1. Introduction
Schistosomiasis, also called bilharzia having been first described by Theodor Bilharz over 150 years ago, is a blood-dwelling trematode fluke worm. With approximately 200 million people infected in over 74 countries, schistosomiasis is recognised as the most important human helminth infection in terms of morbidity and mortality [
1,
2]. Despite over 20 years of highly effective chemotherapeutic (praziquantel) drug treatment integrated with improved sanitation and hygiene measures, this disease is still spreading into new areas of the globe [
1,
3]. Limitations of current treatment regimes, which include high rates of reinfection, the potential development of drug-resistant parasites, the effective administration of drugs requiring a large infrastructure to cover all parts of an area of endemicity, and the associated costs have further supported research for an effective vaccine strategy to complement current treatment for future control and possible elimination of this parasitic disease [
1,
3].
Schistosomiasis immunology has provoked considerable research interest over the past 30 years with many questions remaining unanswered. These relate to the development of the many pathological changes that accompany the infection, how some infected individuals can develop resistance to infection, and lastly, that schistosome worms can survive in the mammalian host for many years despite a strong immune response being generated [
4].
Despite intensive research, identification and development of suitable anti-schistosome vaccine candidates has been slow [
5]; however, an improved understanding of the immune response to
Schistosoma infection in both human and animal models suggests that vaccine development is possible [
3]. The development of schistosomiasis vaccines can be assigned to three types: (1) a prophylactic vaccine aimed at preventing or reducing infection and indirectly transmission and/or leaving no worms in the host; (2) a vaccine aimed to reduce or eliminate reinfection intensity or transmission by interrupting female worm survival or egg production; or (3) a therapeutic vaccine to reduce disease but not affect infection or transmission [
5]. One reason for the slow progress in developing an effective schistosome vaccine is the strong capacity of schistosome parasites to evade a host’s immune response. This arises, in part, from the pathogen’s complexity and its ability to exhibit genetic diversity as well as antigenic variation during the multistage life cycle. Subsequently, to achieve host protection against schistosomiasis, an immune response combining both humoral and cellular responses that target different stages of the parasites life cycle are essential [
6].
Schistosomes appear to have evolved a number of strategies to down-regulate the host’s immune response to promote their own survival [
7]. T cell mediated immunity has been shown to be essential in the fight against schistosomiasis (reviewed by [
3,
4,
7,
8] and references within). Schistosomes, like other parasitic helminths, induce prominent T helper type 2 (Th2, humoral) responses with a quantifiable shift from gamma interferon (IFN-γ) and T helper type 1 (Th1, cellular) responses to an elevated production of interleukin (IL)-4 and Th2 in the spleens of infected mice [
4,
9]. However, disease severity and the immune response is complicated by the host’s genetics, the intensity of the infection, co-infection status, and
in-utero sensitisation to schistosome antigens (compared to a naïve individual) [
4]. Furthermore, humans who are infected with schistosomes, in general, have a Th2 type response, but, on the basis of IFN-γ and IL-5 levels, a Th1-like immune response is generated for some individuals [
4]. IL-10 has also been shown to play an important role in schistosomiasis by preventing the development of Th1 and Th2-mediated pathologies [
4]. Overall, a balance between Th1 and Th2 is required and a skewed response too heavily in either direction has harmful consequences for the host. Indeed, the vast majority of people living with schistosome infections elicit a balanced immune response that holds both the pathology and parasite in harmony (
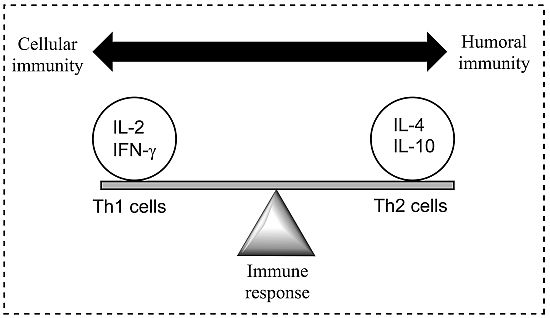
Figure 1) [
8].
Figure 1.
A model demonstrating the balance between Th1 (cell-mediated) and Th2 (humoral) response of the adaptive (specific) immune system; a balance is essential to prevent disease. Both Th1 and Th2 responses are tightly controlled but excessive activation may cause or alter the disease state. Cytokines are most commonly grouped by their functional similarities and one of the most prominent concepts used to discriminate two distinct ways that the specific immune system can react on environmental stimuli is the classification of Th1 and Th2 cell diversity. This classification is based on the cytokine production patterns of T helper cells and reflects the polarization of the immune system to either a cell-mediated (Thl) or a humoral (Th2) immune response [
8].
Figure 1.
A model demonstrating the balance between Th1 (cell-mediated) and Th2 (humoral) response of the adaptive (specific) immune system; a balance is essential to prevent disease. Both Th1 and Th2 responses are tightly controlled but excessive activation may cause or alter the disease state. Cytokines are most commonly grouped by their functional similarities and one of the most prominent concepts used to discriminate two distinct ways that the specific immune system can react on environmental stimuli is the classification of Th1 and Th2 cell diversity. This classification is based on the cytokine production patterns of T helper cells and reflects the polarization of the immune system to either a cell-mediated (Thl) or a humoral (Th2) immune response [
8].
