Phenylethyl Isothiocyanate Extracted from Watercress By-Products with Aqueous Micellar Systems: Development and Optimisation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
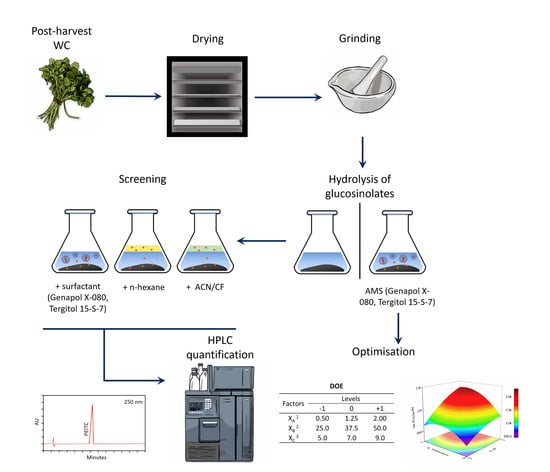
2.2. Extraction Procedures
2.3. Optimisation of PEITC Extraction with AMSs
2.4. PEIT Chromatographic Analysis
2.5. Antioxidant Activity
2.6. Statistical Analysis
3. Results
3.1. Liquid-Liquid Extraction: Comparison of Extractants
3.2. Optimised PEITC Extraction
3.3. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Voutsina, N.; Payne, A.C.; Hancock, R.D.; Clarkson, G.J.J.; Rothwell, S.D.; Chapman, M.A.; Taylor, G. Characterization of the watercress (Nasturtium officinale R. Br.; Brassicaceae) transcriptome using RNASeq and identification of candidate genes for important phytonutrient traits linked to human health. BMC Genom. 2016, 17, 378. [Google Scholar] [CrossRef] [Green Version]
- Blažević, I.; Montaut, S.; Burčul, F.; Rollin, P. Glucosinolates: Novel Sources and Biological Potential. In Glucosinolates; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; Volume 1, pp. 1–58. [Google Scholar]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [Green Version]
- Mitsiogianni, M.; Koutsidis, G.; Mavroudis, N.; Trafalis, D.T.; Botaitis, S.; Franco, R.; Zoumpourlis, V.; Amery, T.; Galanis, A.; Pappa, A.; et al. The Role of Isothiocyanates as Cancer Chemo-Preventive, Chemo-Therapeutic and Anti-Melanoma Agents. Antioxidants 2019, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Giallourou, N.S.; Rowland, I.R.; Rothwell, S.D.; Packham, G.; Commane, D.M.; Swann, J.R. Metabolic targets of watercress and PEITC in MCF-7 and MCF-10A cells explain differential sensitisation responses to ionising radiation. Eur. J. Nutr. 2019, 58, 2377–2391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herr, I.; Lozanovski, V.; Houben, P.; Schemmer, P.; Büchler, M.W. Sulforaphane and related mustard oils in focus of cancer prevention and therapy. Wien. Med. Wochenschr. 2013, 163, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Farhana, N.; Aripin, B.; Surugau, N. Effects of Temperature and pH on Myrosinase Activity and Gluconasturtiin Hydrolysis Products in Watercress. Trans. Sci. Technol. 2016, 3, 449–454. [Google Scholar]
- Van Eylen, D.; Oey, I.; Hendrickx, M.; Loey, A. Van Effects of pressure/temperature treatments on stability and activity of endogenous broccoli (Brassica oleracea L. cv. Italica) myrosinase and on cell permeability. J. Food Eng. 2008, 89, 178–186. [Google Scholar] [CrossRef]
- Tanongkankit, Y.; Sablani, S.S.; Chiewchan, N.; Devahastin, S. Microwave-assisted extraction of sulforaphane from white cabbages: Effects of extraction condition, solvent and sample pretreatment. J. Food Eng. 2013, 117, 151–157. [Google Scholar] [CrossRef]
