Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Extraction Techniques
2.3.1. Soxhlet Extraction (SE)
2.3.2. Ultrasound-Assisted Extraction
2.3.3. Microwave-Assisted Extraction
2.3.4. Supercritical Fluid Extraction
2.3.5. Extraction Yield
2.4. Chemical and Antioxidant Characterization of Grape Seeds Oils
2.4.1. Fatty Acid Profiles
2.4.2. Functional Quality
2.4.3. Tocopherols
2.5. Determination of In Vitro Antioxidant Capacity
2.5.1. DPPH Assay
2.5.2. ABTS Assay
2.6. Statistical Analysis
3. Results
3.1. Influence of SFE Operating Parameters on Total Extraction Yield
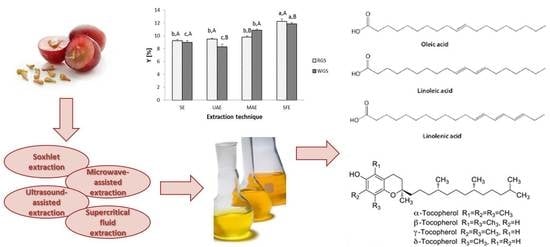
3.2. Influence of Different Extraction Techniques on Total Extraction Yield
3.3. Fatty Acid Profile and Functional Quality
3.4. Tocopherol Content
3.5. In Vitro Antioxidant Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Duba, K.S.; Fiori, L. Supercritical CO2 extraction of grape seed oil: Effect of process parameters on the extraction kinetics. J. Supercrit. Fluids 2015, 98, 33–43. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordiga, M.; Travaglia, F.; Locatelli, M.; Arlorio, M.; Coïsson, J.D. Spent grape pomace as a still potential by-product. Int. J. Food Sci. Technol. 2015, 50, 2022–2031. [Google Scholar] [CrossRef]
- Rodríguez, J.M.L.; Ruiz, D.F. Grape Seeds: Nutrient Content, Antioxidant Properties and Health Benefits; Nova Science Publishers, Inc.: New York, NY, USA, 2016. [Google Scholar]
- Dwyer, K.; Hosseinian, F.; Rod, M. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- Domínguez, J.; Martínez-Cordeiro, H.; Lores, M. Earthworms and grape marc: Simultaneous production of a high-quality biofertilizer and bioactive-rich seeds. In Grape and Wine Biotechnology; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Fidelis, M.; De Moura, C.; Junior, T.K.; Pap, N.; Mattila, P.H.; Mäkinen, S.; Putnik, P.; Kovačević, D.B.; Tian, Y.; Yang, B.; et al. Fruit seeds as sources of bioactive compounds: Sustainable production of high value-added ingredients from by-products within circular economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Huang, J.; Chen, Y.; Hou, Q.; Huang, M. Effect of grape seed extract combined with modified atmosphere packaging on the quality of roast chicken. Poult. Sci. 2020, 99, 1598–1605. [Google Scholar] [CrossRef]
- Ben Mohamed, H.; Duba, K.S.; Fiori, L.; Abdelgawed, H.; Tlili, I.; Tounekti, T.; Zrig, A. Bioactive compounds and antioxidant activities of different grape (Vitis vinifera L.) seed oils extracted by supercritical CO2 and organic solvent. Lwt Food Sci. Technol. 2016, 74, 557–562. [Google Scholar] [CrossRef]
- Karaman, S.; Karasu, S.; Tornuk, F.; Toker, O.S.; Geçgel, Ü.; Sagdic, O.; Ozcan, N.; Gül, O. Recovery potential of cold press byproducts obtained from the edible oil industry: Physicochemical, bioactive, and antimicrobial properties. J. Agric. Food Chem. 2015, 63, 2305–2313. [Google Scholar] [CrossRef]
