.; Boffetta, P.; La Vecchia, C.; Boyle, P. Mouthwash and oral cancer risk—Quantitative meta-analysis of epidemiologic studies. Ann. Agric. Environ. Med. 2012, 19, 173–180. [Google Scholar]
- Aceves Argemí, R.; González Navarro, B.; Ochoa García-Seisdedos, P.; Estrugo Devesa, A.; López-López, J. Mouthwash with alcohol and oral oarcinogenesis: Systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2020, 20, 101407. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, J.; Grenier, D. Neutralizing effect of green tea epigallocatechin-3-gallate on nicotine-induced toxicity and chemokine (C-C motif) ligand 5 secretion in human oral epithelial cells and fibroblasts. J. Investig. Clin. Dent. 2012, 3, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Huang, F.M.; Tai, K.W.; Yang, L.C.; Chou, M.Y. Mechanisms of cytotoxicity of nicotine in human periodontal ligament fibroblast cultures in vitro. J. Periodontal Res. 2002, 37, 279–285. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.N.; Rupasinghe, H.P.V. Novel long chain fatty acid derivatives of quercetin-3-O-glucoside reduce cytotoxicity induced by cigarette smoke toxicants in human fetal lung fibroblasts. Eur. J. Pharmacol. 2016, 781, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Kovács, A.; Erős, I.; Csóka, I. Optimization and development of stable w/o/w cosmetic multiple emulsions by means of the Quality by Design approach. Int. J. Cosmet. Sci. 2016, 38, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Peres, D.D.; Ariede, M.B.; Candido, T.M.; de Almeida, T.S.; Lourenço, F.R.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; Baby, A.R. Quality by design (QbD), Process Analytical Technology (PAT), and design of experiment applied to the development of multifunctional sunscreens. Drug Dev. Ind. Pharm. 2017, 43, 246–256. [Google Scholar] [CrossRef]
- International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use Pharmaceutical Development Q8(R2). Available online: https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf (accessed on 28 April 2020).
- Bogdan, C.; Iurian, S.; Tomuta, I.; Moldovan, M. Improvement of skin condition in striae distensae: Development, characterization and clinical efficacy of a cosmetic product containing Punica granatum seed oil and Croton lechleri resin extract. Drug Des. Devel. Ther. 2017, 11, 521–531. [Google Scholar] [CrossRef] [Green Version]
| By-Product Type | Extraction Techniques | Solvent | Analytical Methods | Main Compounds | Reference | |
|---|---|---|---|---|---|---|
| Pomace |  | SLE—conventional shaker, UAE, Sequential UAE + SLE | Water 100%, EtOH 100%, Water:EtOH (3:1, 1:1, 1:3) | HPLC-UV/Vis | Phenolic acids: gallic acid, protocatechuic acid, hydroxybenzoic and hydroxycinnamic acids Flavanols: (+)-catechin, (−)-epicatechin Anthocyanins Flavonol derivatives: quercetin, laricitrin and syringetin derivatives Stilbenes: trans-resveratrol, piceatannol | [6,7,18,41,43,44,45,46,47,48,49,50,51,52] |
| EA, EtOH-based extraction | Ezymatic digestion, EtOH 95% (v/v) | HPLC-DAD | ||||
| EA, DoE | Aqueous enzymatic digestion, pH, t °C | HPLC-DAD | ||||
| PEF + SLE | Water: EtOH (1:1) | HPLC-UV/Vis | ||||
| SLE | Water: EtOH (1:1), 120 min, 60 °C | HPLC-UV/Vis | ||||
| EA, EtOH-based extraction | Enzymatic digestion or water, EtOH | HPLC-DAD | ||||
| SAS of EtOH extract, DoE | EtOH | HPLC-DAD | ||||
