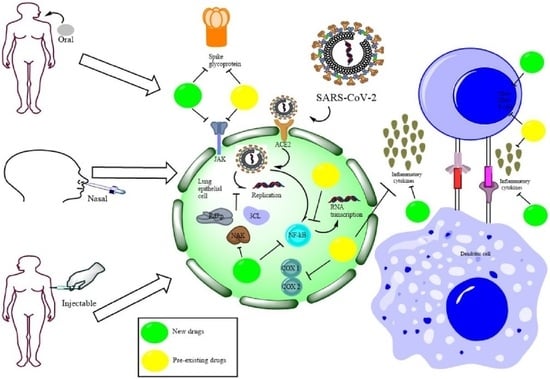
A Clinical Insight on New Discovered Molecules and Repurposed Drugs for the Treatment of COVID-19
Abstract
:1. Introduction
2. New Discovered Molecules
2.1. Molnupiravir
2.1.1. General Description
2.1.2. Mechanism of Action
2.1.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
2.1.4. Regulatory Approval and Marketing Authorization
2.2. Paxlovid™
2.2.1. General Description
2.2.2. Mechanism of Action
2.2.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
2.2.4. Regulatory Approval and Marketing Authorization
2.3. Baricitinib
2.3.1. General Description
2.3.2. Mechanism of Action
2.3.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
2.3.4. Regulatory Approval and Marketing Authorization
2.4. Wharton’s Jelly Mesenchymal Stem Cells (WJ-MSC)
2.4.1. General Description
2.4.2. Mechanism of Action
2.4.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
2.4.4. Regulatory Approval and Marketing Authorization
2.5. Convalescent Plasma (CP)
2.5.1. General Description
2.5.2. Mechanism of Action
2.5.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
2.5.4. Regulatory Approval and Marketing Authorization
2.6. Sarilumab
2.6.1. General Description
2.6.2. Mechanism of Action
2.6.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
2.7. Tocilizumab
2.7.1. General Description
2.7.2. Mechanism of Action
2.7.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
2.7.4. Regulatory Approval and Marketing Authorization
2.8. Bevacizumab
2.8.1. General Description
2.8.2. Mechanism of Action
2.8.3. Clinical Trial, Route of Administration, Dose and Dosage Form
2.8.4. Regulatory Approval and Marketing Authorization
3. Comparative Analysis of Newly Discovered Molecules
4. Repurposed Drugs
4.1. Dexamethasone
4.1.1. General Description
4.1.2. Mechanism of Action
4.1.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
4.1.4. Regulatory Approval
4.2. Naproxen
4.2.1. General Description
4.2.2. Mechanism of Action
4.2.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
4.2.4. Regulatory Approval
4.3. Remdesivir
4.3.1. General Description
4.3.2. Mechanism of Action
4.3.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
4.3.4. Regulatory Approval
4.4. Hydroxychloroquine
4.4.1. General Description
4.4.2. Mechanism of Action
4.4.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
4.4.4. Regulatory Approval
4.5. Favipiravir
4.5.1. General Description
4.5.2. Mechanism of Action
4.5.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
4.5.4. Regulatory Approval
4.6. Umifenovir (Arbidol)
4.6.1. General Description
4.6.2. Mechanism of Action
4.6.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
4.6.4. Regulatory Approval
4.7. Azvudine
4.7.1. General Description
4.7.2. Mechanism of Action
4.7.3. Clinical Trial, Route of Administration, Dose, and Dosage Form
4.7.4. Regulatory Approval
5. Comparative Analysis of Repurposed Drugs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, K.; Nguyen, D.D.; Chen, J.; Wang, R.; Wei, G.-W. Repositioning of 8565 Existing Drugs for COVID-19. J. Phys. Chem. Lett. 2020, 11, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Banerjee, D.; Singh, A.; Saharan, V.A. A Comprehensive Investigation Regarding the Differentiation of the Procurable COVID-19 Vaccines. AAPS PharmSciTech 2022, 23, 95. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. The ‘Very, Very Bad Look’ of Remdesivir, the First FDA-Approved COVID-19 Drug. Science, 28 MOctoberay 2020. Available online: https://www.science.org/content/article/very-very-bad-look-remdesivir-first-fda-approved-Covid-19-drug(accessed on 31 October 2022).
- World Health Organization. WHO Recommends against the Use of Remdesivir in COVID-19 Patients. Available online: https://www.who.int/news-room/feature-stories/detail/who-recommends-against-the-use-of-remdesivir-in-Covid-19-patients (accessed on 31 October 2022).
- Acharjee, S. COVID-19: The Bitter Truth about Using Hydroxychloroquine as a Preventive Drug. India Today, 25 May 2020. Available online: https://www.indiatoday.in/india-today-insight/story/covid-19-the-bitter-truth-about-using-hydroxychloroquine-as-a-preventive-drug-1659116-2020-03-24(accessed on 15 July 2022).
- Kaul, R. Stay away from Indiscriminate Use of Anti-Malaria Drug: Experts on COVID-19. Hindustan Times, 25 March 2020. Available online: https://www.hindustantimes.com/india-news/stay-away-from-indiscriminate-use-of-anti-malaria-drug-experts-on-covid-19/story-UY2zFNCcWivkdw7zToWHcI.html(accessed on 19 December 2022).
- India News. Assam Doctor Who Took Anti-Malaria Drug amid COVID-19 Outbreak Dies. Hindustan Times, 30 March 2020. Available online: https://www.hindustantimes.com/india-news/assam-doctor-who-took-anti-malaria-drug-amid-covid-19-outbreak-dies/story-mHqu2dLT8gKxLohCk9JkeP.html(accessed on 19 December 2022).
- Terry, M. Amidst Controversy, FDA Issues EUA Allowing Anti-Malaria Drugs to Be Used to Treat COVID-19. Biospace. Biospace, 30 March 2020. Available online: https://www.biospace.com/article/fda-allows-anti-malaria-drug-chloroquine-to-be-used-to-treat-covid-19/(accessed on 19 December 2022).
- World Health Organization. WHO Recommends Two New Drugs to Treat COVID-19. Available online: https://www.who.int/news/item/14-01-2022-who-recommends-two-new-drugs-to-treat-Covid-19 (accessed on 31 October 2022).
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Butler, C.C.; Hobbs, F.D.R.; Gbinigie, O.A.; Rahman, N.M.; Hayward, G.; Richards, D.B.; Dorward, J.; Lowe, D.M.; Standing, J.F.; Breuer, J.; et al. Molnupiravir plus Usual Care versus Usual Care Alone as Early Treatment for Adults with COVID-19 at Increased Risk of Adverse Outcomes (PANORAMIC): An Open-Label, Platform-Adaptive Randomised Controlled Trial. Lancet 2022, 401, 281–293. [Google Scholar] [CrossRef]
- Kozlov, M. Merck’s COVID Pill Loses Its Lustre: What That Means for the Pandemic. Nature, 13 December 2021. Available online: https://www.nature.com/articles/d41586-021-03667-0(accessed on 3 March 2022).
