Redox-Sensitive Glyoxalase 1 Up-Regulation Is Crucial for Protecting Human Lung Cells from Gold Nanoparticles Toxicity
Abstract
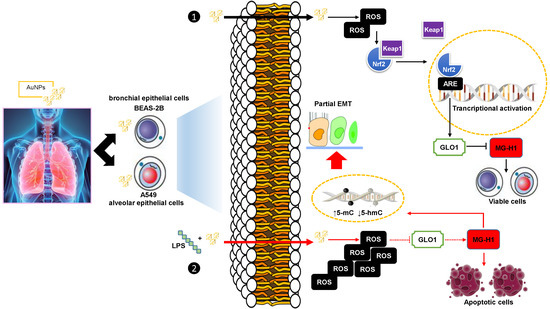
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Citrate-Stabilized AuNPs
2.3. Cell Culture and Treatments
2.4. Cell Viability Assay
2.5. Apoptosis Detection
2.6. Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) Analysis of Cell Uptake
2.7. Transmission Electron Microscopy (TEM) Morphology Measurements
2.8. Cell Lysate and Nuclear Extracts Preparation
2.9. Glo1 Enzyme Activity Assessment
2.10. Detection of Methylglyoxal (MG)-H1 Protein Adducts
2.11. Cell Transfection and siRNA-Mediated Gene Silencing
2.12. Assessment of Cellular Levels of Reactive Oxidative Species
2.13. Nrf2 Activation and HO-1 Detection
2.14. RNA Isolation, Reverse Transcription, and Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analyses
2.15. Genomic DNA Preparation
2.16. Analysis of Global DNA Methylation and Hydroxymethylation
2.17. Statistical Analysis
3. Results
3.1. Biological Characterization of AuNPs-Cell Interaction
3.2. Exposure to AuNPs Does Not Cause Acute Toxicity in BEAS-2B and A549 Cells
3.3. Exposure to AuNPs Induces Glyoxalase-1 (Glo1) Upregulation and MG-H1 Decreased Intracellular Levels in BEAS-2B and A549 Cells
3.4. Glo1/MG-H1 Axis Sustains BEAS-2B and A549 Cell Viability upon AuNPs Exposure
3.5. The Upregulation of Glo1 and the Associated Decrease in MG-H1 Levels Caused by AuNPs Exposure Are Part of a Cell Adaptive Response to Oxidative Stress Involving the Master Redox-Sensitive Transcriptional Regulator Nrf2
3.6. BEAS-2B and A549 Cells Challenged with a Pro-Inflammatory/Pro-Oxidative Stimulus Become Susceptible to the Effects of AuNPs and Show Increased ROS Production, Glo1 Inactivation, and Substantial Dicarbonyl Stress Onset
3.7. BEAS-2B and A549 Cells That Survived the LPS-Induced Insult underwent a “Metastable” Phenotype Associated with DNA Epigenetic Changes, through Global DNA Methylation and Hydroxymethylation, Driven by MG-H1 Accumulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leso, V.; Fontana, L.; Mauriello, M.C.; Iavicoli, I. Occupational risk assessment of engineered nanomaterials: Limits, challenges and opportunities. Curr. Nanosci. 2017, 13, 55–78. [Google Scholar] [CrossRef] [Green Version]
- Iavicoli, I.; Fontana, L.; Leso, V.; Macrini, M.C.; Pelclova, D. Fractional Exhaled Nitric Oxide and Nanomaterial Exposure in Workplaces. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Panzarini, E.; Mariano, S.; Carata, E.; Mura, F.; Rossi, M.; Dini, L. Intracellular Transport of Silver and Gold Nanoparticles and Biological Responses: An Update. Int. J. Mol. Sci. 2018, 19, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuaid, H.N.; Muir, M.F.; Taggart, L.E.; McMahon, S.J.; Coulter, J.A.; Hyland, W.B.; Jain, S.; Butterworth, K.T.; Schettino, G.; Prise, K.M.; et al. Imaging and radiation effects of gold nanoparticles in tumour cells. Sci. Rep. 2016, 6, 19442. [Google Scholar] [CrossRef]
- Schmid, G. The relevance of shape and size of Au55 clusters. Chem. Soc. Rev. 2008, 37, 1909–3190. [Google Scholar] [CrossRef]
- Sperling, R.A.; Rivera Gil, P.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef]
- Falagan-Lotsch, P.; Grzincic, E.M.; Murphy, C.J. One low-dose exposure of gold nanoparticles induces long-term changes in human cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13318–13323. [Google Scholar] [CrossRef] [Green Version]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef]
