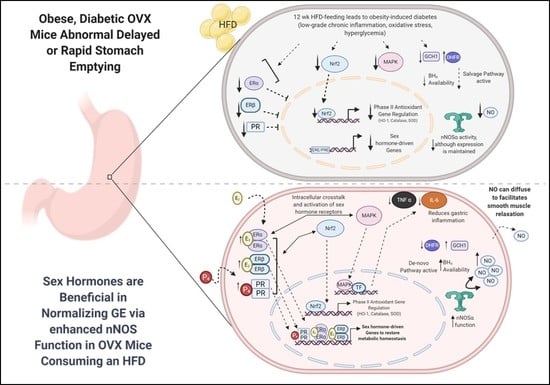
Supplementation of 17β-Estradiol Normalizes Rapid Gastric Emptying by Restoring Impaired Nrf2 and nNOS Function in Obesity-Induced Diabetic Ovariectomized Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Surgical Ovariectomy, HFD Feeding, and 17β-Estradiol and Progesterone Replacement
2.3. Intraperitoneal Glucose Tolerance Test
2.4. Measurement of 2 h Solid Gastric Emptying
2.5. Electric Field Stimulation to Elicit Nitrergic Relaxation in Gastric Antrum Neuromuscular Specimens
2.6. Estimation of Serum 17β-Estradiol, Progesterone, Insulin, and Total Nitrite Concentration
2.7. Gel Electrophoresis and Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Supplementation of E2 and P4 Prevent Body Weight Gain, Adiposity and Hyperglycemia in Obese, Diabetic OVX Mice
3.2. E2 and P4 Prevent Insulin Resistance and Glucose Intolerance in HFD-fed OVX Mice
3.3. Serum Estradiol, Progesterone, Testosterone, Adiponectin, and Total Nitrite Concentration
3.4. Supplementation of E2 Restored Impaired Gastric Emptying and Nitrergic Relaxation
3.5. nNOSα Expression Is Maintained in OVX+HFD Mice, but BH4 Cofactor Biosynthesis Is Impaired in Gastric Antrum
3.6. E2 and P4 Supplementation Regulates MAPK, ER and PR Levels in OVX+HFD Mice
3.7. Gastric Pro-inflammatory Cytokine (IL-6 and TNFα) are Elevated in HFD-fed Mice and Restored by E2 and P4 Supplementation
3.8. E2 and P4 Regulate Nrf2 and Phase II Antioxidant Enzymes Expression in OVX+HFD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krishnasamy, S.; Abell, T.L. Diabetic Gastroparesis: Principles and Current Trends in Management. Diabetes Ther. 2018, 9, 1–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Block, C.; De Leeuw, I.; Pelckmans, P.; Van Gaal, L. Current Concepts in Gastric Motility in Diabetes Mellitus. Curr. Diabetes Rev. 2006, 2, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Anaparthy, R.; Pehlivanov, N.; Grady, J.; Yimei, H.; Pasricha, P.J. Gastroparesis and gastroparesis-like syndrome: Response to therapy and its predictors. Dig. Dis. Sci. 2009, 54, 1003–1010. [Google Scholar] [CrossRef] [Green Version]
- Grover, M.; Farrugia, G.; Lurken, M.S.; Bernard, C.E.; Faussone–Pellegrini, M.S.; Smyrk, T.C.; Parkman, H.P.; Abell, T.L.; Snape, W.J.; Hasler, W.L.; et al. Cellular Changes in Diabetic and Idiopathic Gastroparesis. Gastroenterology 2011, 140, 1575–1585. [Google Scholar] [CrossRef] [Green Version]
- Ördög, T. Interstitial cells of Cajal in diabetic gastroenteropathy. Neurogastroenterol. Motil. 2007, 20, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, P.A. Gastrointestinal Complications of Diabetes Mellitus. J. Pharm. Care Pain Symptom Control 1999, 7, 11–35. [Google Scholar] [CrossRef]
- Kong, M.F.; Horowitz, M. Gastric Emptying in Diabetes Mellitus: Relationship to Blood-Glucose Control. Clin. Geriatr. Med. 1999, 15, 321–338. [Google Scholar] [CrossRef]
- Toda, N.; Herman, A.G. Gastrointestinal Function Regulation by Nitrergic Efferent Nerves. Pharmacol. Rev. 2005, 57, 315–338. [Google Scholar] [CrossRef]