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. Molnupiravir in COVID-19: A Systematic Review of Literature. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102329. [Google Scholar] [CrossRef]
- Kabinger, F.; Stiller, C.; Schmitzová, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Höbartner, C.; Cramer, P. Mechanism of Molnupiravir-Induced SARS-CoV-2 Mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef]
- Toots, M.; Yoon, J.-J.; Hart, M.; Natchus, M.G.; Painter, G.R.; Plemper, R.K. Quantitative Efficacy Paradigms of the Influenza Clinical Drug Candidate EIDD-2801 in the Ferret Model. Transl. Res. 2020, 218, 16–28. [Google Scholar] [CrossRef]
- Pourkarim, F.; Pourtaghi-Anvarian, S.; Rezaee, H. Molnupiravir: A New Candidate for COVID-19 Treatment. Pharmacol. Res. Perspect. 2022, 10, e00909. [Google Scholar] [CrossRef]
- Khiali, S.; Khani, E.; B Rouy, S.; Entezari-Maleki, T. Comprehensive Review on Molnupiravir in COVID-19: A Novel Promising Antiviral to Combat the Pandemic. Future Microbiol. 2022, 17, 377–391. [Google Scholar] [CrossRef]
- Wahl, A.; Gralinski, L.E.; Johnson, C.E.; Yao, W.; Kovarova, M.; Dinnon, K.H.; Liu, H.; Madden, V.J.; Krzystek, H.M.; De, C.; et al. SARS-CoV-2 Infection is Effectively Treated and Prevented by EIDD-2801. Nature 2021, 591, 451–457. [Google Scholar] [CrossRef]
- Rosenke, K.; Hansen, F.; Schwarz, B.; Feldmann, F.; Haddock, E.; Rosenke, R.; Barbian, K.; Meade-White, K.; Okumura, A.; Leventhal, S.; et al. Orally Delivered MK-4482 Inhibits SARS-CoV-2 Replication in the Syrian Hamster Model. Nat. Commun. 2021, 12, 2295. [Google Scholar] [CrossRef]
- Painter, W.P.; Holman, W.; Bush, J.A.; Almazedi, F.; Malik, H.; Eraut, N.C.J.E.; Morin, M.J.; Szewczyk, L.J.; Painter, G.R. Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity against SARS-CoV-2. Antimicrob. Agents Chemother. 2021, 65, e02428-20. [Google Scholar] [CrossRef]
- Khoo, S.H.; Fitzgerald, R.; Fletcher, T.; Ewings, S.; Jaki, T.; Lyon, R.; Downs, N.; Walker, L.; Tansley-Hancock, O.; Greenhalf, W.; et al. Optimal Dose and Safety of Molnupiravir in Patients with Early SARS-CoV-2: A Phase I, Open-Label, Dose-Escalating, Randomized Controlled Study. J. Antimicrob. Chemother. 2021, 76, 3286–3295. [Google Scholar] [CrossRef]
- Lee, C.-C.; Hsieh, C.-C.; Ko, W.-C. Molnupiravir—A Novel Oral Anti-SARS-CoV-2 Agent. Antibiotics 2021, 10, 1294. [Google Scholar] [CrossRef]
- Fischer, W.; Eron, J.J.; Holman, W.; Cohen, M.S.; Fang, L.; Szewczyk, L.J.; Sheahan, T.P.; Baric, R.; Mollan, K.R.; Wolfe, C.R.; et al. Molnupiravir, an Oral Antiviral Treatment for COVID-19. medRxiv 2021. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Molnupiravir Reduces Risk of Hospital Admission or Death by 50% in Patients at Risk, MSD Reports. BMJ 2021, 375, n2422. [Google Scholar] [CrossRef]
- Merck &, C. Merck and Ridgeback’s Molnupiravir, an Oral COVID-19 Antiviral Medicine, Receives First Authorization in the World. Available online: https://www.merck.com/news/merck-and-ridgebacks-molnupiravir-an-oral-covid-19-antiviral-medicine-receives-first-authorization-in-the-world/ (accessed on 19 December 2022).
- EMC. Lagevrio 200 Mg Hard Capsules. Available online: https://www.medicines.org.uk/emc/product/13044/smpc#gref (accessed on 20 November 2022).
- European Medicines Agency. EMA Issues Advice on Use of Lagevrio (Molnupiravir) for the Treatment of COVID-19. Available online: https://www.ema.europa.eu/en/news/ema-issues-advice-use-lagevrio-molnupiravir-treatment-covid-19 (accessed on 20 November 2022).
- US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain (accessed on 19 December 2022).
- Sun Pharma. Sun Pharma Geared to Introduce Molnupiravir, Licensed from MSD and Ridgeback, under the Brand Name Molxvir® in India. Available online: https://sunpharma.com/wp-content/uploads/2021/11/Press-Release-Sun-Pharma-gearing-up-to-introduce-molnupiravir-under-the-brand-name-Molxvir®-in-India.pdf (accessed on 19 December 2022).
- Torrent Pharma. Torrent Pharma to Launch Molnupiravir under the Brand Name Molnutor® in India. Available online: https://torrentpharma.com/pdf/investors/Press_Release_Molnupiravir_Final.pdf (accessed on 19 December 2022).
- Cipla. Cipla Receives Emergency Use Authorisation (EUA) to Launch Oral Antiviral Drug Cipmolnu® (Molnupiravir 200 Mg) in India for Treatment of Adult Patients with COVID-19, with SpO2 > 93% and Who Have High Risk of Progression of the Disease Including Hospitaliza. Available online: https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra (accessed on 19 December 2022).
- Pfizer. Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate (accessed on 3 April 2022).
- National Institute of Health. Ritonavir-Boosted Nirmatrelvir (Paxlovid). Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir--paxlovid-/ (accessed on 25 March 2022).
- U.S. National Library of Medicine. Relative Bioavailability Study of PF-07321332/Ritonavir Oral Powder Relative to the Commercial Tablets in Healthy Participants. Available online: https://clinicaltrials.gov/ct2/show/NCT05263921?term=covid+19&cond=nirmatrelvir&draw=2&rank=3 (accessed on 25 March 2022).
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- European Medicines Agency. Paxlovid. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/paxlovid (accessed on 3 April 2022).
- Pilla, V. Torrent & Aurobindo Receive Licenses to Sell Pfizer’s COVID Pill Paxlovid in India, Low Income Countries. Available online: https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/torrent-aurobindo-receive-licenses-to-sell-pfizers-covid-pill-paxlovid-in-india-low-income-countries/articleshow/90297321.cms (accessed on 3 April 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 44205240, Baricitinib. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Baricitinib (accessed on 3 April 2022).