- Villiers, C.L.; Freitas, H.; Couderc, R.; Villiers, M.B.; Marche, P.N. Analysis of the toxicity of gold nanoparticles on the immune system: Effect on dendritic cell functions. J. Nanopart. Res. 2009, 12, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, J.E.J.; Zhang, J.; Wang, X.; Kawazoe, N.; Chen, G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale 2016, 8, 7992–8007. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Liu, D.; Fong, C.C.; Zhang, J.; Yang, M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Song, M.S.; Ryu, P.D.; Lam, A.T.N.; Joo, S.W.; Lee, S.Y. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/β-catenin signaling pathway. Int. J. Nanomed. 2015, 10, 4383–4392. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Zamry, A.A.; Tan, H.T.; Wong, K.K.; Lim, J.; Mohamud, R. Targeting dendritic cells through gold nanoparticles: A review on the cellular uptake and subsequent immunological properties. Mol. Immunol. 2017, 91, 123–133. [Google Scholar] [CrossRef] [PubMed]
- May, S.; Hirsch, C.; Rippl, A.; Bohmer, N.; Kaiser, J.P.; Diener, L.; Wichser, A.; Bürkle, A.; Wick, P. Transient DNA damage following exposure to gold nanoparticles. Nanoscale 2018, 10, 15723–15735. [Google Scholar] [CrossRef] [Green Version]
- Bachand, G.D.; Allen, A.; Bachand, M.; Achyuthan, K.E.; Seagrave, J.C.; Brozik, S.M. Cytotoxicity and inflammation in human alveolar epithelial cells following exposure to occupational levels of gold and silver nanoparticles. J. Nanopart. Res. 2012, 14, 1212. [Google Scholar] [CrossRef]
- Antognelli, C.; Gambelunghe, A.; Muzi, G.; Talesa, V.N. Glyoxalase I drives epithelial-to-mesenchymal transition via argpyrimidine-modified Hsp70, miR-21 and SMAD signalling in human bronchial cells BEAS-2B chronically exposed to crystalline silica Min-U-Sil 5: Transformation into a neoplastic-like phenotype. Free Radic. Biol. Med. 2016, 92, 110–125. [Google Scholar] [CrossRef]
- Antognelli, C.; Gambelunghe, A.; Muzi, G.; Talesa, V.N. Peroxynitrite-mediated glyoxalase I epigenetic inhibition drives apoptosis in airway epithelial cells exposed to crystalline silica via a novel mechanism involving argpyrimidine-modified Hsp70, JNK, and NF-κB. Free Radic. Biol. Med. 2015, 84, 128–141. [Google Scholar] [CrossRef]
- Marinucci, L.; Balloni, S.; Fettucciari, K.; Bodo, M.; Talesa, V.N.; Antognelli, C. Nicotine induces apoptosis in human osteoblasts via a novel mechanism driven by H2O2 and entailing Glyoxalase 1-dependent MG-H1 accumulation leading to TG2-mediated NF-kB desensitization: Implication for smokers-related osteoporosis. Free Radic. Biol. Med. 2018, 117, 6–17. [Google Scholar] [CrossRef]
- Antognelli, C.; Talesa, V.N. Glyoxalases in Urological Malignancies. Int. J. Mol. Sci. 2018, 19, 415. [Google Scholar] [CrossRef] [Green Version]
- Antognelli, C.; Trapani, E.; Delle Monache, S.; Perrelli, A.; Daga, M.; Pizzimenti, S.; Barrera, G.; Cassoni, P.; Angelucci, A.; Trabalzini, L.; et al. KRIT1 loss-of-function induces a chronic Nrf2-mediated adaptive homeostasis that sensitizes cells to oxidative stress: Implication for Cerebral Cavernous Malformation disease. Free Radic. Biol. Med. 2018, 115, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Trapani, E.; Delle Monache, S.; Perrelli, A.; Fornelli, C.; Retta, F.; Cassoni, P.; Talesa, V.N.; Retta, S.F. Data in support of sustained upregulation of adaptive redox homeostasis mechanisms caused by KRIT1 loss-of-function. Data Brief 2017, 16, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Palumbo, I.; Aristei, C.; Talesa, V.N. Glyoxalase I inhibition induces apoptosis in irradiated MCF-7 cells via a novel mechanism involving Hsp27, p53 and NF-κB. Br. J. Cancer 2014, 111, 395–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antognelli, C.; Mancuso, F.; Frosini, R.; Arato, I.; Calvitti, M.; Calafiore, R.; Talesa, V.N.; Luca, G. Testosterone and Follicle Stimulating Hormone-Dependent Glyoxalase 1 Up-Regulation Sustains the Viability of Porcine Sertoli Cells through the Control of Hydroimidazolone- and Argpyrimidine-Mediated NF-κB Pathway. Am. J. Pathol. 2018, 188, 2553–2563. [Google Scholar] [CrossRef] [Green Version]
