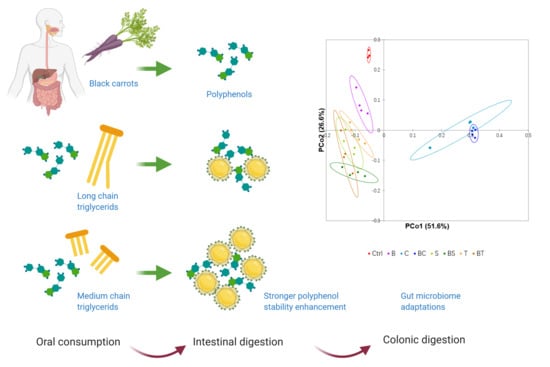
Dietary Lipids Influence Bioaccessibility of Polyphenols from Black Carrots and Affect Microbial Diversity under Simulated Gastrointestinal Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. In Vitro Gastrointestinal Digestion
2.4. In Vitro Colonic Fermentation
2.5. Phytochemical and Antioxidant Assays
2.6. Separation and Analysis of Polyphenols
2.7. The 16S rRNA Sequencing and Analysis
2.8. Short Chain Fatty Acids Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Bioaccessibility of Individual Phenolic Compounds and Effects of Lipids in Different Digestive Compartments
3.2. Antioxidant and Phytochemical Assays
3.3. The Carrot-Oil Matrix Modulates the Gut Microbiota and Affects Short Chain Fatty Acid Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Selby-Pham, S.N.; Cottrell, J.J.; Dunshea, F.R.; Ng, K.; Bennett, L.E.; Howell, K.S. Dietary phytochemicals promote health by enhancing antioxidant defence in a pig model. Nutrients 2017, 9, 758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.-C.; Sheen, J.-M.; Hu, W.L.; Hung, Y.-C. Polyphenols and oxidative stress in atherosclerosis-related ischemic heart disease and stroke. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordestgaard, B.G.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Ahn, I.-S.; Kim, S.-O.; Kong, C.-S.; Chung, H.-Y.; Do, M.-S.; Park, K.-Y. Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J. Med. Food 2007, 10, 552–556. [Google Scholar] [CrossRef]
- Kemperman, R.A.; Bolca, S.; Roger, L.C.; Vaughan, E.E. Novel approaches for analysing gut microbes and dietary polyphenols: Challenges and opportunities. Microbiology 2010, 156, 3224–3231. [Google Scholar] [CrossRef] [Green Version]
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57, 59–81. [Google Scholar] [CrossRef]
- Lambert, J.E.; Parks, E.J. Postprandial metabolism of meal triglyceride in humans. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Agans, R.; Gordon, A.; Kramer, D.L.; Perez-Burillo, S.; Rufián-Henares, J.A.; Paliy, O. Dietary fatty acids sustain the growth of the human gut microbiota. Appl. Environ. Microbiol. 2018, 84, e01525-18. [Google Scholar] [CrossRef] [Green Version]
- Ortega, N.; Macià, A.; Romero, M.-P.; Reguant, J.; Motilva, M.-J. Matrix composition effect on the digestibility of carob flour phenols by an in-vitro digestion model. Food Chem. 2011, 124, 65–71. [Google Scholar] [CrossRef]
- Shishikura, Y.; Khokhar, S.; Murray, B.S. Effects of tea polyphenols on emulsification of olive oil in a small intestine model system. J. Agric. Food Chem. 2006, 54, 1906–1913. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Padayachee, A.; Comino, T.; Chhan, R.; Zhang, P.; Ng, K.; Cottrell, J.J.; Dunshea, F.R. Effect of a polyphenol-rich plant matrix on colonic digestion and plasma antioxidant capacity in a porcine model. J. Funct. Foods 2019, 57, 211–221. [Google Scholar] [CrossRef]
- US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 28; Nutrient Data Laboratory. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/methods-and-application-of-food-composition-laboratory/mafcl-site-pages/sr11-sr28 (accessed on 16 August 2020).