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Rawling, M.; Savory, E.; Stebbing, J. Baricitinib as Potential Treatment for 2019-nCoV Acute Respiratory Disease. Lancet 2020, 395, e30–e31. [Google Scholar] [CrossRef]
- Stebbing, J.; Krishnan, V.; De Bono, S.; Ottaviani, S.; Casalini, G.; Richardson, P.J.; Monteil, V.; Lauschke, V.M.; Mirazimi, A.; Youhanna, S.; et al. Mechanism of Baricitinib Supports Artificial Intelligence-Predicted Testing in COVID-19 Patients. EMBO Mol. Med. 2020, 12, e12697. [Google Scholar] [CrossRef]
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; E Kartman, C.; Krishnan, V.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Alatorre-Alexander, J.; Pellegrini, R.D.C.; et al. Efficacy and Safety of Baricitinib for the Treatment of Hospitalised Adults with COVID-19 (COV-BARRIER): A Randomised, Double-Blind, Parallel-Group, Placebo-Controlled Phase 3 Trial. Lancet Respir. Med. 2021, 9, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Eli Lilly. Baricitinib Receives Emergency Use Authorization from the FDA for the Treatment of Hospitalized Patients with COVID-19. Available online: https://investor.lilly.com/news-releases/news-release-details/baricitinib-receives-emergency-use-authorization-fda-treatment (accessed on 19 December 2022).
- BerGenBio ASA. BerGenBio and Oslo University Hospital Announce the AXL Inhibitor Bemcentinib Will Be Studied in the EU Funded EU-SolidAct Trial in Hospitalised COVID-19 Patients. Cission PR Newswire, 27 January 2022. Available online: https://www.prnewswire.com/news-releases/bergenbio-and-oslo-university-hospital-announce-the-axl-inhibitor-bemcentinib-will-be-studied-in-the-eu-funded-eu-solidact-trial-in-hospitalised-covid-19-patients-301469371.html(accessed on 4 November 2022).
- Kim, N.; Cho, S.-G. Clinical Applications of Mesenchymal Stem Cells. Korean J. Intern. Med. 2013, 28, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, G. Editorial Connecting the Dots: The Promises of Wharton’s Jelly Mesenchymal Stem Cells for Tissue Repair and Regeneration. Open Tissue Eng. Regen. Med. J. 2011, 4, 3–5. [Google Scholar] [CrossRef]
- Carvello, M.; Lightner, A.; Yamamoto, T.; Kotze, P.G.; Spinelli, A. Mesenchymal Stem Cells for Perianal Crohn’s Disease. Cells 2019, 8, 764. [Google Scholar] [CrossRef]
- Saleh, M.; Vaezi, A.A.; Aliannejad, R.; Sohrabpour, A.A.; Kiaei, S.Z.F.; Shadnoush, M.; Siavashi, V.; Aghaghazvini, L.; Khoundabi, B.; Abdoli, S.; et al. Cell Therapy in Patients with COVID-19 Using Wharton’s Jelly Mesenchymal Stem Cells: A Phase 1 Clinical Trial. Stem Cell Res. Ther. 2021, 12, 410. [Google Scholar] [CrossRef]
- Golchin, A.; Seyedjafari, E.; Ardeshirylajimi, A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev. Rep. 2020, 16, 427–433. [Google Scholar] [CrossRef]
- Khanh, V.C.; Fukushige, M.; Chang, Y.H.; Hoang, N.N.; Yamashita, T.; Obata-Yasuoka, M.; Hamada, H.; Osaka, M.; Hiramatsu, Y.; Ohneda, O. Wharton’s Jelly Mesenchymal Stem Cell-Derived Extracellular Vesicles Reduce SARS-CoV2-Induced Inflammatory Cytokines under High Glucose and Uremic Toxin Conditions. Stem Cells Dev. 2021, 30, 758–772. [Google Scholar] [CrossRef]
- Paladino, F.V.; Rodrigues, J.D.M.; da Silva, A.; Goldberg, A.C. The Immunomodulatory Potential of Wharton’s Jelly Mesenchymal Stem/Stromal Cells. Stem Cells Int. 2019, 2019, 3548917. [Google Scholar] [CrossRef]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Mebarki, M.; Abadie, C.; Larghero, J.; Cras, A. Human Umbilical Cord-Derived Mesenchymal Stem/Stromal Cells: A Promising Candidate for the Development of Advanced Therapy Medicinal Products. Stem Cell Res. Ther. 2021, 12, 152. [Google Scholar] [CrossRef]
- Monsel, A.; Hauw-Berlemont, C.; Mebarki, M.; Heming, N.; Mayaux, J.; Tchoumba, O.N.; Diehl, J.-L.; Demoule, A.; Annane, D.; Marois, C.; et al. Treatment of COVID-19-Associated ARDS with Mesenchymal Stromal Cells: A Multicenter Randomized Double-Blind Trial. Crit. Care 2022, 26, 48. [Google Scholar] [CrossRef]
- US National Library of Medicine. Efficacy of Infusions of MSC From Wharton Jelly in the SARS-CoV-2 (COVID-19) Related Acute Respiratory Distress Syndrome (MSC-COVID19). Available online: https://clinicaltrials.gov/ct2/show/NCT04625738?term=Wharton%27s+Jelly+Mesenchymal+stem+cells&cond=COVID-19&draw=2&rank=2 (accessed on 19 December 2022).
- Clinical Center. Convalescent Plasma. Available online: https://clinicalcenter.nih.gov/blooddonor/donationtypes/convalescent_plasma.html (accessed on 22 January 2023).
- Rojas, M.; Rodríguez, Y.; Monsalve, D.M.; Acosta-Ampudia, Y.; Camacho, B.; Gallo, J.E.; Rojas-Villarraga, A.; Ramírez-Santana, C.; Díaz-Coronado, J.C.; Manrique, R.; et al. Convalescent Plasma in COVID-19: Possible Mechanisms of Action. Autoimmun. Rev. 2020, 19, 102554. [Google Scholar] [CrossRef]
- Franchini, M.; Glingani, C.; Liumbruno, G.M. Potential Mechanisms of Action of Convalescent Plasma in COVID-19. Diagnosis 2021, 8, 413–420. [Google Scholar] [CrossRef]
- Roback, J.D.; Guarner, J. Convalescent Plasma to Treat COVID-19 Possibilities and Challenges. JAMA 2020, 323, 1561–1562. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Hyperimmune Plasma for Critical Patients with COVID-19 (COV19-PLASMA). Available online: https://clinicaltrials.gov/ct2/show/NCT04321421 (accessed on 19 December 2022).
- Perotti, C.; Del Fante, C.; Baldanti, F.; Franchini, M.; Percivalle, E.; Nepita, E.V.; Seminari, E.; De Silvestri, A.; Bruno, R.; Klersy, C. Plasma from Donors Recovered from the New Coronavirus 2019 as Therapy for Critical Patients with COVID-19 (COVID-19 plasma study): A Multicentre Study Protocol. Intern. Emerg. Med. 2020, 15, 819–824. [Google Scholar] [CrossRef]
- Piechotta, V.; Chai, K.L.; Valk, S.J.; Doree, C.; Monsef, I.; Wood, E.M.; Lamikanra, A.; Kimber, C.; McQuilten, Z.; So-Osman, C.; et al. Convalescent Plasma or Hyperimmune Immunoglobulin for People with COVID-19: A Living Systematic Review. Cochrane Database Syst. Rev. 2020, 7, CD013600. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Expanded Access to Convalescent Plasma for the Treatment of Patients with COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04338360 (accessed on 19 December 2022).