- Koo, J.R.; Vaziri, N.D. Effects of diabetes, insulin and antioxidants on NO synthase abundance and NO interaction with reactive oxygen species. Kidney Int. 2003, 63, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Huang, Z.; Lin, Y.; Zhang, Z.; Fang, D.; Zhang, D.D. The Protective Role of Nrf2 in Streptozotocin-Induced Diabetic Nephropathy. Diabetes 2010, 59, 850–860. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, Y.; Toyomasu, Y.; Saravanaperumal, S.A.; Bardsley, M.R.; Smestad, J.A.; Lorincz, A.; Eisenman, S.T.; Cipriani, G.; Nelson Holte, M.H.; Al Khazal, F.J.; et al. Hyperglycemia Increases Interstitial Cells of Cajal via MAPK1 and MAPK3 Signaling to ETV1 and KIT, Leading to Rapid Gastric Emptying. Gastroenterology 2017, 153, 521–535. [Google Scholar] [CrossRef] [Green Version]
- Gangula, P.R.R.; Sekhar, K.R.; Mukhopadhyay, S. Gender Bias in Gastroparesis: Is Nitric Oxide the Answer? Dig. Dis. Sci. 2011, 56, 2520–2527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutson, W.R.; Roehrkasse, R.L.; Wald, A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology 1989, 96, 11–17. [Google Scholar] [CrossRef]
- Chen, T.S.; Doong, M.L.; Wang, S.W.; Tsai, S.C.; Lu, C.C.; Shih, H.C.; Chen, Y.H.; Chang, F.Y.; Lee, S.D.; Wang, P.S. Gastric emptying and gastrointestinal transit during lactation in rats. Am. J. Physiol. 1997, 272 Pt 1, G626–G631. [Google Scholar] [CrossRef] [PubMed]
- Verrengia, M.; Sachdeva, P.; Gaughan, J.; Fisher, R.S.; Parkman, H.P. Variation of symptoms during the menstrual cycle in female patients with gastroparesis. Neurogastroenterol. Motil. 2011, 23, 625. [Google Scholar] [CrossRef] [PubMed]
- Zia, J.K.; Heitkemper, M.M. Upper Gastrointestinal Tract Motility Disorders in Women, Gastroparesis, and Gastroesophageal Reflux Disease. Gastroenterol. Clin. N. Am. 2016, 45, 239–251. [Google Scholar] [CrossRef]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen Signaling Multiple Pathways to Impact Gene Transcription. Curr. Genom. 2006, 7, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Bryzgalova, G.; Lundholm, L.; Portwood, N.; Gustafsson, J.Å.; Khan, A.; Efendic, S.; Dahlman-Wright, K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am. J. Physiol. Metab. 2008, 295, E904–E912. [Google Scholar] [CrossRef] [Green Version]
- Scordalakes, E.M.; Shetty, S.J.; Rissman, E.F. Roles of estrogen receptor α and androgen receptor in the regulation of neuronal nitric oxide synthase. J. Comp. Neurol. 2002, 453, 336–344. [Google Scholar] [CrossRef]
- Sato, S.; Braham, C.S.; Putnam, S.K.; Hull, E.M. Neuronal nitric oxide synthase and gonadal steroid interaction in the MPOA of male rats: Co-localization and testosterone-induced restoration of copulation and nNOS-immunoreactivity. Brain Res. 2005, 1043, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.H.; Lin, G.; Fang, M.; Rudd, J.A. Localization of estrogen receptor ERα, ERβ and GPR30 on myenteric neurons of the gastrointestinal tract and their role in motility. Gen. Comp. Endocrinol. 2019, 272, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Molero, L. Expression of estrogen receptor subtypes and neuronal nitric oxide synthase in neutrophils from women and men Regulation by estrogen. Cardiovasc. Res. 2002, 56, 43–51. [Google Scholar] [CrossRef]