- Pérez-Burillo, S.; Rufián-Henares, J.; Pastoriza, S. Towards an improved global antioxidant response method (GAR+): Physiological-resembling in vitro digestion-fermentation method. Food Chem. 2018, 239, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Augustin, M.A.; Sanguansri, L.; Shen, Z.; Ng, K.; Ajlouni, S. Enhanced bioaccessibility of curcuminoids in buttermilk yogurt in comparison to curcuminoids in aqueous dispersions. J. Food Sci. 2016, 81, H769–H776. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Zabielski, R.; Hammon, H.M.; Metges, C.C. Nutritional programming of gastrointestinal tract development. Is the pig a good model for man? Nutr. Res. Rev. 2010, 23, 4–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A. Lc-esi-qtof/ms characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M. Binding of polyphenols to plant cell wall analogues–Part 1: Anthocyanins. Food Chem. 2012, 134, 155–161. [Google Scholar] [CrossRef]
- Chandra, A.; Rana, J.; Li, Y. Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC-MS. J. Agric. Food Chem. 2001, 49, 3515–3521. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Tian, G.; Wu, X.; Chen, D.; Yu, B.; He, J. Adaptation of gut microbiome to different dietary nonstarch polysaccharide fractions in a porcine model. Mol. Nutr. Food Res. 2017, 61, 1700012. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.; Carle, R.; Schieber, A. Quantification of anthocyanins in black carrot extracts (Daucus carota ssp. sativus var. atrorubens Alef.) and evaluation of their color properties. Eur. Food Res. Technol. 2004, 219, 479–486. [Google Scholar] [CrossRef]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red cabbage–stability to simulated gastrointestinal digestion. Phytochemistry 2007, 68, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Mikkelsen, D.; Gidley, M.J. Lack of release of bound anthocyanins and phenolic acids from carrot plant cell walls and model composites during simulated gastric and small intestinal digestion. Food Funct. 2013, 4, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Gumienna, M.; Lasik, M.; Czarnecki, Z. Bioconversion of grape and chokeberry wine polyphenols during simulated gastrointestinal in vitro digestion. Int. J. Food Sci. Nutr. 2011, 62, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H.; Honda, Y.; Nakagawa, S.; Ashida, H.; Kanazawa, K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 2003, 51, 571–581. [Google Scholar] [CrossRef]
- Kim, I.; Moon, J.K.; Hur, S.J.; Lee, J. Structural changes in mulberry (Morus Microphylla. Buckl) and chokeberry (Aronia melanocarpa) anthocyanins during simulated in vitro human digestion. Food Chem. 2020, 318. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Pérez-Vicente, A.; Gil-Izquierdo, A.; García-Viguera, C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312. [Google Scholar] [CrossRef]
- Woodward, G.; Kroon, P.; Cassidy, A.; Kay, C. Anthocyanin stability and recovery: Implications for the analysis of clinical and experimental samples. J. Agric. Food Chem. 2009, 57, 5271–5278. [Google Scholar] [CrossRef]
- Oliveira, H.; Perez-Gregório, R.; de Freitas, V.; Mateus, N.; Fernandes, I. Comparison of the in vitro gastrointestinal bioavailability of acylated and non-acylated anthocyanins: Purple-fleshed sweet potato vs red wine. Food Chem. 2019, 276, 410–418. [Google Scholar] [CrossRef]