- Lachman, J.; Hejtmánková, A.; Hejtmánková, K.; Horníčková, Š.; Pivec, V.; Skála, O.; Dědina, M.; Přibyl, J. Towards complex utilisation of winemaking residues: Characterisation of grape seeds by total phenols, tocols and essential elements content as a by-product of winemaking. Ind. Crop. Prod. 2013, 49, 445–453. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J. Antioxidant and antiproliferative properties of a tocotrienol-rich fraction from grape seeds. Food Chem. 2009, 114, 1386–1390. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2019, 57, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, M.; Morgan, M.T.; Dia, V.; D’Souza, D.H. Heat sensitization of hepatitis A virus and Tulane virus using grape seed extract, gingerol and curcumin. Food Microbiol. 2020, 90, 103461. [Google Scholar] [CrossRef] [PubMed]
- Konuskan, D.B.; Kamiloglu, O.; Demirkeser, O. Fatty acid composition, total phenolic content and antioxidant activity of grape seed oils obtained by cold-pressed and solvent extraction. Indian J. Pharm. Educ. Res. 2019, 53, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Bélliard, T.; Castello, J.; Van Hecke, É.; Lanoisellé, J.-L. Grape seed oil extraction: Interest of supercritical fluid extraction and gas-assisted mechanical extraction for enhancing polyphenol co-extraction in oil. Comptes Rendus Chim. 2014, 17, 284–292. [Google Scholar] [CrossRef]
- De Lopes Menezes, M.; Johann, G.; Diório, A.; Pereira, N.C.; da Silva, E.A. Phenomenological determination of mass transfer parameters of oil extraction from grape biomass waste. J. Clean. Prod. 2018, 176, 130–139. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M. Effect of cold press and soxhlet extraction systems on fatty acid, tocopherol contents, and phenolic compounds of various grape seed oils. J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef] [Green Version]
- Passos, C.P.; Silva, R.M.; Da Silva, F.A.; Coimbra, M.A.; Silva, C.M. Supercritical fluid extraction of grape seed (Vitis vinifera L.) oil. Effect of the operating conditions upon oil composition and antioxidant capacity. Chem. Eng. J. 2010, 160, 634–640. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2019, 19, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Barba, F.J.; Zhu, Z.Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Böger, B.R.; Salviato, A.; Valezi, D.F.; Di Mauro, E.; Georgetti, S.R.; Kurozawa, L.E. Optimization of ultrasound-assisted extraction of grape-seed oil to enhance process yield and minimize free radical formation. J. Sci. Food Agric. 2018, 98, 5019–5026. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V.; Gariépy, Y.; Thangavel, K. Optimization of microwave-assisted extraction of phenolic antioxidants from grape seeds (Vitis vinifera). Food Bioprocess Technol. 2012, 6, 441–455. [Google Scholar] [CrossRef]