- Rao, J.N. Estrogens and Gastroparesis: A Clinical Relevance. Dig. Dis. Sci. 2013, 58, 1449–1451. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.Y.; Chen, L.B.; Liu, P.Y.; Xie, D.P.; Wang, P.S. Effects of progesterone on gastric emptying and intestinal transit in male rats. World J. Gastroenterol. 2002, 8, 338. [Google Scholar] [CrossRef]
- Xiao, Z.L.; Pricolo, V.; Biancani, P.; Behar, J. Role of progesterone signaling in the regulation of G-protein levels in female chronic constipation. Gastroenterology 2005, 128, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt, K.; Waite, L.; Abboud, F.M.; Conklin, J.L. Nongenomic effects of progesterone on human intestinal smooth muscle cells. Am. J. Physiol. Liver Physiol. 1996, 271, G370–G376. [Google Scholar] [CrossRef]
- Al-Shboul, O.; Mustafa, A.; Al-hashimi, F. Non-genomic effects of progesterone on Rho kinase II in rat gastric smooth muscle cells. J. Smooth Muscle Res. 2013, 49, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, N.G.; Sousa, L.P.; Sousa, M.O.; Pietrani, N.T.; Fernandes, A.P.; Gomes, K.B. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2013, 99, 85–92. [Google Scholar] [CrossRef]
- Uruno, A.; Furusawa, Y.; Yagishita, Y.; Fukutomi, T.; Muramatsu, H.; Negishi, T.; Sugawara, A.; Kensler, T.W.; Yamamoto, M. The Keap1-Nrf2 System Prevents Onset of Diabetes Mellitus. Mol. Cell. Biol. 2013, 33, 2996–3010. [Google Scholar] [CrossRef] [Green Version]
- Hogan, A.M.; Collins, D.; Baird, A.W.; Winter, D.C. Estrogen and its role in gastrointestinal health and disease. Int. J. Colorectal Dis. 2009, 24, 1367–1375. [Google Scholar] [CrossRef]
- Chen, T.S.; Doong, M.L.; Chang, F.Y.; Lee, S.D.; Wang, P.S. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am. J. Physiol. Liver Physiol. 1995, 268, G171–G176. [Google Scholar] [CrossRef]
- Pratha, V.S. Principles of Gender-Specific Medicine; Elsevier Academic Press: Cambridge, MA, USA, 2004; ISBN 9780124409057. [Google Scholar]
- Pae, M.; Baek, Y.; Lee, S.; Wu, D. Loss of ovarian function in association with a high-fat diet promotes insulin resistance and disturbs adipose tissue immune homeostasis. J. Nutr. Biochem. 2018, 57, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhab, M.A.; Hamzawy, M.A.; El-Denshary, E.S.M.; Bahgat, A.K.; Hassan, N.S.; Aly, S.E. Hepatoprotective Effect of Estradiol and -Lipoic Acid in Rats. Glob. J. Pharmacol. 2014, 8, 694–702. [Google Scholar] [CrossRef]
- Shimozawa, N.; Okajima, K.; Harada, N.; Arai, M.; Ishida, Y.; Shimada, S.; Kurihara, H.; Nakagata, N. Contribution of Sensory Neurons to Sex Difference in the Development of Stress-Induced Gastric Mucosal Injury in Mice. Gastroenterology 2006, 131, 1826–1834. [Google Scholar] [CrossRef]
- Ingberg, E.; Theodorsson, E.; Theodorsson, A.; Ström, J.O. Effects of high and low 17β-estradiol doses on focal cerebral ischemia in rats. Sci. Rep. 2016, 6, 202–228. [Google Scholar] [CrossRef] [Green Version]