- Senefeld, J.W.; Johnson, P.W.; Kunze, K.L.; Bloch, E.M.; van Helmond, N.; Golafshar, M.A.; Klassen, S.A.; Klompas, A.M.; Sexton, M.A.; Soto, J.C.D.; et al. Access to and Safety of COVID-19 Convalescent Plasma in the United States Expanded Access Program: A National Registry Study. PLoS Med. 2021, 18, e1003872. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Recommendations for Investigational COVID-19 Convalescent Plasma. Available online: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-applications-inds-cber-regulated-products/recommendations-investigational-covid-19-convalescent-plasma (accessed on 19 December 2022).
- Drugbank. Sarilumab: Uses, Interactions, Mechanism of Action. Available online: https://go.drugbank.com/drugs/DB11767 (accessed on 16 July 2022).
- Cooper, S. Sarilumab for the Treatment of Rheumatoid Arthritis. Immunotherapy 2016, 8, 249–250. [Google Scholar] [CrossRef]
- Lamb, Y.N.; Deeks, E.D. Sarilumab: A Review in Moderate to Severe Rheumatoid Arthritis. Drugs 2018, 78, 929–940. [Google Scholar] [CrossRef]
- Scott, L.J. Sarilumab: First Global Approval. Drugs 2017, 77, 705–712. [Google Scholar] [CrossRef]
- June, R.R.; Olsen, N.J. Room for More IL-6 Blockade? Sarilumab for the Treatment of Rheumatoid Arthritis. Expert Opin. Biol. Ther. 2016, 16, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients with COVID-19. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04315298 (accessed on 17 December 2022).
- Regeneron Pharmaceuticals Inc. Regeneron and Sanofi Announce FDA Approval of Kevzara® (Sarilumab) for the Treatment of Moderately to Severely Active Rheumatoid Arthritis in Adult Patients. Available online: https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-announce-fda-approval-kevzarar-sarilumab (accessed on 16 July 2022).
- Regeneron Pharmaceuticals Inc Regeneron and Sanofi Begin Global Kevzara® (Sarilumab) Clinical Trial Program in Patients with Severe COVID-19. Available online: https://newsroom.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-begin-global-kevzarar-sarilumab-clinical (accessed on 16 July 2022).
- Sivapalasingam, S.; Lederer, D.J.; Bhore, R.; Hajizadeh, N.; Criner, G.; Hosain, R.; Mahmood, A.; Giannelou, A.; Somersan-Karakaya, S.; O’Brien, M.P.; et al. Efficacy and Safety of Sarilumab in Hospitalized Patients with Coronavirus Disease 2019: A Randomized Clinical Trial. Clin. Infect. Dis. 2022, 75, e380–e388. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Sarilumab COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04327388 (accessed on 14 December 2022).
- Lescure, F.-X.; Honda, H.; Fowler, R.A.; Lazar, J.S.; Shi, G.; Wung, P.; Patel, N.; Hagino, O.; Bazzalo, I.J.; Casas, M.M.; et al. Sarilumab in Patients Admitted to Hospital with Severe or Critical COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Respir. Med. 2021, 9, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Drugbank. Tocilizumab: Uses, Interactions, Mechanism of Action. Available online: https://go.drugbank.com/drugs/DB06273 (accessed on 16 July 2022).
- Sebba, A. Tocilizumab: The First Interleukin-6-Receptor Inhibitor. Am. J. Health Syst. Pharm. 2008, 65, 1413–1418. [Google Scholar] [CrossRef]
- Drugs.com. Tocilizumab. Available online: https://www.drugs.com/ingredient/tocilizumab.html (accessed on 17 July 2022).
- ACTEMRA®. Starting SC Injections. Available online: https://www.actemra.com/ra/treatment/sc-injections.html (accessed on 22 January 2023).
- Parsons, L. FDA Approves Roche’s Actemra COVID-19 Trial. PM Live, 24 March 2020. Available online: https://www.pmlive.com/pharma_news/fda_approves_roches_actemra_covid-19_trial_1329887(accessed on 17 July 2022).
- U.S. National Library of Medicine. A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients with Severe COVID-19 Pneumonia (COVACTA). Available online: https://clinicaltrials.gov/ct2/show/NCT04320615 (accessed on 19 December 2022).
- Rosas, I.O.; Bräu, N.; Waters, M.; Go, R.C.; Malhotra, A.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Savic, S.; Douglas, I.S.; et al. Tocilizumab in Patients Hospitalised with COVID-19 Pneumonia: Efficacy, Safety, Viral Clearance, and Antibody Response from a Randomised Controlled Trial (COVACTA). Eclinicalmedicine 2022, 47, 101409. [Google Scholar] [CrossRef]
- United States Food & Drug Administration. Frequently Asked Questions on the Emergency Use Authorization for Actemra (Tocilizumab) for Treatment of COVID-19. Available online: https://www.fda.gov/media/150345/download (accessed on 22 January 2023).
- Drugs.com. Avastin FDA Approval History. Available online: https://www.drugs.com/history/avastin.html (accessed on 19 December 2022).
- Kazazi-Hyseni, F.; Beijnen, J.H.; Schellens, J.H.M. Bevacizumab. Oncologist 2010, 15, 819–825. [Google Scholar] [CrossRef]
- Drugbank. Bevacizumab: Uses, Interactions, Mechanism of Action. Available online: https://go.drugbank.com/drugs/DB00112 (accessed on 17 July 2022).
- Pang, J.; Xu, F.; Aondio, G.; Li, Y.; Fumagalli, A.; Lu, M.; Valmadre, G.; Wei, J.; Bian, Y.; Canesi, M.; et al. Efficacy and Tolerability of Bevacizumab in Patients with Severe COVID-19. Nat. Commun. 2021, 12, 814. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Bevacizumab in Severe or Critical Patients with COVID-19 Pneumonia (BEST-CP). Available online: https://clinicaltrials.gov/ct2/show/NCT04275414 (accessed on 19 December 2022).
- Haddad, F.; Dokmak, G.; Karaman, R. A Comprehensive Review on the Efficacy of Several Pharmacologic Agents for the Treatment of COVID-19. Life 2022, 12, 1758. [Google Scholar] [CrossRef]
- Zou, R.; Peng, L.; Shu, D.; Zhao, L.; Lan, J.; Tan, G.; Peng, J.; Yang, X.; Liu, M.; Zhang, C.; et al. Antiviral Efficacy and Safety of Molnupiravir against Omicron Variant Infection: A Randomized Controlled Clinical Trial. Front. Pharmacol. 2022, 13, 939573. [Google Scholar] [CrossRef]
- EMC. Paxlovid 150 Mg/100 Mg Film-Coated Tablets. Available online: https://www.medicines.org.uk/emc/product/13145/smpc (accessed on 14 January 2023).
- Drugbank. Dexamethasone. Available online: https://go.drugbank.com/drugs/DB01234 (accessed on 14 December 2022).