- Makarova, N.V.; Valiulina, D.F.; Eremeeva, N.B. Comparative studies of extraction methods of biologically-active substances with antioxidant properties from grape seed (Vitis vinifera L.). Proc. Univ. Appl. Chem. Biotechnol. 2020, 10, 140–148. [Google Scholar] [CrossRef]
- Duba, K.S.; Fiori, L. Solubility of grape seed oil in supercritical CO2: Experiments and modeling. J. Chem. Thermodyn. 2016, 100, 44–52. [Google Scholar] [CrossRef]
- Coelho, J.P.; Filipe, R.M.; Robalo, M.P.; Stateva, R.P. Recovering value from organic waste materials: Supercritical fluid extraction of oil from industrial grape seeds. J. Supercrit. Fluids 2018, 141, 68–77. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Prado, J.M.; Dalmolin, I.; Carareto, N.D.D.; Basso, R.C.; Meirelles, A.J.A.; Vladimir Oliveira, J.; Batista, E.A.C.; Meireles, M.A.A. Supercritical fluid extraction of grape seed: Process scale-up, extract chemical composition and economic evaluation. J. Food Eng. 2012, 109, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Gayas, B.; Kaur, G.; Gul, K. Ultrasound-assisted extraction of apricot kernel oil: Effects on functional and rheological properties. J. Food Process Eng. 2017, 40, e12439. [Google Scholar] [CrossRef]
- Zeković, Z.; Pintać, D.; Majkić, T.; Vidović, S.; Mimica-Dukić, N.; Teslić, N.; Versari, A.; Pavlić, B. Utilization of sage by-products as raw material for antioxidants recovery-Ultrasound versus microwave-assisted extraction. Ind. Crop. Prod. 2017, 99, 49–59. [Google Scholar] [CrossRef]
- Pekić, B.; Zeković, Z.; Petrović, L.; Tolić, A. Behavior of (–)-α-bisabolol and (–)-α-bisabololoxides A and B in camomile flower extraction with supercritical carbon dioxide. Sep. Sci. Technol. 1995, 30, 3567–3576. [Google Scholar] [CrossRef]
- Ivanov, D.; Čolović, R.; Bera, O.; Lević, J.; Sredanović, S. Supercritical fluid extraction as a method for fat content determination and preparative technique for fatty acid analysis in mesh feed for pigs. Eur. Food Res. Technol. 2011, 233, 343–350. [Google Scholar] [CrossRef]
- Cunha, V.M.B.; Silva, M.P.d.; Sousa, S.H.B.d.; Bezerra, P.d.N.; Menezes, E.G.O.; Silva, N.J.N.d.; Banna, D.A.D.d.S.; Araújo, M.E.; Carvalho Junior, R.N.D. Bacaba-de-leque (Oenocarpus distichus Mart.) oil extraction using supercritical CO2 and bioactive compounds determination in the residual pulp. J. Supercrit. Fluids 2019, 144, 81–90. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Eisenmenger, M.; Dunford, N.T.; Eller, F.; Taylor, S.; Martinez, J. Pilot-scale supercritical carbon dioxide extraction and fractionation of wheat germ oil. J. Am. Oil Chem. Soc. 2006, 83, 863–868. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pavlić, B.; Bera, O.; Teslić, N.; Vidović, S.; Parpinello, G.; Zeković, Z. Chemical profile and antioxidant activity of sage herbal dust extracts obtained by supercritical fluid extraction. Ind. Crop. Prod. 2018, 120, 305–312. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Peter Savage, G. Chemical, rheological and nutritional characteristics of sesame and olive oils blended with linseed oil. Adv. Pharm. Bull. 2018, 8, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Siano, F.; Straccia, M.C.; Paolucci, M.; Fasulo, G.; Boscaino, F.; Volpe, M.G. Physico-chemical properties and fatty acid composition of pomegranate, cherry and pumpkin seed oils. J. Sci. Food Agric. 2016, 96, 1730–1735. [Google Scholar] [CrossRef]