| MAE, DoE | EtOH 30–60%, 5–15 min | UHPLC-UV/Vis | ||||
| SLE, reflux method DoE | EtOH 40–60%, 50–65–80 °C | LC-MS | ||||
| HVED, US-SLE, PEF | Water | HPLC-UV/Vis | ||||
| Seeds |  | SPE | Water | HPLC-DAD HPLC-MS | Proanthocyanidins Phenolic acids: gallic acid, protocatechuic acid, syringic acid Polyunsaturated fatty acids: linoleic acid, oleic acid, palmitic acid, stearic acid Tocopherols Stilbenes: trans-resveratrol | [7,24,26,53,54,55,56,57] |
| SLE | 70% EtOH, 60 °C, 5 h | HPLC-UV/Vis | ||||
| SLE, maceration 2–10 days | EtOH 0–15% (v/v), water | HPLC-DAD | ||||
| SLE | Water, 70% EtOH | HPLC-UV-MS | ||||
| Skins |  | MAE, SLE, DoE | EtOH 40–80%, 50–70 °C | UV/Vis | Anthocyanins (red grapes): cyanidin, peonidin, petunidin, delphinidin, and malvidin Procyanidins Phenolic acids: hydroxycinnamic acids, gallic acid Catechins: catechin, epicatechin, epigallocatechin Flavonols: quercetin, quercetin derivatives kaempferol, kaempferol derivatives Stilbenes: trans-resveratrol, trans-polydatin | [20,25,53,58,59,60,61,62,63,64] |
| SLE, UAE, MAE | EtOH 8–92%, solid:liquid ratio (1:3–1:17) | HPLC-DAD | ||||
| PHWE | Water, 40–120 °C, 15 MPa, 3 × 5 min | HPLC-DAD | ||||
| SLE, MAE, UAE | Deep eutectic solvents, SLE-12 h MAE 50–90 °C, 15–90 min UAE 30–90 °C, 15–90 min | HPLC-DAD | ||||
| ASE, SLE, DoE | ASE EtOH 20–60%, 5–25 min, 40–80 °C SLE EtOH 49%, 5 h, 50° | HPLC-UV/Vis UHPLC-UV/Vis | ||||
| Stems |  | PLE, DoE | 0–100% EtOH, 40–120 °C, 1–11 min | RP-HPLC-DAD-MS; PLC-UV/Vis | Stilbenes: trans-resveratrol, piceatannol Flavanols: (+)-catechin Phenolic acids: gallic acid Procyanidins: procyanidin B3 | [19,33,65] |
| Superheated liquid EtOH, Supercritical EtOH extraction | EtOH, 60–300 °C, 1 h | Microplate reader (UV/Vis) | ||||
| Tendrils |  | SLE, reflux method | 50% EtOH, 60 °C, 30 min | LC-MS/MS | Phenolic acids: gallic acid, protocatechuic acid, caffeic acid, ellagic acid, caftaric acid Flavonols: rutin, quercetin-3-O-glucuronide Organic acids: fumaric acid, citric acid | [34,35] |
| SLE, reflux method | 70% EtOH, 60 °C, 20 h | HPLC-DAD | ||||
| Leaves |  | SLE, reflux method | 50% EtOH, 60 °C, 30 min | LC-MS/MS | Flavones: quercetin, kaempferol Anthocyans: anthocyanidins, procyanidins Flavonols: quercetol Glycoside flavonoids: hyperoside, isoquercitrin, quercitrin Flavanols: catechin, gallocatechin, epigallocatechin Stilbenes: trans-resveratrol | [34,36,37,66,67] |
| SLE, kinetic maceration | Water, 25 min | LC/MS-MS UV/Vis-LC-DAD | ||||
| UAE | 20–60% EtOH, 30–70 °C, 5–55 min solid:liquid ratio (1:10–1:30) | HPLC, UV/Vis | ||||
| Thermomaceration (skins, seeds, leaves canes), DoE | Grape must, 20–60 °C, 0–24 h | HPLC–DAD LC-MS/MS | ||||
| Canes |  | OH, conventional heating | 45% EtOH, 80 °C, 20–90 min | UHPLC-UV/Vis | Flavanols: (+)-catechin, (−)-epicatechin Phenolic acids: caffeic acid, trans-p-coutaric acid, trans-caftaric acid, gallic acid, syringic acid Stilbenes: trans-resveratrol Minerals: K, Ca, Fe, Mg, P, Zn | [39,40,41,68] |
| SLE | 60% EtOH, 80 °C, 30 min | HPLC-DAD | ||||
| SLE, SLDE, MAE, PLE | Water, SLE 100 °C, 15–60 min SLDE 25−27 °C, 8 bar, MAE 100 °C, 5–15 min, PLE 30–100 °C, 50–100 bar, 10–30 min | HPLC-DAD-MS HS-SBSE-GC-MS | ||||
| By-Product Type | Microbial Strains | Concentration Tested | Reference |
|---|---|---|---|
| Pomace | Staphylococcus aureus, Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, Candida parapsilosis, Candida krusei | 3.9–2000 μg/mL | [82] |