- Rhen, T.; Cidlowski, J.A. Antiinflammatory Action of Glucocorticoids—New Mechanisms for Old Drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. COVID-19-Associated ARDS Treated with Dexamethasone: Alliance COVID-19 Brasil III (CoDEX). Available online: https://clinicaltrials.gov/ct2/show/NCT04327401 (accessed on 19 December 2022).
- U.S. National Library of Medicine. Efficacy of Dexamethasone Treatment for Patients with ARDS Caused by COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04325061 (accessed on 14 December 2022).
- World Health Organization. Coronavirus Disease (COVID-19): Dexamethasone. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-Covid-19-dexamethasone (accessed on 23 January 2023).
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in the Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Drugbank. Naproxen: Uses, Interactions, Mechanism of Action. Available online: https://go.drugbank.com/drugs/DB00788 (accessed on 14 July 2022).
- Giménez, M.; Pujol, J.; Ali, Z.; López-Solà, M.; Contreras-Rodríguez, O.; Deus, J.; Ortiz, H.; Soriano-Mas, C.; Llorente-Onaindia, J.; Monfort, J. Naproxen Effects on Brain Response to Painful Pressure Stimulation in Patients with Knee Osteoarthritis: A Double-Blind, Randomized, Placebo-Controlled, Single-Dose Study. J. Rheumatol. 2014, 41, 2240–2248. [Google Scholar] [CrossRef]
- Terrier, O.; Dilly, S.; Pizzorno, A.; Chalupska, D.; Humpolickova, J.; Bouřa, E.; Berenbaum, F.; Quideau, S.; Lina, B.; Fève, B.; et al. Antiviral Properties of the NSAID Drug Naproxen Targeting the Nucleoprotein of SARS-CoV-2 Coronavirus. Molecules 2021, 26, 2593. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Efficacy of Addition of Naproxen in the Treatment of Critically III Patients Hospitalized for COVID-19 Infection (ENACOVID). Available online: https://clinicaltrials.gov/ct2/show/NCT04325633 (accessed on 15 December 2022).
- Drugbank. Remdesivir: Uses, Interactions, Mechanism of Action. Available online: https://go.drugbank.com/drugs/DB14761 (accessed on 15 July 2022).
- Amirian, E.S.; Levy, J.K. Current Knowledge about the Antivirals Remdesivir (GS-5734) and GS-441524 as Therapeutic Options for Coronaviruses. One Health 2020, 9, 100128. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Woolner, E.; Perry, J.K.; Feng, J.Y.; Porter, D.P.; Götte, M. Remdesivir is a Direct-Acting Antiviral That Inhibits RNA-Dependent RNA Polymerase from Severe Acute Respiratory Syndrome Coronavirus 2 with High Potency. J. Biol. Chem. 2020, 295, 6785–6797. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.X.; et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Frediansyah, A.; Nainu, F.; Dhama, K.; Mudatsir, M.; Harapan, H. Remdesivir and Its Antiviral Activity against COVID-19: A Systematic Review. Clin. Epidemiol. Glob. Health 2021, 9, 123–127. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734TM) in Participants with Severe Coronavirus Disease (COVID-19). Available online: https://clinicaltrials.gov/ct2/show/NCT04292899 (accessed on 19 December 2022).
- A Olender, S.; Perez, K.K.; Go, A.S.; Balani, B.; Price-Haywood, E.G.; Shah, N.S.; Wang, S.; Walunas, T.L.; Swaminathan, S.; Slim, J.; et al. Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care. Clin. Infect. Dis. 2021, 73, e4166–e4174. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Adaptive COVID-19 Treatment Trial (ACTT). Available online: https://clinicaltrials.gov/ct2/show/NCT04280705 (accessed on 4 December 2022).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- United States Food & Drug Administration. FDA Approves First Treatment for COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 23 January 2023).
- Gilead Sciences. FDA Approves Veklury® (Remdesivir) for the Treatment of Non-Hospitalized Patients at High Risk for COVID-19 Disease Progression. Available online: https://www.gilead.com/news-and-press/press-room/press-releases/2022/1/fda-approves-veklury-remdesivir-for-the-treatment-of-nonhospitalized-patients-at-high-risk-for-covid19-disease-progression (accessed on 23 January 2023).
- European Medicines Agency. Meeting Highlights from the Committee for Medicinal Products for Human Use (CHMP) 13–16 December 2021. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-committee-medicinal-products-human-use-chmp-13-16-december-2021 (accessed on 23 January 2023).
- Drugbank. Hydroxychloroquine: Uses, Interactions, Mechanism of Action. Available online: https://go.drugbank.com/drugs/DB01611 (accessed on 15 July 2022).
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. Hydroxychloroquine in Patients with COVID-19: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, V.; Hristova, S.; Uzunova, K.; Vekov, T. A Review on the Mechanism of Action of Favipiravir and Hydroxychloroquine in COVID-19. Res. Rev. Insights 2021, 5, 1–7. [Google Scholar] [CrossRef]
- Satarker, S.; Ahuja, T.; Banerjee, M.; E, V.B.; Dogra, S.; Agarwal, T.; Nampoothiri, M. Hydroxychloroquine in COVID-19: Potential Mechanism of Action against SARS-CoV-2. Curr. Pharmacol. Rep. 2020, 6, 203–211. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Outcomes Related to COVID-19 Treated with Hydroxychloroquine among In-Patients with Symptomatic Disease (ORCHID). Available online: https://clinicaltrials.gov/ct2/show/NCT04332991 (accessed on 4 August 2022).
- Casey, J.D.; Johnson, N.J.; Semler, M.W.; Collins, S.P.; Aggarwal, N.R.; Brower, R.G.; Chang, S.Y.; Eppensteiner, J.; Filbin, M.; Gibbs, K.W.; et al. Rationale and Design of ORCHID: A Randomized Placebo-Controlled Clinical Trial of Hydroxychloroquine for Adults Hospitalized with COVID-19. Ann. Am. Thorac. Soc. 2020, 17, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Lupkin, S. Malaria Drugs Get FDA “Emergency Use Authorization” For COVID-19. NPR. 30 March 2020. Available online: https://www.npr.org/sections/coronavirus-live-updates/2020/03/30/823987540/fda-oks-addition-to-stockpile-of-malaria-drugs-for-covid-19 (accessed on 15 July 2022).
- Self, W.H.; Semler, M.W.; Leither, L.M.; Casey, J.D.; Angus, D.C.; Brower, R.G.; Chang, S.Y.; Collins, S.P.; Eppensteiner, J.C.; Filbin, M.R.; et al. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients with COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 2165–2176. [Google Scholar] [CrossRef]
- Shippey, E.A.; Wagler, V.D.; Collamer, A.N. Hydroxychloroquine: An Old Drug with New Relevance. Clevel. Clin. J. Med. 2018, 85, 459–467. [Google Scholar] [CrossRef]
- Devaux, C.A.; Rolain, J.-M.; Colson, P.; Raoult, D. New Insights on the Antiviral Effects of Chloroquine against Coronavirus: What to Expect for COVID-19? Int. J. Antimicrob. Agents 2020, 55, 105938. [Google Scholar] [CrossRef]
- Sandler, R. FDA Authorizes Anti-Malarial Drugs Chloroquine and Hydroxychloroquine for Emergency Coronavirus Treatment. Forbes, 30 March 2020. Available online: https://www.forbes.com/sites/rachelsandler/2020/03/30/fda-approves-anti-malarial-drugs-chloroquine-and-hydroxychloroquine-for-emergency-coronavirus-treatment/?sh=2ec6be865e5d (accessed on 15 July 2022).