- Ortega, N.; Reguant, J.; Romero, M.-P.; Macia, A.; Motilva, M.-J. Effect of fat content on the digestibility and bioaccessibility of cocoa polyphenol by an in vitro digestion model. J. Agric. Food Chem. 2009, 57, 5743–5749. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Erlejman, A.; Verstraeten, S.; Fraga, C.; Oteiza, P. The interaction of flavonoids with membranes: Potential determinant of flavonoid antioxidant effects. Free Radic. Res. 2004, 38, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/peptide binding and precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.J.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, M.; McClements, D.J. Factors affecting lipase digestibility of emulsified lipids using an in vitro digestion model: Proposal for a standardised pH-stat method. Food Chem. 2011, 126, 498–505. [Google Scholar] [CrossRef]
- Huo, T.; Ferruzzi, M.G.; Schwartz, S.J.; Failla, M.L. Impact of fatty acyl composition and quantity of triglycerides on bioaccessibility of dietary carotenoids. J. Agric. Food Chem. 2007, 55, 8950–8957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colle, I.J.; Van Buggenhout, S.; Lemmens, L.; Van Loey, A.M.; Hendrickx, M.E. The type and quantity of lipids present during digestion influence the in vitro bioaccessibility of lycopene from raw tomato pulp. Food Res. Int. 2012, 45, 250–255. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.; Faria, A.; de Freitas, V.; Calhau, C.; Mateus, N. Multiple-approach studies to assess anthocyanin bioavailability. Phytochem. Rev. 2015, 14, 899–919. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E. Phenolic acids-their properties, occurrence in plant materials, absorption and metabolism. Bromatol. Chem. Toksykol. 2006, 39, 103. [Google Scholar]
- Olthof, M.R.; Hollman, P.C.; Buijsman, M.N.; Van Amelsvoort, J.M.; Katan, M.B. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Khairallah, J.; Sadeghi Ekbatan, S.; Sabally, K.; Iskandar, M.M.; Hussain, R.; Nassar, A.; Sleno, L.; Rodes, L.; Prakash, S.; Donnelly, D.J. Microbial biotransformation of a polyphenol-rich potato extract affects antioxidant capacity in a simulated gastrointestinal model. Antioxidants 2018, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- De Ferrars, R.; Czank, C.; Zhang, Q.; Botting, N.; Kroon, P.; Cassidy, A.; Kay, C. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Ma, S.; Wang, M.; Feng, X.-Y. Characterization of free, conjugated, and bound phenolic acids in seven commonly consumed vegetables. Molecules 2017, 22, 1878. [Google Scholar] [CrossRef] [Green Version]
- Howard, L.; Wong, A.; Perry, A.; Klein, B. β-Carotene and ascorbic acid retention in fresh and processed vegetables. J. Food Sci. 1999, 64, 929–936. [Google Scholar] [CrossRef]
- Escarpa, A.; González, M. Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal. Chim. Acta 2001, 427, 119–127. [Google Scholar] [CrossRef]
- Ferrario, C.; Statello, R.; Carnevali, L.; Mancabelli, L.; Milani, C.; Mangifesta, M.; Duranti, S.; Lugli, G.A.; Jimenez, B.; Lodge, S. How to feed the mammalian gut microbiota: Bacterial and metabolic modulation by dietary fibers. Front. Microbiol. 2017, 8, 1749. [Google Scholar] [CrossRef] [PubMed]