- Modder, U.; Riggs, B.; Spelsberg, T.; Fraser, D.; Atkinson, E.; Arnold, R.; Khosla, S. Dose-response of estrogen on bone versus the uterus in ovariectomized mice. Eur. J. Endocrinol. 2004, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sampath, C.; Sprouse, J.C.; Freeman, M.L.; Gangula, P.R. Activation of Nrf2 attenuates delayed gastric emptying in obesity induced diabetic (T2DM) female mice. Free Radic. Biol. Med. 2019, 135, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Gangula, P.R.R.; Mukhopadhyay, S.; Ravella, K.; Cai, S.; Channon, K.M.; Garfield, R.E.; Pasricha, P.J. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am. J. Physiol. Liver Physiol. 2010, 298, G692–G699. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Sekhar, K.R.; Hale, A.B.; Channon, K.M.; Farrugia, G.; Freeman, M.L.; Gangula, P.R. Loss of NRF2 impairs gastric nitrergic stimulation and function. Free Radic. Biol. Med. 2011, 51, 619–625. [Google Scholar] [CrossRef] [Green Version]
- Seth, R.K.; Das, S.; Pourhoseini, S.; Dattaroy, D.; Igwe, S.; Ray, J.B.; Fan, D.; Michelotti, G.A.; Diehl, A.M.; Chatterjee, S. M1 Polarization Bias and Subsequent Nonalcoholic Steatohepatitis Progression Is Attenuated by Nitric Oxide Donor DETA NONOate via Inhibition of CYP2E1-Induced Oxidative Stress in Obese Mice. J. Pharmacol. Exp. Ther. 2015, 352, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Ludgero-Correia, A.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Faria, T.S. Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition 2012, 28, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallou-Kabani, C.; Vigé, A.; Gross, M.S.; Rabès, J.P.; Boileau, C.; Larue-Achagiotis, C.; Tomé, D.; Jais, J.P.; Junien, C. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity 2007, 15, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Fraulob, J.C.; Ogg-Diamantino, R.; Fernandes-Santos, C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. A mouse model of metabolic syndrome: Insulin resistance, fatty liver and Non-Alcoholic Fatty Pancreas Disease (NAFPD) in C57BL/6 mice fed a high fat diet. J. Clin. Biochem. Nutr. 2010, 46, 212–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambliss, K.L.; Shaul, P.W. Estrogen Modulation of Endothelial Nitric Oxide Synthase. Endocr. Rev. 2002, 23, 665–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarosiek, I.; Bashashati, M.; Biswas, S.; Shankar, N.; Gomez, Y.; Bright, T.; McCallum, R.W.; Sarosiek, J.; Terreros, D. Significant Alterations within the Plasma Cytokine Profile in Gastroparesis: A Case-control Study. Gastroenterology 2017, 152, S520. [Google Scholar] [CrossRef]
- Grover, M.; Gibbons, S.J.; Nair, A.A.; Bernard, C.E.; Zubair, A.S.; Eisenman, S.T.; Wilson, L.A.; Miriel, L.; Pasricha, P.J.; Parkman, H.P.; et al. Transcriptomic signatures reveal immune dysregulation in human diabetic and idiopathic gastroparesis. BMC Med. Genom. 2018, 11, 62. [Google Scholar] [CrossRef]
- Kashyap, P.; Farrugia, G. Oxidative stress: Key player in gastrointestinal complications of diabetes. Neurogastroenterol. Motil. 2011, 23, 111–114. [Google Scholar] [CrossRef]