- Tangolar, S.G.; Özogul, F.; Tangolar, S.; Yağmur, C. Tocopherol content in fifteen grape varieties obtained using a rapid HPLC method. J. Food Compos. Anal. 2011, 24, 481–486. [Google Scholar] [CrossRef]
- Sabir, A.; Unver, A.; Kara, Z. The fatty acid and tocopherol constituents of the seed oil extracted from 21 grape varieties (Vitis spp.). J. Sci. Food Agric. 2012, 92, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Bravi, M.; Spinoglio, F.; Verdone, N.; Adami, M.; Aliboni, A.; D’Andrea, A.; De Santis, A.; Ferri, D. Improving the extraction of α-tocopherol-enriched oil from grape seeds by supercritical CO2. Optimisation of the extraction conditions. J. Food Eng. 2007, 78, 488–493. [Google Scholar] [CrossRef]
- Sovilj, M. Critical review of supercritical carbon dioxide extraction of selected oil seeds. Acta Period. Technol. 2010, 10, 105–120. [Google Scholar] [CrossRef]
- Molero Gómez, A.; Pereyra López, C.; Martinez de la Ossa, E. Recovery of grape seed oil by liquid and supercritical carbon dioxide extraction: A comparison with conventional solvent extraction. Chem. Eng. J. Biochem. Eng. J. 1996, 61, 227–231. [Google Scholar] [CrossRef]
- Jokić, S.; Bijuk, M.; Aladić, K.; Bilić, M.; Molnar, M. Optimisation of supercritical CO2 extraction of grape seed oil using response surface methodology. Int. J. Food Sci. Technol. 2016, 51, 403–410. [Google Scholar] [CrossRef]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochemistry 2013, 20, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Hejtmánková, A.; Táborský, J.; Kotíková, Z.; Pivec, V.; Střalková, R.; Vollmannová, A.; Bojňanská, T.; Dědina, M. Evaluation of oil content and fatty acid composition in the seed of grapevine varieties. Lwt Food Sci. Technol. 2015, 63, 620–625. [Google Scholar] [CrossRef]
- Wang, S.; Yang, R.; Li, H.; Jiang, J.; Zhang, L.; Zhang, Q.; Li, P. Evaluation and comparison of in vitro antioxidant activities of unsaponifiable fraction of 11 kinds of edible vegetable oils. Food Sci. Nutr. 2018, 6, 2355–2362. [Google Scholar] [CrossRef]



| Sample | Extraction Technique | Process Conditions |
|---|---|---|
| Red grape seeds (RGS) | ||
| RGS-SFE1 | Supercritical fluid extraction | 250 bar, 40 °C, 0.3 kg h−1 |
| RGS-SFE2 | 300 bar, 40 °C, 0.3 kg h−1 | |
| RGS-SFE3 | 350 bar, 40 °C, 0.3 kg h−1 | |
| RGS-SFE4 | 350 bar, 50 °C, 0.3 kg h−1 | |
| RGS-SFE5 | 350 bar, 60 °C, 0.3 kg h−1 | |
| RGS-SFE6 | 350 bar, 60 °C, 0.2 kg h−1 | |
| RGS-SFE7 | 350 bar, 60 °C, 0.4 kg h−1 | |