- Nograšek, B.; Accetto, T.; Fanedl, L.; Avguštin, G. Description of a novel pectin-degrading bacterial species Prevotella pectinovora sp. nov., based on its phenotypic and genomic traits. J. Microbiol. 2015, 53, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, K.A.; Boesmans, L.; Boets, E. Modulating the microbiota in inflammatory bowel diseases: Prebiotics, probiotics or faecal transplantation? Proc. Nutr. Soc. 2014, 73, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Zentek, J.; Buchheit-Renko, S.; Männer, K.; Pieper, R.; Vahjen, W. Intestinal concentrations of free and encapsulated dietary medium-chain fatty acids and effects on gastric microbial ecology and bacterial metabolic products in the digestive tract of piglets. Arch. Anim. Nutr. 2012, 66, 14–26. [Google Scholar] [CrossRef]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [Green Version]
- Nalina, T.; Rahim, Z. The crude aqueous extract of Piper betle L. and its antibacterial effect towards Streptococcus mutans. Am. J. Biochem. Biotechnol. 2007, 3, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Božik, M.; Hovorková, P.; Klouček, P. Antibacterial effect of carvacrol and coconut oil on selected pathogenic bacteria. Sci. Agric. Bohem. 2018, 49, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Falcão-e-Cunha, L.; Peres, H.; Freire, J.P.; Castro-Solla, L. Effects of alfalfa, wheat bran or beet pulp, with or without sunflower oil, on caecal fermentation and on digestibility in the rabbit. Anim. Feed. Sci. Technol. 2004, 117, 131–149. [Google Scholar] [CrossRef]
- Day, L.; Xu, M.; Øiseth, S.K.; Hemar, Y.; Lundin, L. Control of morphological and rheological properties of carrot cell wall particle dispersions through processing. Food Bioprocess Technol. 2010, 3, 928–934. [Google Scholar] [CrossRef]
- Hinnebusch, B.F.; Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 2002, 132, 1012–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Rose, D.J. Long-term dietary pattern of fecal donor correlates with butyrate production and markers of protein fermentation during in vitro fecal fermentation. Nutr. Res. 2014, 34, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Schlörmann, W.; Birringer, M.; Lochner, A.; Lorkowski, S.; Richter, I.; Rohrer, C.; Glei, M. In Vitro fermentation of nuts results in the formation of butyrate and c9, t11 conjugated linoleic acid as chemopreventive metabolites. Eur. J. Nutr. 2016, 55, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parry, J. Pacific islanders pay heavy price for abandoning traditional diet. World Health Organ. Bull. World Health Organ. 2010, 88, 484. [Google Scholar]
- Wallace, T.C. Health effects of coconut oil—A narrative review of current evidence. J. Am. Coll. Nutr. 2019, 38, 97–107. [Google Scholar] [CrossRef]
- Jones, R.B.; Alderete, T.L.; Kim, J.S.; Millstein, J.; Gilliland, F.D.; Goran, M.I. High intake of dietary fructose in overweight/obese teenagers associated with depletion of Eubacterium and Streptococcus in gut microbiome. Gut Microbes 2019, 10, 712–719. [Google Scholar] [CrossRef]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [Green Version]



| Fatty Acid (%) | Coconut Oil | Sunflower Oil | Beef Tallow |
|---|---|---|---|
| C8 | 7.0 | - | - |
| C10 | 8.0 | - | - |
| C12 | 48.0 | - | - |
| C14 | 16.0 | - | 3.0 |
| C16 | 9.5 | 5.0 | 26.0 |
| C16:1 | - | - | 3.0 |