- Mundell, E.J. FDA Approves Malaria Drugs to Treat COVID-19, Despite Little Proof They Work. Clinical Collection, 31 March 2020. Available online: https://www.clinicalconnection.com/health-news/news-article/51148/fda-approves-malaria-drugs-to-treat-covid-19-despite-little-proof-they-work (accessed on 19 December 2022).
- European Medicines Agency. COVID-19: Chloroquine and Hydroxychloroquine Only to Be Used in Clinical Trials or Emergency Use Programmes. Available online: https://www.ema.europa.eu/en/news/covid-19-chloroquine-hydroxychloroquine-only-be-used-clinical-trials-emergency-use-programmes (accessed on 15 July 2022).
- Peng, S.; Wang, H.; Wang, Z.; Wang, Q. Progression of Antiviral Agents Targeting Viral Polymerases. Molecules 2022, 27, 7370. [Google Scholar] [CrossRef]
- Drugbank. Favipiravir. Available online: https://go.drugbank.com/drugs/DB12466 (accessed on 16 July 2022).
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a Broad Spectrum Inhibitor of Viral RNA Polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 449–463. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Oral Favipiravir Compared to Standard Supportive Care in Subjects with Mild. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04346628 (accessed on 4 August 2022).
- Holubar, M.; Subramanian, A.; Purington, N.; Hedlin, H.; Bunning, B.; Walter, K.S.; Bonilla, H.; Boumis, A.; Chen, M.; Clinton, K.; et al. Favipiravir for Treatment of Outpatients with Asymptomatic or Uncomplicated Coronavirus Disease 2019: A Double-Blind, Randomized, Placebo-Controlled, Phase 2 Trial. Clin. Infect. Dis. 2022, 75, 1883–1892. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Efficacy and Safety of Favipiravir in Management of COVID-19 (FAV-001). Available online: https://clinicaltrials.gov/ct2/show/NCT04349241 (accessed on 14 December 2022).
- Hassanipour, S.; Arab-Zozani, M.; Amani, B.; Heidarzad, F.; Fathalipour, M.; Martinez-De-Hoyo, R. The Efficacy and Safety of Favipiravir in Treatment of COVID-19: A Systematic Review and Meta-Analysis of Clinical Trials. Sci. Rep. 2021, 11, 11022. [Google Scholar] [CrossRef]
- Eunethta. Favipiravir for the Treatment of COVID-19. Available online: https://eunethta.eu/doi (accessed on 21 December 2022).
- Agrawal, U.; Raju, R.; Udwadia, Z.F. Favipiravir: A New and Emerging Antiviral Option in COVID-19. Med. J. Armed Forces India 2020, 76, 370–376. [Google Scholar] [CrossRef]
- Blaising, J.; Polyak, S.J.; Pécheur, E.-I. Arbidol as a Broad-Spectrum Antiviral: An Update. Antivir. Res. 2014, 107, 84–94. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, L.; Liu, Y.; Luo, R.; Zeng, L.; Telegina, I.; Vlassov, V. Arbidol for Preventing and Treating Influenza in Adults and Children. Cochrane Database Syst. Rev. 2017, 2017, CD011489. [Google Scholar] [CrossRef]
- Drugbank. Umifenovir. Available online: https://go.drugbank.com/drugs/DB13609 (accessed on 16 July 2022).
- U.S. National Library of Medicine. The Efficacy of Lopinavir plus Ritonavir and Arbidol against Novel Coronavirus Infection ELACOI). Available online: https://clinicaltrials.gov/ct2/show/NCT04252885 (accessed on 14 December 2022).
- Li, Y.; Xie, Z.; Lin, W.; Cai, W.; Wen, C.; Guan, Y.; Mo, X.; Wang, J.; Wang, Y.; Peng, P.; et al. Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial. Med 2020, 1, 105–113.e4. [Google Scholar] [CrossRef]
- Yu, B.; Chang, J. The first Chinese Oral Anti-COVID-19 Drug Azvudine Launched. Innovation 2022, 3, 100321. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Li, Y.-H.; Wang, L.-L.; Liu, H.-Q.; Lu, S.-Y.; Liu, Y.; Li, K.; Liu, B.; Li, S.-Y.; Shao, F.-M.; et al. Azvudine is a Thymus-Homing Anti-SARS-CoV-2 Drug Effective in Treating COVID-19 Patients. Signal Transduct. Target. Ther. 2021, 6, 414. [Google Scholar] [CrossRef]
- Granlen News. HIV RT Inhibitor Azvudine Received IND Approval. Available online: http://www.granlen.com/contents/94/9.html (accessed on 14 January 2023).
- Global Times. China’s First Domestic Oral Anti-COVID-19 Drug Is Priced at Less than 300 Yuan per Bottle/35 Tablets. Available online: https://www.globaltimes.cn/page/202208/1272362.shtml (accessed on 14 January 2023).
- Ren, Z.; Luo, H.; Yu, Z.; Song, J.; Liang, L.; Wang, L.; Wang, H.; Cui, G.; Liu, Y.; Wang, J.; et al. A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study. Adv. Sci. 2020, 7, e2001435. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Azvudine|C9H11FN6O4—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Azvudine (accessed on 13 January 2023).