- Antognelli, C.; Moretti, S.; Frosini, R.; Puxeddu, E.; Sidoni, A.; Talesa, V.N. Methylglyoxal Acts as a Tumor-Promoting Factor in Anaplastic Thyroid Cancer. Cells 2019, 8, 547. [Google Scholar] [CrossRef] [Green Version]
- Pu, Y.; Liu, Y.Q.; Zhou, Y.; Qi, Y.F.; Liao, S.P.; Miao, S.K.; Zhou, L.M.; Wan, L.H. Dual role of RACK1 in airway epithelial mesenchymal transition and apoptosis. J. Cell. Mol. Med. 2020, 24, 3656–3668. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Leopold, K. Ligand-assisted extraction for separation and preconcentration of gold nanoparticles from waters. Anal. Chem. 2012, 84, 4340–4349. [Google Scholar] [CrossRef]
- Feichtmeier, N.S.; Walther, P.; Leopold, K. Uptake, effects, and regeneration of barley plants exposed to gold nanoparticles. Environ. Sci. Pollut. Res. Int. 2015, 22, 8549–8558. [Google Scholar] [CrossRef]
- Antognelli, C.; Frosini, R.; Santolla, M.F.; Peirce, M.J.; Talesa, V.N. Oleuropein-Induced Apoptosis Is Mediated by Mitochondrial Glyoxalase 2 in NSCLC A549 Cells: A Mechanistic Inside and a Possible Novel Nonenzymatic Role for an Ancient Enzyme. Oxid. Med. Cell. Longev. 2019, 2019, 8576961. [Google Scholar] [CrossRef] [Green Version]
- Thornalley, P.J.; Yurek-George, A.; Argirov, O.K. Kinetics and mechanism of the reaction of aminoguanidine with the alpha-oxoaldehydes glyoxal, methylglyoxal, and 3-deoxyglucosone under physiological conditions. Biochem. Pharmacol. 2000, 60, 55–65. [Google Scholar] [CrossRef]
- Ong, K.J.; MacCormack, T.J.; Clark, R.J.; Ede, J.D.; Ortega, V.A.; Felix, L.C.; Dang, M.K.M.; Ma, G.; Fenniri, H.; Veinot, J.G.C.; et al. Widespread nanoparticle-assay interference: Implications for nanotoxicity testing. PLoS ONE 2014, 9, e90650. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B.; Aronsson, A.C.; Marmstal, E.; Tibellin, G. Glyoxalase I (rat liver). Methods Enzymol. 1981, 77, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.; Cheng, R.Y.; Revelo, M.P.; Mitzner, W.; Tang, W. Hydroxymethylation as a Novel Environmental Biosensor. Curr. Environ. Health Rep. 2014, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tabish, A.M.; Poels, K.; Hoet, P.; Godderis, L. Epigenetic factors in cancer risk: Effect of chemical carcinogens on global DNA methylation pattern in human TK6 cells. PLoS ONE 2012, 7, e34674. [Google Scholar] [CrossRef] [Green Version]
- Antognelli, C.; Gambelunghe, A.; Talesa, V.N.; Muzi, G. Reactive oxygen species induce apoptosis in bronchial epithelial BEAS-2B cells by inhibiting the antiglycation glyoxalase I defence: Involvement of superoxide anion, hydrogen peroxide and NF-kappa B. Apoptosis 2014, 19, 102–116. [Google Scholar] [CrossRef]
- Xue, M.; Rabbani, N.; Momiji, H.; Imbasi, P.; Anwar, M.M.; Kitteringham, N.; Park, B.K.; Souma, T.; Moriguchi, T.; Yamamoto, M.; et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012, 443, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.S.; Cheng, Y.H.; Chiou, C.H.; Chang, T.L. Resveratrol upregulates Nrf2 expression to attenuate methylglyoxal-induced insulin resistance in Hep G2 cells. J. Agric. Food Chem. 2012, 60, 9180–9187. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, C.; Wan, G.; Wang, X.; Cheng, X.; Ou, Y. Phycocyanin prevents methylglyoxal-induced mitochondrial-dependent apoptosis in INS-1 cells by Nrf2. Food Funct. 2016, 7, 1129–1137. [Google Scholar] [CrossRef]