| C18 | - | 6.0 | 14.0 |
| C18:1 | 6.5 | 30.0 | 47.0 |
| C18:2 | - | 59.0 | 3.0 |
| C18:3 | - | - | 1.0 |
| Identified | R | Gastric Fraction | |||
| Compound | value | B (BA%) | BC (BA%) | BS (BA%) | BT (BA%) |
| Cy 3-xylglcgal | 110.9 | 18.1b (16.3%) | 42.2a (38.1%) | 45.0a (40.6%) | 10.1b (9.10%) |
| Cy 3-xylgal | 850.3 | 64.1bc (7.50%) | 209.5a (24.6%) | 194.7ab (22.9%) | 48.8c (5.70%) |
| Cy 3-xyl (sin) glcgal | 126.4 | 39.6a (31.3%) | 51.0a (40.3%) | 45.7a (36.1%) | 8.50b (6.7%) |
| Cy 3-xyl (fer) glcgal | 1483.8 | 711.6a (48.0%) | 823.9a (55.5%) | 732.6a (49.4%) | 140.5b (9.5%) |
| Cy 3-xyl (cmr) glcgal | 55.6 | 26.6ab (47.9%) | 39.9a (71.7%) | 32.5a (58.4%) | 6.90b (12.3%) |
| Total anthocyanins | 2627 | 859.9a (32.7%) | 1166a (44.4%) | 1050a (40.0%) | 214.7b (8.20%) |
| Neochlorogenic acid | 996.2 | 13.0 (1.30%) | 17.1 (1.70%) | 17.5 (1.80%) | 7.60 (0.80%) |
| Chlorogenic acid | 2470.8 | 1899ab (76.9%) | 2303a (93.2%) | 2002a (81.0%) | 861.7b (34.9%) |
| Caffeic acid | 109.4 | 63.5a (58.0%) | 51b (46.6%) | 47.1b (43.0%) | 14.3c (13.0%) |
| Ferulic acid | 135.4 | 55.8a (41.2%) | 40.3b (29.8%) | 44.3b (32.8%) | 13.2c (9.80%) |
| p-coumaric acid | - | - | - | - | - |
| Total phenolic acids | 3712 | 2031a (54.7%) | 2411a (65.0%) | 2111a (56.9%) | 896.9b (24.2%) |
| Identified | R | Small Intestinal Fraction | |||
| compound | value | B (BA%) | BC (BA%) | BS (BA%) | BT (BA%) |
| Cy 3-xylglcgal | 110.9 | 0.25b (0.23%) | 1.76a (1.59%) | 1.35a (1.22%) | 1.70a (1.54%) |
| Cy 3-xylgal | 850.3 | 0.95b (0.11%) | 9.78a (1.15%) | 3.68b (0.43%) | 3.02b (0.36%) |
| Cy 3-xyl (sin) glcgal | 126.4 | 0.80c (0.63%) | 3.54a (2.8%) | 2.84ab (2.24%) | 2.09b (1.66%) |
| Cy 3-xyl (fer) glcgal | 1483.8 | 23.0c (1.55%) | 117a (7.9%) | 73.3b (4.94%) | 69.1b (4.66%) |
| Cy 3-xyl (cmr) glcgal | 55.6 | 0.84c (1.51%) | 3.00a (5.4%) | 1.47bc (2.65%) | 1.77b (3.18%) |
| Total anthocyanins | 2627 | 25.8c (0.98%) | 135.3a (5.15%) | 82.7b (3.15%) | 77.7b (2.96%) |
| Neochlorogenic acid | 996.2 | 7.73c (0.78%) | 5.65c (0.57%) | 23.7b (2.37%) | 39.3a (3.95%) |
| Chlorogenic acid | 2470.8 | 101.6b (4.1%) | 805.6a (32.6%) | 599.5a (24.3%) | 862.5a (34.9%) |
| Caffeic acid | 109.4 | 172.7b (157.9%) | 202.3a (184.9%) | 210.1a (192.1%) | 206.8a (189.1%) |
| Ferulic acid | 135.4 | 247.5b (182.8%) | 236.1b (174.4%) | 308.2a (227.7%) | 246.0b (181.7%) |
| p-coumaric acid | - | 144.5c (n.a.) | 168.5b (n.a.) | 204.0a (n.a.) | 178.5b (n.a.) |
| Total phenolic acids | 3712 | 673.9b (18.2%) | 1418a (38.2%) | 1346a (36.3%) | 1533a (41.3%) |
| Identified | R | Colonic Fraction | |||
| compound | value | B (BA%) | BC (BA%) | BS (BA%) | BT (BA%) |
| Cy 3-xylglcgal | 110.9 | - | - | - | - |
| Cy 3-xylgal | 850.3 | - | - | - | - |
| Cy 3-xyl (sin) glcgal | 126.4 | - | - | - | - |
| Cy 3-xyl (fer) glcgal | 1483.8 | - | - | - | - |
| Cy 3-xyl (cmr) glcgal | 55.6 | - | - | - | - |
| Total anthocyanins | 2627 | - | - | - | - |
| Neochlorogenic acid | 996.2 | - | - | - | - |
| Chlorogenic acid | 2470.8 | - | - | - | - |
| Caffeic acid | 109.4 | - | - | - | - |
| Ferulic acid | 135.4 | - | 90.3a (66.7%) | - | - |
| p-coumaric acid | - | - | - | - | - |
| Total phenolic acids | 3712 | - | 90.3a (2.43%) | - | - |
| Genera | Controls | Samples | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ctrl | C | S | T | B | BC | BS | BT | ||