| RGS-SFE315 | 350 bar, 60 °C, 0.4 kg h−1, 315 < d < 800 µm | |
| RGS-SFE800 | 350 bar, 60 °C, 0.4 kg h−1, d > 800 µm | |
| RGS-UAE | Ultrasound-assisted extraction | solvent: n-hexane, 40 kHz, 50 °C, 40 min, 60 W L−1 |
| RGS-MAE | Microwave-assisted extraction | solvent: n-hexane, 600 W, 15 min |
| RGS-SE | Soxhlet extraction | solvent: n-hexane, 6 h, 15 exchanges of extract |
| White grape seeds (WGS) | ||
| WGS-SFE | Supercritical fluid extraction | 350 bar, 60 °C, 0.4 kg h−1 |
| WGS-UAE | Ultrasound-assisted extraction | solvent: n-hexane, 40 kHz, 50 °C, 40 min, 60 W L−1 |
| WGS-MAE | Microwave-assisted extraction | solvent: n-hexane, 600 W, 15 min |
| WGS-SE | Soxhlet extraction | solvent: n-hexane, 6 h, 15 exchanges of extract |
| Fatty Acid | Palmitic (C16:0) | Palmitoleic (C16:1) | Stearic (18:0) | Oleic (C18:1n9C) | Linoleic (C18:2n6C) | γ-Linolenic (C18:3n6C) | α-Linolenic (C18:3n3C) | Heneicosanoic (C21:0) | Saturated Fatty Acids | Monounsaturated Fatty Acids | Polyunsaturated Fatty Acids | Unsaturated Fatty Acids | Ratio S/U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red grape seeds | |||||||||||||
| RGS-SFE | 7.93 | 0.14 | 3.79 | 13.39 | 73.58 | 0.18 | 0.66 | 0.34 | 12.06 | 13.53 | 74.42 | 87.94 | 0.14 |
| RGS-UAE | 7.20 | 0.12 | 3.83 | 13.72 | 74.15 | 0.21 | 0.52 | 0.25 | 11.28 | 13.84 | 74.88 | 88.72 | 0.13 |
| RGS-MAE | 7.33 | 0.14 | 4.33 | 16.18 | 71.09 | 0.24 | 0.45 | 0.25 | 11.91 | 16.31 | 71.78 | 88.09 | 0.14 |
| RGS-SE | 7.42 | 0.13 | 3.93 | 14.03 | 73.45 | 0.23 | 0.55 | 0.26 | 11.61 | 14.16 | 74.23 | 88.39 | 0.13 |
| White grape seeds | |||||||||||||
| WGS-SFE | 7.73 | 0.14 | 4.29 | 17.57 | 69.37 | 0.23 | 0.41 | 0.25 | 12.27 | 17.71 | 70.02 | 87.73 | 0.14 |
| WGS-UAE | 7.66 | 0.17 | 4.24 | 18.39 | 68.65 | 0.24 | 0.42 | 0.23 | 12.13 | 18.56 | 69.31 | 87.87 | 0.14 |
| WGS-MAE | 7.29 | 0.13 | 4.27 | 17.91 | 69.83 | 0.00 | 0.39 | 0.19 | 11.74 | 18.03 | 70.22 | 88.26 | 0.13 |
| WGS-SE | 7.51 | 0.16 | 4.37 | 18.47 | 68.61 | 0.25 | 0.40 | 0.24 | 12.11 | 18.62 | 69.27 | 87.89 | 0.14 |
| Sample | AI | TI | H/H |
|---|---|---|---|
| Red grape seeds | |||
| SFE7-RGS | 0.090 | 0.257 | 11.07 |
| SE-RGS | 0.081 | 0.242 | 12.28 |
| UAE-RGS | 0.083 | 0.258 | 11.97 |
| MAE-RGS | 0.084 | 0.249 | 11.86 |
| White grape seeds | |||
| SFE1-WGS | 0.088 | 0.268 | 11.30 |
| SE-WGS | 0.087 | 0.264 | 11.42 |
| UAE-WGS | 0.083 | 0.256 | 12.09 |
| MAE-WGS | 0.085 | 0.264 | 11.65 |
| Sample | Parameter | α-Tocopherol | γ-Tocopherol | Total Tocopherols |
|---|---|---|---|---|
| Pressure (bar) | ||||
| RGS1-SFE | 250 | 5.65 ± 0.20 a | 1.35 ± 0.09 a | 7.00 ± 0.29 a |
| RGS2-SFE | 300 | 5.15 ± 0.10 b | 1.29 ± 0.04 a | 6.45 ± 0.14 b |
| RGS3-SFE | 350 | 5.05 ± 0.07 b | 1.35 ± 0.02 a | 6.40 ± 0.05 b |
| Temperature (°C) | ||||
| RGS3-SFE | 40 | 5.05 ± 0.07 b | 1.35 ± 0.02 b | 6.40 ± 0.05 b |
| RGS4-SFE | 50 | 4.85 ± 0.07 b | 1.26 ± 0.02 c | 6.11 ± 0.07 b |
| RGS5-SFE | 60 | 6.18 ± 0.33 a | 1.76 ± 0.02 a | 7.94 ± 0.31 a |