- Tseng, Y.T.; Tsai, Y.H.; Fülöp, F.; Chang, F.R.; Lo, Y.C. 2-Iodo-4’-Methoxychalcone Attenuates Methylglyoxal-Induced Neurotoxicity by Activation of GLP-1 Receptor and Enhancement of Neurotrophic Signal, Antioxidant Defense and Glyoxalase Pathway. Molecules 2019, 24, 2249. [Google Scholar] [CrossRef] [Green Version]
- Oyewole, A.O.; Wilmot, M.C.; Fowler, M.; Birch-Machin, M.A. Comparing the effects of mitochondrial targeted and localized antioxidants with cellular antioxidants in human skin cells exposed to UVA and hydrogen peroxide. FASEB J. 2014, 28, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pooladanda, V.; Thatikonda, S.; Bale, S.; Pattnaik, B.; Sigalapalli, D.K.; Bathini, N.B.; Singh, S.B.; Godugu, C. Nimbolide protects against endotoxin-induced acute respiratory distress syndrome by inhibiting TNF-α mediated NF-κB and HDAC-3 nuclear translocation. Cell Death Dis. 2019, 10, 81. [Google Scholar] [CrossRef]
- Li, Y.; Cohenford, M.A.; Dutta, U.; Dain, J.A. The structural modification of DNA nucleosides by nonenzymatic glycation: An in vitro study based on the reactions of glyoxal and methylglyoxal with 2’-deoxyguanosine. Anal. Bioanal. Chem. 2008, 390, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Bollati, V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009, 21, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.Y.; Ryu, P.D.; Joo, S.W.; Lee, S.Y. Alteration of MicroRNA Expression Profiles by Surface-Modified Gold Nanoparticles in Human Lung Adenocarcinoma Cells. J. Nanosci. Nanotechnol. 2018, 18, 3024–3030. [Google Scholar] [CrossRef]
- Björck, H.M.; Du, L.; Pulignani, S.; Paloschi, V.; Lundströmer, K.; Kostina, A.S.; Österholm, C.; Malashicheva, A.; Kostareva, A.; Evangelista, A.; et al. Mechanistic Interrogation of Bicuspid Aortic Valve associated Aortopathy (MIBAVA) Leducq Consortium. Altered DNA methylation indicates an oscillatory flow mediated epithelial-to-mesenchymal transition signature in ascending aorta of patients with bicuspid aortic valve. Sci. Rep. 2018, 8, 2777. [Google Scholar] [CrossRef] [Green Version]
- Pistore, C.; Giannoni, E.; Colangelo, T.; Rizzo, F.; Magnani, E.; Muccillo, L.; Giurato, G.; Mancini, M.; Rizzo, S.; Riccardi, M.; et al. DNA methylation variations are required for epithelial-to-mesenchymal transition induced by cancer-associated fibroblasts in prostate cancer cells. Oncogene 2017, 36, 5551–5566. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Shi, M.; Zhu, Y.; Ma, Y.; Zhong, Y.; Xiong, C.; Chen, H.; Peng, C. TET1-mediated DNA hydroxymethylation activates inhibitors of the Wnt/β-catenin signaling pathway to suppress EMT in pancreatic tumor cells. J. Exp. Clin. Cancer Res. 2019, 38, 348. [Google Scholar] [CrossRef] [Green Version]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell. Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sanchez-Perez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, W.C.; Shieh, J.M.; Wu, W.B. P38 MAPK and Nrf2 Activation Mediated Naked Gold Nanoparticle Induced Heme Oxygenase-1 Expression in Rat Aortic Vascular Smooth Muscle Cells. Arch. Med. Res. 2020, 51, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Leso, V.; Schulte, P.A. Biomarkers of susceptibility: State of the art and implications for occupational exposure to engineered nanomaterials. Toxicol. Appl. Pharmacol. 2016, 299, 112–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nokin, M.J.; Durieux, F.; Bellier, J.; Peulen, O.; Uchida, K.; Spiegel, D.A.; Cochrane, J.R.; Hutton, C.A.; Castronovo, V. Hormetic potential of methylglyoxal, a side-product of glycolysis, in switching tumours from growth to death. Sci. Rep. 2017, 7, 11722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nass, N.; Vogel, K.; Hofmann, B.; Presek, P.; Silber, R.E.; Simm, A. Glycation of PDGF results in decreased biological activity. Int. J. Biochem. Cell Biol. 2010, 42, 749–754. [Google Scholar] [CrossRef]