- Fusari, C.M.; Ramirez, D.A.; Camargo, A.B. Simplified analytical methodology for glucosinolate hydrolysis products: A miniaturized extraction technique and multivariate optimization. Anal. Methods 2019, 11, 309–316. [Google Scholar] [CrossRef]
- Pusateri, D.J.; Kizer, T.R.; Lowry, A.N. Extraction of Non-Polar Isothiocyanates from Plants. US Patent 6,824,796, 30 November 2004. [Google Scholar]
- Rodrigues, L.; Silva, I.; Poejo, J.; Serra, A.T.; Matias, A.A.; Simplício, A.L.; Bronze, M.R.; Duarte, C.M.M. Recovery of antioxidant and antiproliferative compounds from watercress using pressurized fluid extraction. RSC Adv. 2016, 6, 30905–30918. [Google Scholar] [CrossRef]
- Pereira, L.; Silva, P.; Duarte, M.; Rodrigues, L.; Duarte, C.; Albuquerque, C.; Serra, A. Targeting Colorectal Cancer Proliferation, Stemness and Metastatic Potential Using Brassicaceae Extracts Enriched in Isothiocyanates: A 3D Cell Model-Based Study. Nutrients 2017, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Molina-Bolívar, J.A.; Aguiar, J.; Ruiz, C.C. Light scattering and fluorescence probe studies on micellar properties of Triton X-100 in KCl solutions. Mol. Phys. 2001, 99, 1729–1741. [Google Scholar] [CrossRef]
- Tani, H.; Kamidate, T.; Watanabe, H. Aqueous Micellar Two-Phase Systems for Protein Separation. Anal. Sci. 1998, 14, 875–888. [Google Scholar] [CrossRef] [Green Version]
- Vieira, F.A.; Guilherme, R.J.R.; Neves, M.C.; Abreu, H.; Rodrigues, E.R.O.; Maraschin, M.; Coutinho, J.A.P.; Ventura, S.P.M. Single-step extraction of carotenoids from brown macroalgae using non-ionic surfactants. Sep. Purif. Technol. 2017, 172, 268–276. [Google Scholar] [CrossRef]
- Katsoyannos, E.; Chatzilazarou, A.; Gortzi, O.; Lalas, S.; Konteles, S.; Tataridis, P. Application of cloud point extraction using surfactants in the isolation of physical antioxidants (phenols) from olive mill wastewater. Proc. Fresenius Environ. Bull. 2006, 15, 1122–1125. [Google Scholar]
- Cordisco, E.; Haidar, C.N.; Coscueta, E.R.; Nerli, B.B.; Malpiedi, L.P. Integrated extraction and purification of soy isoflavones by using aqueous micellar systems. Food Chem. 2016, 213, 514–520. [Google Scholar] [CrossRef]
- Alibrahim, M. Cloud point extraction of polycyclic aromatic hydrocarbons in aqueous solution with nonionic surfactants. Tenside Surfactants Deterg. 2014, 51, 333–338. [Google Scholar] [CrossRef]
- Gortzi, O.; Lalas, S.; Chatzilazarou, A.; Katsoyannos, E.; Papaconstandinou, S.; Dourtoglou, E. Recovery of natural antioxidants from olive mill wastewater using Genapol-X080. JAOCS J. Am. Oil Chem. Soc. 2008, 85, 133–140. [Google Scholar] [CrossRef]
- Raja, S.; Murty, V.R.; Thivaharan, V.; Rajasekar, V.; Ramesh, V. Aqueous Two Phase Systems for the Recovery of Biomolecules—A Review. Sci. Technol. 2012, 1, 7–16. [Google Scholar] [CrossRef]
- Cordisco, E.; Haidar, C.N.; Goñi, R.; Nerli, B.B.; Malpiedi, L.P. Physicochemical characterization of aqueous micellar systems formed by environmentally friendly salts. Fluid Phase Equilib. 2015, 393, 111–116. [Google Scholar] [CrossRef]