| Megasphaera | 2.87b | 15.2ab | 31.1ab | 26.8ab | 8.39b | 1.14b | 44.7a | 47.0a | <0.0001 |
| Escherichia | 0.39c | 29.4b | 5.01c | 4.81c | 9.15c | 47.8a | 8.91c | 0.42c | <0.0001 |
| Muribaculaceae | 16.4a | 0.06c | 17.9a | 11.8ab | 10.6ab | 0.01c | 3.83bc | 9.84ab | <0.0001 |
| Prevotella | 6.61bc | 0.06cd | 3.02bcd | 6.07bcd | 15.85a | 0.01d | 9.61ab | 6.93b | <0.0001 |
| Streptococcus | 10.3a | 3.66bc | 0.38c | 0.55c | 4.82b | 3.18bc | 0.26c | 0.29c | <0.0001 |
| Dialister | 1.32c | 5.50a | 2.22bc | 1.24c | 0.48c | 7.77ab | 1.06c | 2.20bc | <0.0001 |
| Acidaminococcus | 0.63c | 0.01c | 3.13abc | 4.21ab | 2.95abc | - | 6.00a | 2.18bc | <0.0001 |
| Prevotellaceae NK3B31 group | 2.71b | - | 0.39c | 1.60bc | 5.93a | - | 4.64a | 1.78bc | <0.0001 |
| Oribacterium | 1.13b | 1.79ab | 0.33b | 1.85ab | 2.99ab | 2.86ab | 1.25b | 4.37a | <0.0001 |
| Parasutterella | 0.82b | 0.04b | 4.99a | 4.68a | 2.78ab | 0.01b | 1.93ab | 1.07b | <0.0001 |
| Subdoligranulum | 1.79bc | 5.09ab | 0.26c | 0.42c | 1.20bc | 6.42a | 0.21c | 0.18c | <0.0001 |
| Clostridium sensu stricto 1 | 0.84b | 5.87a | 1.13b | 1.61b | 0.57b | 3.84ab | 0.37b | 0.45b | <0.0001 |
| Allisonella | 1.41c | 0.11c | 4.35a | 4.09ab | 1.77bc | 0.07c | 1.42c | 1.39c | <0.0001 |
| Desulfovibrio | 1.97bc | 0.20c | 1.39c | 4.69a | 3.74ab | 0.06c | 1.17c | 1.27c | <0.0001 |
| Rikenellaceae RC9 gut group | 8.27a | - | 1.07b | 0.47b | 0.18b | - | 0.08b | 0.50b | <0.0001 |
| Erysipelotrichaceae UCG-006 | 0.79 | 1.93 | 1.08 | 1.38 | 2.62 | 1.52 | 0.16 | 0.58 | 0.003 |
| Lactobacillus | 0.78b | 0.60b | 0.63b | 0.76b | 4.00a | 0.33b | 2.09ab | 0.16b | <0.0001 |
| Phascolarctobacterium | 0.74b | 2.81a | 0.74b | 1.14ab | 0.83b | 1.81ab | 0.39b | 0.71b | <0.0001 |
| Short chain fatty acids | |||||||||
| Acetate | 64.3a | 31.2d | 32.3cd | 42.6bc | 67.5a | 37.2bcd | 40.0bcd | 46.8b | <0.0001 |
| Propionate | 15.0c | 4.00d | 19.7bc | 16.3c | 24.1b | 3.17d | 23.3b | 31.4a | <0.0001 |
| Butyrate | 22.1c | 3.57d | 34.5a | 27.2bc | 24.5bc | 2.99d | 34.1a | 30.1ab | <0.0001 |
| Total SCFAs | 101.4abc | 37.9d | 86.4c | 86.1c | 116.0a | 43.4d | 97.3bc | 108.3ab | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, C.; Suleria, H.A.R.; Dunshea, F.R.; Howell, K. Dietary Lipids Influence Bioaccessibility of Polyphenols from Black Carrots and Affect Microbial Diversity under Simulated Gastrointestinal Digestion. Antioxidants 2020, 9, 762. https://doi.org/10.3390/antiox9080762
Gu C, Suleria HAR, Dunshea FR, Howell K. Dietary Lipids Influence Bioaccessibility of Polyphenols from Black Carrots and Affect Microbial Diversity under Simulated Gastrointestinal Digestion. Antioxidants. 2020; 9(8):762. https://doi.org/10.3390/antiox9080762
Chicago/Turabian StyleGu, Chunhe, Hafiz A. R. Suleria, Frank R. Dunshea, and Kate Howell. 2020. "Dietary Lipids Influence Bioaccessibility of Polyphenols from Black Carrots and Affect Microbial Diversity under Simulated Gastrointestinal Digestion" Antioxidants 9, no. 8: 762. https://doi.org/10.3390/antiox9080762
APA StyleGu, C., Suleria, H. A. R., Dunshea, F. R., & Howell, K. (2020). Dietary Lipids Influence Bioaccessibility of Polyphenols from Black Carrots and Affect Microbial Diversity under Simulated Gastrointestinal Digestion. Antioxidants, 9(8), 762. https://doi.org/10.3390/antiox9080762