- Bansode, S.B.; Chougale, A.D.; Joshi, R.S.; Giri, A.P.; Bodhankar, S.L.; Harsulkar, A.M.; Kulkarni, M.J. Proteomic analysis of protease resistant proteins in the diabetic rat kidney. Mol. Cell. Proteom. 2013, 12, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, Y.; Rao, P.; Huang, K.; Luo, D.; Cai, X.; Xiao, J. Blockade of receptors of advanced glycation end products ameliorates diabetic osteogenesis of adipose-derived stem cells through DNA methylation and Wnt signalling pathway. Cell Prolif. 2018, 51, e12471. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Tang, L.; Luo, M.; Zhou, J.; Guo, B.; Liu, Y.; Chen, B. Size- and time-dependent alteration in metabolic activities of human hepatic cytochrome P450 isozymes by gold nanoparticles via microsomal coincubations. Nanoscale Res. Lett. 2014, 9, 642. [Google Scholar] [CrossRef] [Green Version]
- Shih, J.Y.; Yang, P.C. The EMT regulator slug and lung carcinogenesis. Carcinogenesis 2011, 32, 1299–12304. [Google Scholar] [CrossRef] [Green Version]
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.J.; Murphy, G.F.; Lian, C.G. Melanoma epigenetics: Novel mechanisms, markers, and medicines. Lab. Investig. 2014, 94, 822–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Gene | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| Glo1 (NM_006708.3) | CTCTCCAGAAAAGCTACACTTGAG | CGAGGGTCTGAATTGCCATTG |
| HO-1 (NM_002133.3) | AAGATTGCCCAGAAAGCCCTGGAC | AACTGTCGCCACCAGAAAGCTGAG |
| ZO-1 (NM_001301025.3) | GCGTCACCTACCACCTCGTCGTC | GCCTGCTGCCTTCTCCCACTCTG |
| E-cad (NM_001317184.2) | TTGCGGAAGTCAGTTCAG | CAGAGCCAAGAGGAGACC |
| Vim (NM_003380.5) | GCACACAGCAAGGCGATGG | GGAGCGAGAGTGGCAGAGG |
| α-SMA (NM_001141945.2) | GGCATCATCACCAACTGGGACGAC | AGCACCGCCTGGATAGCCACATAC |
| Snail (NM_005985.4) | GACCACTATGCCGCGCTCTT | TCGCTGTAGTTAGGCTTCCGATT |
| Slug (NM_003068.5) | AGCAGCTGCACTGCGATGCC | ACACAGCAGCCAGATTCCTC |
| Twist (NM_000474.4) | GGAGTCCGCAGTCTTACGAG | TCTGGAGGACCTGGTAGAGG |
| β-actin (NM_001101.5) | CACTCTTCCAGCCTTCCTTCC | ACAGCACTGTGTTGGCGTAC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambelunghe, A.; Giovagnoli, S.; Di Michele, A.; Boncompagni, S.; Dell’Omo, M.; Leopold, K.; Iavicoli, I.; Talesa, V.N.; Antognelli, C. Redox-Sensitive Glyoxalase 1 Up-Regulation Is Crucial for Protecting Human Lung Cells from Gold Nanoparticles Toxicity. Antioxidants 2020, 9, 697. https://doi.org/10.3390/antiox9080697
Gambelunghe A, Giovagnoli S, Di Michele A, Boncompagni S, Dell’Omo M, Leopold K, Iavicoli I, Talesa VN, Antognelli C. Redox-Sensitive Glyoxalase 1 Up-Regulation Is Crucial for Protecting Human Lung Cells from Gold Nanoparticles Toxicity. Antioxidants. 2020; 9(8):697. https://doi.org/10.3390/antiox9080697
Chicago/Turabian StyleGambelunghe, Angela, Stefano Giovagnoli, Alessandro Di Michele, Simona Boncompagni, Marco Dell’Omo, Kerstin Leopold, Ivo Iavicoli, Vincenzo Nicola Talesa, and Cinzia Antognelli. 2020. "Redox-Sensitive Glyoxalase 1 Up-Regulation Is Crucial for Protecting Human Lung Cells from Gold Nanoparticles Toxicity" Antioxidants 9, no. 8: 697. https://doi.org/10.3390/antiox9080697
APA StyleGambelunghe, A., Giovagnoli, S., Di Michele, A., Boncompagni, S., Dell’Omo, M., Leopold, K., Iavicoli, I., Talesa, V. N., & Antognelli, C. (2020). Redox-Sensitive Glyoxalase 1 Up-Regulation Is Crucial for Protecting Human Lung Cells from Gold Nanoparticles Toxicity. Antioxidants, 9(8), 697. https://doi.org/10.3390/antiox9080697