- Sharma, S.; Kori, S.; Parmar, A. Surfactant mediated extraction of total phenolic contents (TPC) and antioxidants from fruits juices. Food Chem. 2015, 185, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Coscueta, E.R.; Pellegrini Malpiedi, L.; Nerli, B.B. Micellar systems of aliphatic alcohol ethoxylates as a sustainable alternative to extract soybean isoflavones. Food Chem. 2018, 264, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Haidar, C.N.; Coscueta, E.; Cordisco, E.; Nerli, B.B.; Malpiedi, L.P. Aqueous micellar two-phase system as an alternative method to selectively remove soy antinutritional factors. LWT 2018, 93, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Coscueta, E.R.; Campos, D.A.; Osório, H.; Nerli, B.B.; Pintado, M. Enzymatic soy protein hydrolysis: A tool for biofunctional food ingredient production. Food Chem. X 2019, 1, 100006. [Google Scholar] [CrossRef]
- Gonçalves, B.; Falco, V.; Moutinho-Pereira, J.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of Elevated CO2 on Grapevine (Vitis vinifera L.): Volatile Composition, Phenolic Content, and in vitro Antioxidant Activity of Red Wine. J. Agric. Food Chem. 2009, 57, 265–273. [Google Scholar] [CrossRef]
- Kutner, M.H.; Nachtsheim, C.J.; Neter, J.; Li, W. Applied Linear Statistical Models, 5th ed.; McGraw Hill: New York, NY, USA, 2005. [Google Scholar]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
- Gunst, R.F.; Myers, R.H.; Montgomery, D.C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments. Technometrics 1996, 38, 285. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Holban, A.M. Ingredients Extraction by Physicochemical Methods in Food. In Handbook of Food Bioengineering; Elsevier Science: Amsterdam, The Netherlands, 2017; ISBN 9780128112021. [Google Scholar]
- Sarkar, R.; Mukherjee, S.; Biswas, J.; Roy, M. Phenethyl isothiocyanate, by virtue of its antioxidant activity, inhibits invasiveness and metastatic potential of breast cancer cells: HIF-1α as a putative target. Free Radic. Res. 2016, 50, 84–100. [Google Scholar] [CrossRef]
- Valgimigli, L.; Iori, R. Antioxidant and pro-oxidant capacities of ITCs. Environ. Mol. Mutagen. 2009, 50, 222–237. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Shibutani, M.; Kuroiwa, K.; Takagi, H.; Inoue, K.; Nishikawa, H.; Miki, T.; Hirose, M. Chemoprevention of acrylamide toxicity by antioxidative agents in rats—Effective suppression of testicular toxicity by phenylethyl isothiocyanate. Arch. Toxicol. 2005, 79, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Kim, J. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okulicz, M.; Bialik, I.; Chichlowska, J. The time-dependent effect of gluconasturtiin and phenethyl isothiocyanate on metabolic and antioxidative parameters in rats. J. Anim. Physiol. Anim. Nutr. (Berl.) 2005, 89, 367–372. [Google Scholar] [CrossRef] [PubMed]


| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| XA 1 | 0.50 | 1.25 | 2.00 |
| XB 2 | 25.0 | 37.5 | 50.0 |
| XC 3 | 5.0 | 7.0 | 9.0 |
| Extractant | Conc. (µg/g WC DB) | |
|---|---|---|
| WC extracts | Genapol X-080 | 1168 ± 187 a |
| Tergitol 15-S-7 | 1287 ± 83 a,b | |
| n-hexane | 1682 ± 156 b | |
| ACN/CF | 1270 ± 169 a,b |
| Run | Factors | Response † | |||
|---|---|---|---|---|---|
| XA 1 | XB 2 | XC 3 | Y (µg PEITC/g WC DB) | ||
| Genapol X-080 | Tergitol 15-S-7 | ||||
| 1 | 1.25 | 50.0 | 5.0 | 641 ± 102 | 590 ± 68 |
| 2 | 0.50 | 37.5 | 5.0 | 654 ± 69 | 798 ± 86 |
| 3 | 2.00 | 37.5 | 9.0 | 2552 ± 247 | 2842 ± 229 |
| 4 | 1.25 | 37.5 | 7.0 | 1450 ± 175 | 1669 ± 173 |
| 5 | 0.50 | 37.5 | 9.0 | 1168 ± 122 | 1346 ± 97 |
| 6 | 1.25 | 50.0 | 9.0 | 939 ± 79 | 1090 ± 63 |
| 7 | 1.25 | 25.0 | 9.0 | 2235 ± 149 | 2102 ± 156 |
| 8 | 1.25 | 37.5 | 7.0 | 1640 ± 181 | 1760 ± 163 |
| 9 | 0.50 | 25.0 | 7.0 | 852 ± 70 | 891 ± 65 |
| 10 | 2.00 | 25.0 | 7.0 | 2346 ± 169 | 2441 ± 185 |
| 11 | 2.00 | 50.0 | 7.0 | 940 ± 111 | 932 ± 96 |
| 12 | 2.00 | 37.5 | 5.0 | 988 ± 144 | 971 ± 85 |
| 13 | 1.25 | 25.0 | 5.0 | 838 ± 68 | 824 ± 84 |
| 14 | 0.50 | 50.0 | 7.0 | 748 ± 69 | 887 ± 70 |
| 15 | 1.25 | 37.5 | 7.0 | 1593 ± 142 | 1722 ± 103 |
| Coefficient | Estimated Coefficient Values | |
|---|---|---|
| Genapol X-080 | Tergitol 15-S-7 | |
| −6605.12 | −7396.17 | |
| 645.722 | 504.556 | |
| 211.279 | 259.502 | |
| 895.638 | 903.736 | |
| −34.7289 | −40.1244 | |
| 174.833 | 220.611 | |
| −1.61313 | −2.432 | |
| −10.99 | −7.79 | |
| −33.3045 | −44.6458 | |
| Statistics | ||
| Lack of fit 1 | 0.351 | 0.082 |
| R2 | 0.943 | 0.953 |
| R2adj | 0.930 | 0.943 |
| RSD | 167 | 150 |
| Sample | ORAC (μmol TE/mg PEITC) | ABTS (μmol TE/mg PEITC) |
|---|---|---|
| PEITC | 1.9 ± 0.7 | 1.1 ± 0.2 |
| AMS1 1 | 134.9 ± 4.0 a | 86.4 ± 3.8 a |
| AMS2 2 | 123.3 ± 5.1 a | 81.2 ± 2.6 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coscueta, E.R.; Reis, C.A.; Pintado, M. Phenylethyl Isothiocyanate Extracted from Watercress By-Products with Aqueous Micellar Systems: Development and Optimisation. Antioxidants 2020, 9, 698. https://doi.org/10.3390/antiox9080698
Coscueta ER, Reis CA, Pintado M. Phenylethyl Isothiocyanate Extracted from Watercress By-Products with Aqueous Micellar Systems: Development and Optimisation. Antioxidants. 2020; 9(8):698. https://doi.org/10.3390/antiox9080698
Chicago/Turabian StyleCoscueta, Ezequiel R., Celso A. Reis, and Manuela Pintado. 2020. "Phenylethyl Isothiocyanate Extracted from Watercress By-Products with Aqueous Micellar Systems: Development and Optimisation" Antioxidants 9, no. 8: 698. https://doi.org/10.3390/antiox9080698
APA StyleCoscueta, E. R., Reis, C. A., & Pintado, M. (2020). Phenylethyl Isothiocyanate Extracted from Watercress By-Products with Aqueous Micellar Systems: Development and Optimisation. Antioxidants, 9(8), 698. https://doi.org/10.3390/antiox9080698