| Streptococcus mutans, Streptococcus sobrinus, Lactobacillus rhamnosus, Actinomyces viscosus, Fusobacterium nucleatum, Porphyromonas gingivalis | 2000–8000 μg/mL | [87] | |
| Streptococcus mutans | 62.5–500 µg/mL | [88] | |
| Black grape skin | Staphylococcus aureus, Enterococcus faecalis, Enterobacter aerogenes, Salmonella typhimurium, Escherichia coli, Penicillium chrysogenum, Penicillium expansum, Aspergillus niger, Aspergillus versicolor | 260 mg, 540 mg, 1080 mg TAE (tannic acid equivalents)/mL | [84] |
| Thompson seedless raisins | Streptococcus mutans, Porphyromonas gingivalis | 3.9–500 mg/mL | [85] |
| Seeds | Streptococcus mutans, Streptococcus sobrinus, Lactobacillus rhamnosus, Actinomyces viscosus, Porphyromonas gingivalis, Fusobacterium nucleatum | 1000–8000 µg/mL | [89] |
| Streptococcus mutans, Streptococcus sobrinus, Actinomyces viscosus, Lactobacillus rhamnosus, Porphyromonas gingivalis, Fusobacterium nucleatum | 250–8000 µg/mL | [90] | |
| Streptococcus mutans | 1–3 mg/mL | [86] | |
| Actinomyces oris, Fusobacterium nucleatum, Streptococcus oralis, Streptococcus mutans, Veillonella dispar | 10 g/L | [91] | |
| Red wine extract and seed extract | Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Porphyromonas gingivalis, Streptococcus oralis, Veillonella parvula, Actinomyces naeslundii | 20 g/L | [72] |
| By-Product Type | Type of Assay | Observed Effect | Concentration Tested | Reference | |
|---|---|---|---|---|---|
| Leaves | in vitro | HGF cell line | ↓ ROS production | 10–300 µg/mL | [34] |
| ↓ IL-1 beta, IL-6, IL-8 | |||||
| AGS cell line | ↓ IL-8, NF-κB Nuclear Translocation | 1–100 µg/mL | [148] | ||
| HaCaT cell line | ↓ IL-8, NF-κB Nuclear Translocation, VEGF | 1–100 µg/mL | [149] | ||
| in vivo | Swiss albino mice | ↓ Carrageenan-induced paw oedema | 100–400 mg/kg | [151] | |
| ↓ Acetic acid induced vascular permeability | |||||
| ↓ Yeast induced pyrexia | |||||
| Pomace | in vitro | C2C12 cell line | ↑ GCS levels, GST activity | 2.5 and 10 µg/mL | [129] |
| ↓ CAT levels + activity | |||||
| EA.hy926 cell line | ↑ GCS levels, GST activity | 0.068 and 0.250 µg/mL | |||
| 3T3 cell line | ↓ ROS production | 0.73–3.65 mg/mL | [153] | ||
| hMSCs | ↓ RANKL/OPG ratio | 10 and 20 µg/mL | [154] | ||
| ↑ BMP2 and Runx2 expression | |||||
| in vivo | Wistar rats treated with CCl4 | ↑ CAT, SOD, peroxidase activity | 50 mg/kg | [152] | |
| ↓ MDA levels | |||||
| Root | in vitro | Huh7 cell line | ↑ Nrf2 transactivation | 1–50 µg/mL | [145] |
| ↑ HO-1 and GCS | |||||
| RAW264.7 cell line | ↓ IL-1β and iNOS genes | 20 µg/mL | |||
| Tendrils | in vitro | HGF cell line | ↓ ROS production | 10–300 µg/mL | [34] |
| ↓ IL-1 beta, IL-6, IL-8 | |||||
| NCTC 2544 cell line | ↑ GSH levels | 12.5–62.5 mg/mL | [145] | ||
| Seeds | in vitro | RAW 264.7 cell line | ↓ ROS production | 0.5–100 µg/mL | [136] |
| ↓ NO production | |||||
| ↓ iNOS expression | |||||
| HVTs-SM1 cell line | ↓ ROS production | 1–100 µg/mL | [137] | ||
| Monocyte (U937)-Derived Macrophages | ↓ MMP-1, -7, -8, -9, and -13 secretion | 25–100 µg/mL | [138] | ||
| ↓ activation of NF-kB p65 | |||||
| ↓ AP-1 activation | |||||
| ↓ MMP-1 and -9 activity | |||||
| in vivo | Sprague-Dawley rats | ↓ lipid peroxide | diet with 5% grape seed | [139] | |
| ↑ hepatic GST activity | |||||