- Yu, B.; Chang, J. Azvudine (FNC): A Promising Clinical Candidate for COVID-19 Treatment. Signal Transduct. Target. Ther. 2020, 5, 236. [Google Scholar] [CrossRef]
- Noreen, S.; Maqbool, I.; Madni, A. Dexamethasone: Therapeutic Potential, Risks, and Future Projection during COVID-19 Pandemic. Eur. J. Pharmacol. 2021, 894, 173854. [Google Scholar] [CrossRef]
- Kivrak, A.; Ulaş, B.; Kivrak, H. A Comparative Analysis for Anti-Viral Drugs: Their Efficiency against SARS-CoV-2. Int. Immunopharmacol. 2021, 90, 107232. [Google Scholar] [CrossRef] [PubMed]
- The Recovery Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Kocks, J.; Kerkhof, M.; Scherpenisse, J.; van de Maat, A.; van Geer-Postmus, I.; le Rütte, T.; Schaart, J.; Gans, R.O.B.; Kerstjens, H.A. A Potential Harmful Effect of Dexamethasone in Non-Severe COVID-19: Results from the COPPER-Pilot Study. ERJ Open Res. 2022, 8, 00129–2022. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Sayar, S.; Radmanesh, E.; Naghshi, S.; Mousaviasl, S.; Jelvay, S.; Ebrahimzadeh, M.; Mohammadi, A.; Abbasi, S.; Mobarak, S.; et al. Efficacy of Naproxen in the Management of Patients Hospitalized with COVID-19 Infection: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102319. [Google Scholar] [CrossRef]
- Nabati, M.; Parsaee, H. Potential Cardiotoxic Effects of Remdesivir on Cardiovascular System: A Literature Review. Cardiovasc. Toxicol. 2022, 22, 268–272. [Google Scholar] [CrossRef]
- Choi, S.W.; Shin, J.S.; Park, S.-J.; Jung, E.; Park, Y.-G.; Lee, J.; Kim, S.J.; Park, H.-J.; Lee, J.-H.; Park, S.-M.; et al. Antiviral Activity and Safety of Remdesivir against SARS-CoV-2 Infection in Human Pluripotent Stem Cell-Derived Cardiomyocytes. Antivir. Res. 2020, 184, 104955. [Google Scholar] [CrossRef]
- Kaur, R.J.; Charan, J.; Dutta, S.; Sharma, P.; Bhardwaj, P.; Sharma, P.; Lugova, H.; Krishnapillai, A.; Islam, S.; Haque, M.; et al. Favipiravir Use in COVID-19: Analysis of Suspected Adverse Drug Events Reported in the WHO Database. Infect. Drug Resist. 2020, 13, 4427–4438. [Google Scholar] [CrossRef]
- Takahashi, H.; Iwasaki, Y.; Watanabe, T.; Ichinose, N.; Okada, Y.; Oiwa, A.; Kobayashi, T.; Moriya, M.; Oda, T. Case Studies of SARS-CoV-2 Treated with Favipiravir among Patients in Critical or Severe Condition. Int. J. Infect. Dis. 2020, 100, 283–285. [Google Scholar] [CrossRef]
- Yamamura, H.; Matsuura, H.; Nakagawa, J.; Fukuoka, H.; Domi, H.; Chujoh, S. Effect of Favipiravir and an Anti-Inflammatory Strategy for COVID-19. Crit. Care 2020, 24, 413. [Google Scholar] [CrossRef]
- Ergür, F.Ö.; Yıldız, M.; Şener, M.U.; Kavurgacı, S.; Ozturk, A. Adverse Effects Associated with Favipiravir in Patients with COVID-19 Pneumonia: A Retrospective Study. Sao Paulo Med. J. 2022, 140, 372–377. [Google Scholar] [CrossRef]
- Alamer, A.; Alrashed, A.A.; Alfaifi, M.; Alosaimi, B.; AlHassar, F.; Almutairi, M.M.; Howaidi, J.; Almutairi, W.; Mohzari, Y.; Sulaiman, T.; et al. Effectiveness and Safety of Favipiravir Compared to Supportive Care in Moderately to Critically Ill COVID-19 Patients: A Retrospective Study with Propensity Score Matching Sensitivity Analysis. Curr. Med. Res. Opin. 2021, 37, 1085–1097. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Paul, S.; Samanta, B. Hydroxychloroquine in the Prophylaxis of COVID 19: A Survey of Safety on the Healthcare Workers in India. Perspect. Clin. Res. 2021, 12, 58–59. [Google Scholar] [CrossRef]
- Cortez, M.; Che, C. Malaria Drug Hydroxychloroquine No Better than Regular Coronavirus Care, Chinese Study Says. The Print, 25 March 2020. Available online: https://theprint.in/health/malaria-drug-hydroxychloroquine-no-better-than-regular-coronavirus-care-chinese-study-says/387587/ (accessed on 19 December 2022).
- Darazam, I.A.; Shokouhi, S.; Mardani, M.; Pourhoseingholi, M.A.; Rabiei, M.M.; Hatami, F.; Shabani, M.; Moradi, O.; Gharehbagh, F.J.; Irvani, S.S.N.; et al. Umifenovir in Hospitalized Moderate to Severe COVID-19 Patients: A Randomized Clinical Trial. Int. Immunopharmacol. 2021, 99, 107969. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 769–777. [Google Scholar] [CrossRef]
- Huang, D.; Yu, H.; Wang, T.; Yang, H.; Yao, R.; Liang, Z. Efficacy and Safety of Umifenovir for Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis. J. Med Virol. 2021, 93, 481–490. [Google Scholar] [CrossRef]
- United States Food & Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19 (accessed on 30 September 2022).
- European Medicines Agency. EMA Receives Application for Marketing Authorisation for Ronapreve (Casirivimab/Imdevimab) for Treatment and Prevention of COVID-19. Available online: https://www.ema.europa.eu/en/news/ema-receives-application-marketing-authorisation-ronapreve-casirivimab-imdevimab-treatment (accessed on 24 January 2023).
- European Medicines Agency. EMA Issues Advice on Use of REGN-COV2 Antibody Combination (Casirivimab/Imdevimab). Available online: https://www.ema.europa.eu/en/news/ema-issues-advice-use-regn-cov2-antibody-combination-casirivimab-imdevimab (accessed on 24 January 2023).