- Cellek, S. Point of NO Return for Nitrergic Nerves in Diabetes: A New Insight into Diabetic Complications. Curr. Pharm. Des. 2004, 10, 3683–3695. [Google Scholar] [CrossRef]
- Zheng, H.; Whitman, S.A.; Wu, W.; Wondrak, G.T.; Wong, P.K.; Fang, D.; Zhang, D.D. Therapeutic Potential of Nrf2 Activators in Streptozotocin-Induced Diabetic Nephropathy. Diabetes 2011, 60, 3055–3066. [Google Scholar] [CrossRef] [Green Version]
- Al Mushref, M.; Srinivasan, S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann. Transl. Med. 2013, 1, 14. [Google Scholar] [CrossRef]
- Barros, R.P.A.; Gustafsson, J.Å. Estrogen Receptors and the Metabolic Network. Cell Metab. 2011, 14, 289–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balistreri, C.R.; Caruso, C.; Candore, G. The Role of Adipose Tissue and Adipokines in Obesity-Related Inflammatory Diseases. Mediat. Inflamm. 2010, 2010, 802078. [Google Scholar] [CrossRef]
- Chakraborty, T.R.; Donthireddy, L.; Adhikary, D.; Chakraborty, S. Long-Term High Fat Diet Has a Profound Effect on Body Weight, Hormone Levels, and Estrous Cycle in Mice. Med. Sci. Monit. 2016, 22, 1601–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerley-Hamilton, J.S.; Trask, H.W.; Ridley, C.J.A.; DuFour, E.; Ringelberg, C.S.; Nurinova, N.; Wong, D.; Moodie, K.L.; Shipman, S.L.; Moore, J.H.; et al. Obesity Is Mediated by Differential Aryl Hydrocarbon Receptor Signaling in Mice Fed a Western Diet. Environ. Health Perspect. 2012, 120, 1252–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- D’Eon, T.M.; Souza, S.C.; Aronovitz, M.; Obin, M.S.; Fried, S.K.; Greenberg, A.S. Estrogen Regulation of Adiposity and Fuel Partitioning. J. Biol. Chem. 2005, 280, 35983–35991. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.; Nathan, L.; Singh, R.; Fu, Y.S.; Chaudhuri, G. E 2 and not P 4 increases NO release from NANC nerves of the gastrointestinal tract: Implications in pregnancy. Am. J. Physiol. Integr. Comp. Physiol. 2001, 280, R1546–R1554. [Google Scholar] [CrossRef] [Green Version]
- Coşkun, J.; Sevinç, A.; Tevetoğlu, I.; Alican, I.; Kurtel, H.; Yeğen, B.Ç. Delayed gastric emptying in conscious male rats following chronic estrogen and progesterone treatment. Res. Exp. Med. 1995, 195, 49–54. [Google Scholar] [CrossRef]
- Showkat Ali, M.; Tiscareno-Grejada, I.; Locovei, S.; Smiley, R.; Collins, T.; Sarosiek, J.; McCallum, R. Gender and Estradiol as Major Factors in the Expression and Dimerization of nNOSα in Rats with Experimental Diabetic Gastroparesis. Dig. Dis. Sci. 2012, 57, 2814–2825. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Raz, L.; Wang, R.; Han, D.; De Sevilla, L.; Yang, F.; Vadlamudi, R.K.; Brann, D.W. Estrogen Attenuates Ischemic Oxidative Damage via an Estrogen Receptor -Mediated Inhibition of NADPH Oxidase Activation. J. Neurosci. 2009, 29, 13823–13836. [Google Scholar] [CrossRef]