| Solvent flow rate (kg h−1) | ||||
| RGS6-SFE | 0.2 | 7.84 ± 0.04 a | 1.65 ± 0.09 a | 9.49 ± 0.09 a |
| RGS5-SFE | 0.3 | 6.18 ± 0.33 b | 1.76 ± 0.02 a | 7.94 ± 0.31 b |
| RGS7-SFE | 0.4 | 5.35 ± 0.10 c | 1.16 ± 0.02 b | 6.51 ± 0.12 c |
| Particle size (µm) | ||||
| RGS315-SFE | 315–800 | 6.18 ± 0.33 a | 1.39 ± 0.09 a | 7.57 ± 0.43 a |
| RGS800-SFE | >800 | 3.63 ± 0.03 b | 0.98 ± 0.06 b | 4.60 ± 0.02 b |
| Sample | α-Tocopherol | γ-Tocopherol | Total Tocopherols |
|---|---|---|---|
| Red grape seeds | |||
| RGS-SFE | 5.35 ± 0.10 b | 1.16 ± 0.02 b | 6.51 ± 0.12 b |
| RGS-SOX | 4.85 ± 0.06 c | 1.48 ± 0.06 a | 6.33 ± 0.03 c |
| RGS-UAE | 6.51 ± 0.05 a | 1.41 ± 0.04 a | 7.92 ± 0.04 a |
| RGS-MAE | 6.51 ± 0.09 a | 1.44 ± 0.04 a | 7.96 ± 0.04 a |
| White grape seeds | |||
| WGS-SFE1 | 0.44 ± 0.03 c | 0.51 ± 0.04 c | 0.95 ± 0.07 d |
| WGS-SOX | 1.47 ± 0.13 b | 0.90 ± 0.09 a | 2.37 ± 0.04 b |
| WGS-UAE | 1.47 ± 0.03 b | 0.71 ± 0.02 b | 2.18 ± 0.02 c |
| WGS-MAE | 1.90 ± 0.03 a | 0.73 ± 0.04 b | 2.63 ± 0.02 a |
| Sample | Parameter | DPPH | ABTS |
|---|---|---|---|
| Pressure (bar) | |||
| RGS1-SFE | 250 | 1.46 ± 0.36 a | 6.26 ± 0.17 a |
| RGS2-SFE | 300 | 1.35 ± 0.07 a | 3.14 ± 0.18 b |
| RGS3-SFE | 350 | 1.58 ± 0.16 a | 3.75 ± 0.56 b |
| Temperature (°C) | |||
| RGS3-SFE | 40 | 1.58 ± 0.16 a | 3.75 ± 0.56 a |
| RGS4-SFE | 50 | 1.61 ± 0.12 a | 3.66 ± 0.10 a |
| RGS5-SFE | 60 | 1.43 ± 0.18 a | 3.78 ± 0.32 a |
| Solvent flow rate (kg CO2 h−1) | |||
| RGS6-SFE | 0.2 | 1.87 ± 0.04 a | 4.12 ± 0.40 a,b |
| RGS5-SFE | 0.3 | 1.43 ± 0.18 b | 3.78 ± 0.32 b |
| RGS7-SFE | 0.4 | 2.25 ± 0.24 a | 4.92 ± 0.33 a |
| Particle size (µm) | |||
| RGS315-SFE | 315–800 | 1.75 ± 0.55 a | 4.60 ± 0.20 a |
| RGS800-SFE | >800 | 1.36 ± 0.25 a | 3.74 ± 0.11 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimić, I.; Teslić, N.; Putnik, P.; Bursać Kovačević, D.; Zeković, Z.; Šojić, B.; Mrkonjić, Ž.; Čolović, D.; Montesano, D.; Pavlić, B. Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants. Antioxidants 2020, 9, 568. https://doi.org/10.3390/antiox9070568
Dimić I, Teslić N, Putnik P, Bursać Kovačević D, Zeković Z, Šojić B, Mrkonjić Ž, Čolović D, Montesano D, Pavlić B. Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants. Antioxidants. 2020; 9(7):568. https://doi.org/10.3390/antiox9070568
Chicago/Turabian StyleDimić, Ivana, Nemanja Teslić, Predrag Putnik, Danijela Bursać Kovačević, Zoran Zeković, Branislav Šojić, Živan Mrkonjić, Dušica Čolović, Domenico Montesano, and Branimir Pavlić. 2020. "Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants" Antioxidants 9, no. 7: 568. https://doi.org/10.3390/antiox9070568
APA StyleDimić, I., Teslić, N., Putnik, P., Bursać Kovačević, D., Zeković, Z., Šojić, B., Mrkonjić, Ž., Čolović, D., Montesano, D., & Pavlić, B. (2020). Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants. Antioxidants, 9(7), 568. https://doi.org/10.3390/antiox9070568