- Keam, S.J. Tixagevimab + Cilgavimab: First Approval. Drugs 2022, 82, 1001–1010. [Google Scholar] [CrossRef]






| Clinicaltrials.gov Identifier | Nature of Clinical Trial | Selection Criteria of Volunteer | No. of Volunteers | Sponsor(s) | Drug Product(s)/Treatment | Phase | Final Outcome |
|---|---|---|---|---|---|---|---|
| NCT04392219 | Randomized, double blind, and placebo controlled | Adults and older adults | 130 | Ridgeback Biotherapeutics at Covance Leeds Clinical Research Unit, UK | Molnupiravir | Phase 1 | Molnupiravir was more tolerable than placebo, and adverse events were also lesser than placebo |
| NCT04405570 | Randomized, double blind, and placebo controlled | Adults and older adults | 204 | Ridgeback and Merck | Molnupiravir | Phase 2 | 400 and 800 mg dose bid was effective with no side effects |
| NCT04575597 | Randomized, double-blind, and placebo-controlled trial | Adults and older adults | 1850 | Merck | Molnupiravir | Phase 2/3 | Effective in reducing death, 6.8% (95% CI) for the drug and 9.7% (95% CI) for placebo |
| NCT04960202 | Randomized and quadruple-blinded trial | Adults and older adults | 2246 | Pfizer | Paxlovid | Phase 2/3 | Less death risk and adverse effects; therapeutic efficacy maintained with percentage point difference of −5.81 |
| (NCT05011513 | Randomized, quadruple blinded, and parallel sequenced | Adults and older adults | 1140 | Paxlovid | Terminated due to lower number of deaths and hospitalized standard-risk patients | ||
| NCT04421027 | Randomized, double blinded, and parallel assigned | Adults and older adults | 1525 | Eli Lilly | Baricitinib | Phase 3 | Mortality was reduced by 38.2% |
| NCT04333368 | Randomized, triple blinded, and parallel assigned | Adults and older adults | 47 | Assistance Publique–Hôpitaux de Paris | Wharton’s jelly mesenchymal stem cells | Phase 1/2 | Adverse effects were reported in 28.6% and 25% of patients from the intervention and placebo group, respectively |
| NCT04321421 | Open-label study | Adults and older adults, having moderate to severe ARDS | 49 | Foundation IRCCS San Matteo Hospital | Convalescent plasma | Result showed uncertainty about beneficial effect of CP | |
| NCT04315298 | Randomized, double blind, and placebo controlled | Adult or older adult patients having confirmed COVID-19 infection | 1912 | Regeneron Pharmaceuticals in collaboration with Sanofi | Sarilumab | Phase 2/3 | The percentage of improvement was 43.2% and 35.5% in the intervention and placebo group, respectively |
| NCT04327388 | Randomized, quadruple blinded, parallel mode | Adults or older adults | 420 | Sanofi in collaboration with Regeneron Pharmaceuticals | Sarilumab | Phase 3 | Adverse effects were reported by 70%, 65%, and 65% of patients with 400 and 200 mg sarilumab and placebo, respectively |
| NCT04320615 | Randomized, double blinded, parallel mode, and multicenter | Adult or older adult patients | 452 | Hoffmann-La Roche | Tocilizumab | Phase 3 | No beneficial outcomes were obtained |
| NCT04275414 | Open label | Adult or older adults having confirmed COVID-19 infection | 27 | Qilu Hospital of Shandong University | Bevacizumab | Phase 2 | Body temperature was normalized in 93% of patients and improvement in PaO2/FiO2 ratios |
| Clinicaltrials.gov Identifier | Nature of Clinical Trial | Selection Criteria of Volunteer | No. of Volunteers | Sponsor(s) | Drug Product(s)/Treatment | Phase | Final Outcome |
|---|---|---|---|---|---|---|---|
| NCT04327401 | Randomized, open label, parallel mode | Adult or older adults | 290 | Hospital Israelita Albert Einstein, Hospital do Coracao, Brazilian Research in Intensive Care Network and Ache Laboratorios Farmaceuticos S.A. | Dexamethasone | Phase 3 | Terminated by the data monitoring committee for the unaccepted recovery results |
| NCT04325633 | Open label, randomized, parallel model | Adult or older adult patients | 584 | Assistance Publique—Hôpitaux de Paris | Naproxen | Phase 3 | Terminated due to insufficient recruitment of participants |
| NCT04292899 | Randomized, open label, parallel | Hospitalized child, adult, or older adults | 6000 | Gilead Sciences | Remdesivir | Phase 3 | After 14 days. 74.4% and 59% of patients survived from the intervention and noninterventional group. |
| NCT04280705 | Randomized, double blinded, and parallel mode | Hospitalized adult or older adult patients | 572 | National Institute of Allergy and Infectious Diseases (NIAID) | Remdesivir | Phase 3 | Adverse events were seen in 24.6% and 31.6% of patients in the interventional and placebo groups, respectively |
| NCT04332991 | Randomized, quadruple blinded, parallel | Adult or older adult patients | 510 | Massachusetts General Hospital in collaboration with National Heart, Lung, and Blood Institute (NHLBI) | Hydroxychloroquine | Phase 3 | No significant differences between the interventional group and the placebo group was found |
| NCT04346628 | Randomized, open label, parallel mode | Adult or older adult patients | 120 | Stanford University | Favipiravir | Phase 2 | No significant differences (95% CI) were observed between groups in mortality and symptom resolution |
| NCT04349241 | Randomized, open label, parallel | Adult or older adult patients | 100 | Faculty of Medicine, Ain Shams University Research Institute- Clinical Research Center | Favipiravir | Phase 3 | Two consecutive SARS-CoV-2-negative reports were observed after the treatment |
| NCT04252885 | Randomized open label, parallel | Adult or older adult patients | 125 | Guangzhou Eighth People’s Hospital | Umifenovir | Phase 4 | Arbidol monotherapy could be beneficial for mild, moderate, and severe COVID-19 patients |
| Drug | Formulation | Approved by and Type of Approval | Date of Approval | Marketed Name | References |
|---|---|---|---|---|---|
| New Drugs | |||||
| Baricitinib | Oral tablets | USFDA (EUA) | October 2022 | Olumiant® | [43] |
| Casirivimab and Imdevimab | Injectable solution | FDA (EUA), EMA (full approval) | November 2020 (USFDA), February 2021 (EMA for REGN-COV2), October 2021 (EMA for Ronapreve) | REGEN-COV® (USFDA), REGN-COV2 (EMA), Ronapreve (EMA) | [167,168,169] |
| Molnupiravir | Oral capsules | USFDA (EUA), MHRA, EMA (EUA) | December 2021 (USFDA), November 2021 (MHRA, EMA) | Lagevrio™ | [26,27,28,29] |
| Nirmatrelvir and Ritonavir | Oral tablets | USFDA (EUA), EMA (EUA) | December 2021 (USFDA, EMA) | Paxlovid™ | [33,37] |
| Tocilizumab | Injectable solution | USFDA (full approval) | December 2022 | Actemra® | [85] |
| Repurposed Drugs | |||||
| Favipiravir | Tablets | DCGI (EUA) | July 2020 | Fabiflu® | [137] |
| Hydroxychloroquine | Oral formulation | USFDA (EUA), EMA (EUA) | March 2020April 2020 | [124,125,126,127] | |
| Remdesivir | Injection, solution | USFDA (EUA), EMA (EUA) | October 2020December 2021 | Veklury® | [113,114,115] |
| Tixagevimab and Cilgavimab | Injection, solution | USFDA (EUA) | December 2021 | Evusheld™ | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, S.; Banerjee, D.; Singh, A.; Kumar, S.; Pooja, D.; Ram, V.; Kulhari, H.; Saharan, V.A. A Clinical Insight on New Discovered Molecules and Repurposed Drugs for the Treatment of COVID-19. Vaccines 2023, 11, 332. https://doi.org/10.3390/vaccines11020332
Banerjee S, Banerjee D, Singh A, Kumar S, Pooja D, Ram V, Kulhari H, Saharan VA. A Clinical Insight on New Discovered Molecules and Repurposed Drugs for the Treatment of COVID-19. Vaccines. 2023; 11(2):332. https://doi.org/10.3390/vaccines11020332
Chicago/Turabian StyleBanerjee, Surojit, Debadri Banerjee, Anupama Singh, Sumit Kumar, Deep Pooja, Veerma Ram, Hitesh Kulhari, and Vikas Anand Saharan. 2023. "A Clinical Insight on New Discovered Molecules and Repurposed Drugs for the Treatment of COVID-19" Vaccines 11, no. 2: 332. https://doi.org/10.3390/vaccines11020332
APA StyleBanerjee, S., Banerjee, D., Singh, A., Kumar, S., Pooja, D., Ram, V., Kulhari, H., & Saharan, V. A. (2023). A Clinical Insight on New Discovered Molecules and Repurposed Drugs for the Treatment of COVID-19. Vaccines, 11(2), 332. https://doi.org/10.3390/vaccines11020332