- Numakawa, T.; Matsumoto, T.; Numakawa, Y.; Richards, M.; Yamawaki, S.; Kunugi, H. Protective Action of Neurotrophic Factors and Estrogen against Oxidative Stress-Mediated Neurodegeneration. J. Toxicol. 2011, 2011, 405194. [Google Scholar] [CrossRef] [PubMed]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, S.N.; Gorelick, D.A. Crosstalk between nuclear and G protein-coupled estrogen receptors. Gen. Comp. Endocrinol. 2018, 261, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Al-Shboul, O.; Nazzal, M.; Mustafa, A.; Al-Dwairi, A.; Alqudah, M.; Abu Omar, A.; Alfaqih, M.; Alsalem, M. Estrogen relaxes gastric muscle cells via a nitric oxide- and cyclic guanosine monophosphate-dependent mechanism: A sex-associated differential effect. Exp. Ther. Med. 2018, 16, 1685–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, R.M.; Rubalcaba, E.; Lippman, M.E.; Cowan, K.H. Effects of Estrogen and Tamoxifen on the Regulation of Dihydrofolate Reductase Gene Expression in a Human Breast Cancer Cell Line. Cancer Res. 1985, 45, 1644–1650. [Google Scholar]
- Sun, X.; Kumar, S.; Tian, J.; Black, S.M. Estradiol Increases Guanosine 5′-Triphosphate Cyclohydrolase Expression Via the Nitric Oxide-Mediated Activation of Cyclic Adenosine 5′-Monophosphate Response Element Binding Protein. Endocrinology 2009, 150, 3742–3752. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.E.; Baumgardt, S.L.; Fang, J.; Paterson, M.; Liu, Y.; Du, J.; Shi, Y.; Qiao, S.; Bosnjak, Z.J.; Warltier, D.C.; et al. Cardiomyocyte GTP Cyclohydrolase 1 Protects the Heart Against Diabetic Cardiomyopathy. Sci. Rep. 2016, 6, 27925. [Google Scholar] [CrossRef] [Green Version]
- Gan, H.T.; Pasricha, P.J.; Chen, J.D.Z. Blockade of p38 mitogen-activated protein kinase pathway ameliorates delayed intestinal transit in burned rats. Am. J. Surg. 2007, 193, 530–537. [Google Scholar] [CrossRef]
- Yang, K.; Qiu, B.Y.; Yan, J.; Yang, Y.X.; Zhang, T.; Chen, X.; Zou, Y.P.; Gan, H.T.; Huang, X.L. Blockade of p38 mitogen-activated protein kinase pathway ameliorates delayed gastric emptying in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014, 23, 696–700. [Google Scholar] [CrossRef]
- Sites, C.K.; Toth, M.J.; Cushman, M.; L’Hommedieu, G.D.; Tchernof, A.; Tracy, R.P.; Poehlman, E.T. Menopause-related differences in inflammation markers and their relationship to body fat distribution and insulin-stimulated glucose disposal. Fertil. Steril. 2002, 77, 128–135. [Google Scholar] [CrossRef]
- Pelekanou, V.; Kampa, M.; Kiagiadaki, F.; Deli, A.; Theodoropoulos, P.; Agrogiannis, G.; Patsouris, E.; Tsapis, A.; Castanas, E.; Notas, G. Estrogen anti-inflammatory activity on human monocytes is mediated through cross-talk between estrogen receptor ERα36 and GPR30/GPER1. J. Leukoc. Biol. 2016, 99, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When NRF2 Talks, Who’s Listening? Antioxid. Redox Signal. 2010, 13, 1649–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansell, P.J.; Lo, S.C.; Newton, L.G.; Espinosa-Nicholas, C.; Zhang, D.D.; Liu, J.H.; Hannink, M.; Lubahn, D.B. Repression of cancer protective genes by 17β-estradiol: Ligand-dependent interaction between human Nrf2 and estrogen receptor α. Mol. Cell. Endocrinol. 2005, 243, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Tamir, S.; Izrael, S.; Vaya, J. The effect of oxidative stress on ERα and ERβ expression. J. Steroid Biochem. Mol. Biol. 2002, 81, 327–332. [Google Scholar] [CrossRef]