| ↑ GSH/GSSG | |||||
| Kunming mice | ↓ Croton oil-induced ear oedema | 10–40 mg/kg proanthocyanidins fraction | [140] | ||
| Wistar rats | ↓ Carrageenan-induced paw oedema | ||||
| ↓ MDA, NOS activity, NO, IL-1β, TNF-α, PGE2 | |||||
| Sprague Dawley rats | ↓ Inflammatory cell number | 200 mg/kg | [141] | ||
| ↑ Connective tissues attachment level | |||||
| ↓ Osteoclast density | |||||
| Stem | in vitro | HVTs-SM1 cell line | ↓ ROS production | 1.1–100 µg/mL | [33] |
| C2C12 cell line | ↓ ROS production | 0.95 µg/mL | [65] | ||
| ↑ GSH levels | |||||
| ↓ lipid and protein peroxidation | |||||
| EA.hy926 cell line | ↓ lipid and protein peroxidation | 0.20 µg/mL | |||
| ↑ GSH levels | |||||
| Type of Oral Care Product | Type of Extract | Bioactive Compounds | Other Actives in the Product | Claimed Effects | Patent/Patent Application Number |
|---|---|---|---|---|---|
| Toothpaste Oral rinse | V. vinifera seed or pulp extracts | Polyphenols | Potassium nitrate Metal cations salts Polyphosphates, pyrophosphates, phosphonates Fluoride ion source Xylitol | Prevention or treatment of halitosis Antimicrobial effect | US 6,706,256 B2 [157] |
| Rinse, wet wipe towelettes or spray for dental appliances hygiene | V. vinifera seed or pulp extracts | Polyphenols | α- hydroxy- acid, hydrogen peroxide, denatured alcohol, or ethanol | Antimicrobial effect Anti-odor effect Stain remover | US 2012/0207806 A1 [158] |
| Oral hygiene composition | V. vinifera seed aqueous extract | Polyphenols, mainly oligo-proanthocyanidin | Inorganic fluorine salts | Anti-biofilm effect Reduced microbial colonization | US 2010/0129297 A1 [159] |
| Oral rinse | V. vinifera seed extract | Polyphenols | Essential oil Hydrogen peroxide Alcohol | Antimicrobial Anti-inflammatory | US 8,273,385 B1 [160] |
| Dentifrice | V. vinifera extract | Not mentioned | Calcium carbonate Red iron oxide | Astringent effect Antibacterial Anti- inflammatory | US 7,736,629 B2 [161] |
| Oral hygiene tablets and capsules | V. vinifera skin extract | Anthocyanins | Other herbal ingredients | Anti-inflammatory Soothing effect Protective effect on gums and mouth tissue | US 8,728,446 B2 [162] |
| Quick-dissolving cleansing agent | V. vinifera extract | Not mentioned | Sodium fluoride | Antioxidant Vasoconstriction Astringent effect | US 6,664,225 B2 [163] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdan, C.; Pop, A.; Iurian, S.M.; Benedec, D.; Moldovan, M.L. Research Advances in the Use of Bioactive Compounds from Vitis vinifera By-Products in Oral Care. Antioxidants 2020, 9, 502. https://doi.org/10.3390/antiox9060502
Bogdan C, Pop A, Iurian SM, Benedec D, Moldovan ML. Research Advances in the Use of Bioactive Compounds from Vitis vinifera By-Products in Oral Care. Antioxidants. 2020; 9(6):502. https://doi.org/10.3390/antiox9060502
Chicago/Turabian StyleBogdan, Cătălina, Anca Pop, Sonia M. Iurian, Daniela Benedec, and Mirela L. Moldovan. 2020. "Research Advances in the Use of Bioactive Compounds from Vitis vinifera By-Products in Oral Care" Antioxidants 9, no. 6: 502. https://doi.org/10.3390/antiox9060502
APA StyleBogdan, C., Pop, A., Iurian, S. M., Benedec, D., & Moldovan, M. L. (2020). Research Advances in the Use of Bioactive Compounds from Vitis vinifera By-Products in Oral Care. Antioxidants, 9(6), 502. https://doi.org/10.3390/antiox9060502



_Cherfan.png)