- Sprouse, J.; Sampath, C.; Gangula, P.R. Mo1585—Role of Sex Hormones and Their Receptors on Gastric Nrf2 and Neuronal Nitric Oxide Function. Gastroenterology 2019, 156. [Google Scholar] [CrossRef]









| Group | n | Δ BW (g) | Visceral Adipose Weight (g) | Fasting Blood Glucose (mg/dL) | Fasting Insulin (ng/mL) |
|---|---|---|---|---|---|
| SHAM+ND | 6 | 3.39 ± 1.01 | 0.33 ± 0.05 | 97.75 ± 4.29 | 0.24 ± 0.05 |
| SHAM+HFD | 6 | 19.25 ± 2.61 * | 2.37 ± 0.24 * | 208.00 ± 11.16 * | 0.98 ± 0.19 * |
| OVX+ND | 6 | 4.39 ± 1.25 | 0.78 ± 0.18 * | 150.60 ± 6.28 * | 0.81 ± 0.09 * |
| OVX+HFD | 6 | 13.82 ± 0.68 # | 3.35 ± 0.22 # | 185.33 ± 8.25 # | 1.09 ± 0.22 # |
| +E2 (0.25 mg/kg) | 6 | 15.15 ± 1.05 | 2.40 ± 0.37 $ | 151.00 ± 3.13 $ | 0.903 ± 0.14 |
| +E2 (1.0 mg/kg) | 6 | 5.78 ± 1.25 $ | 0.17 ± 0.04 $ | 108.33 ± 6.62 $ | 0.867 ± 0.20 |
| +P4 (1.0 mg/kg) | 6 | 10.07 ± 0.75 $ | 0.68 ± 0.11 $ | 70.75 ± 3.42 $ | 1.03 ± 0.46 |
| +P4 (4.0 mg/kg) | 6 | 8.07 ± 1.36 $ | 0.86 ± 0.14 $ | 118.25 ± 5.41 $ | 1.03 ± 0.31 |
| Serum 17β-Estradiol (ng/L) | Serum Total Nitrite (μM) | Serum Progesterone (ng/mL) | Serum Adiponectin (μg/mL) | |
|---|---|---|---|---|
| SHAM+ND | 34.72 ± 4.22 | 19.87 ± 0.39 | 5.69 ± 0.66 | 16.56 ± 2.19 |
| SHAM+HFD | 44.49 ± 3.99 * | 16.05 ± 0.55 * | 8.65 ± 1.04 * | 6.29 ± 0.12 * |
| OVX+ND | 21.80 ± 3.42 * | 15.76 ± 0.64 * | 3.46 ± 1.38 | 11.46 ± 0.54 * |
| OVX+HFD | 39.07 ± 5.05 # | 19.42 ± 0.48 # | 6.41 ± 1.23 # | 5.01 ± 0.16 # |
| +E2 (0.25 mg/kg) | 38.10 ± 1.69 | 21.19 ± 0.64 $ | 2.60 ± 0.59 $ | 9.88 ± 0.23 $ |
| +E2 (1.00 mg/kg) | 44.63 ± 4.27 | 16.66 ± 0.19 $ | 3.19 ± 1.34 $ | 10.14 ± 0.56 $ |
| +P4 (1.00 mg/kg) | 38.41 ± 3.64 | 16.94 ± 0.69 $ | 7.78 ± 1.45 | 6.74 ± 0.71 |
| +P4 (4.00 mg/kg) | 23.67 ± 6.29 $ | 18.87 ± 0.21 | 9.44 ± 0.93 | 9.25 ± 0.57 $ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprouse, J.C.; Sampath, C.; Gangula, P.R. Supplementation of 17β-Estradiol Normalizes Rapid Gastric Emptying by Restoring Impaired Nrf2 and nNOS Function in Obesity-Induced Diabetic Ovariectomized Mice. Antioxidants 2020, 9, 582. https://doi.org/10.3390/antiox9070582
Sprouse JC, Sampath C, Gangula PR. Supplementation of 17β-Estradiol Normalizes Rapid Gastric Emptying by Restoring Impaired Nrf2 and nNOS Function in Obesity-Induced Diabetic Ovariectomized Mice. Antioxidants. 2020; 9(7):582. https://doi.org/10.3390/antiox9070582
Chicago/Turabian StyleSprouse, Jeremy C., Chethan Sampath, and Pandu R. Gangula. 2020. "Supplementation of 17β-Estradiol Normalizes Rapid Gastric Emptying by Restoring Impaired Nrf2 and nNOS Function in Obesity-Induced Diabetic Ovariectomized Mice" Antioxidants 9, no. 7: 582. https://doi.org/10.3390/antiox9070582
APA StyleSprouse, J. C., Sampath, C., & Gangula, P. R. (2020). Supplementation of 17β-Estradiol Normalizes Rapid Gastric Emptying by Restoring Impaired Nrf2 and nNOS Function in Obesity-Induced Diabetic Ovariectomized Mice. Antioxidants, 9(7), 582. https://doi.org/10.3390/antiox9070582