The initial trigger for the Th2 response is unclear and complicated by the different developmental stages of the schistosome parasite. The deposition and entrapment of eggs in the liver, spleen and lungs tissues elicits a Th1 immune response and characterised by the production of IL-12, IFN-γ and tumour necrosis factor (TNF). The early immune response following infection with cercariae is predominantly a Th1 response, targeted at the adult worm [
10]. As the transition of the disease takes place from mild to chronic, a shift to a Th2 response in observed with the production of anti-inflammatory cytokines (IL-4, -5, -10, and -13) and parasite-specific antibodies [
8]. The inflammation and granuloma formation are thought to be responsible for the strong Th2 immune response, rather than the presence of the schistosomula or adult worms [
11]. Additionally, the trapped eggs secrete a range of molecules leading to a marked MHC II CD4
+ T cell inflammation characterised by eosinophils (whose role in the infection process remains undetermined), monocytes, and lymphocytes, in addition to collagen deposition and hepatic fibrosis [
4]. The generic nature of the Th2 bias (where cytokine IL-4 is deemed essential for the polarisation of the Th2 response) during infection suggests the possibility that the vertebrate innate immune system recognises conserved parasite motifs, preferentially triggering a Th2 response [
11]. Furthermore, fibrosis is inhibited in mice that are immunised with IL-12 and egg antigens, with cytokines (IFN-γ and TNF) present, preventing Th2 biasing and natural immunity [
4]. It has been suggested that a vaccine activating macrophage-induced Th1 cytokines (IFN-γ and IL-2) may help in the prevention of schistosomiasis and, although the mechanism is unclear, an IgE antibody-dependent targeted cellular cytotoxicity has also been shown to protect humans. Additionally, further cytokines (
i.e., IL-1, TNF-α, IL-6) have been shown to have supporting roles in the host’s immune response and the mechanism of protection [
3,
4,
7].
Immunisation is considered one of the most effective of the public health interventions. Despite the advantages of prevention over treatment whereby traditional vaccines evolved from a prophylactic role (prevention of disease), a large market exists for vaccines to treat diseases. However, to date, no therapeutic vaccines have been approved [
12]. Elicitation of immunological memory in traditional vaccine-exposed individuals to whole live attenuated organisms or killed micro-organisms against which they were being immunised elicited a strong immune response, associated in part with the capacity of the pathogen to replicate, and to be retained at the administration site. However, disadvantages in this approach resulted in a great deal of research being focused on alternative vaccine development techniques [
12,
13]. Current methods of vaccine design use a subunit approach whereby only the minimal microbial components necessary to stimulate an appropriate immune response are incorporated into the vaccine; however, despite the potential advantages of subunit vaccines, poorly immunogenic vaccines are produced, necessitating administration with powerful adjuvants, and in some cases, the addition of T helper epitopes to elicit a lasting immune response [
12].
An immunological adjuvant, when incorporated into a vaccine formulation, accelerates, prolongs or enhances the quality of specific immune responses to vaccine antigens [
14]. The mechanism of action for adjuvants include: (1) increasing the biological or immunologic half-life of vaccine antigens; (2) improving antigen delivery to antigen-presenting cells (APCs), as well as antigen processing and presentation by the APCs; and (3) inducing the production of immunomodulatory cytokines. Through modulation of cytokine responses, adjuvant formulations can be designed that favour the development of Th1 or Th2 immune responses to vaccine antigens [
14]. However, despite the important role of adjuvants, relatively few have been incorporated successfully into vaccines intended for human administration [
12].
This review summarises adjuvants in preclinical and clinical
Schistosoma research where adjuvants are evaluated for their role in effective vaccine development focusing on their pharmaceutical and immunological properties. Adjuvants can be classified by their sources, mechanisms of action or chemical properties.
Table 1 lists the types of adjuvants under preclinical and clinical development for use with schistosome vaccines. Further, a description of
Schistosoma antigen candidates cited in this review are summarised in
Table 2. A compilation of
Schistosoma vaccine candidates clinically tested and classified by adjuvant is presented in
Table 3,
Table 4,
Table 5,
Table 6,
Table 7 and
Table 8 [
6].
3. Perspectives and Conclusions
Adjuvant selection has a large impact on the effectiveness of the vaccine and the use of adjuvants to aid in the stimulation of the immune system is a critical step and a major variable affecting vaccine development. However, there is still a tendency to employ the few approved ones with the wrong intention to rapidly progress to clinical trials. Before adjuvant selection can be made, a comprehensive understanding of the immune system, level of protection and the desired immune response is required—or, more importantly, it is important to know which immune responses should be avoided. It is also essential to examine the type of immune response that the candidate vaccine antigen induces in the target host under natural infection without inclusion of any adjuvant [
4]. For schistosomiasis, this is complicated as very few individuals develop natural resistance to reinfection with the schistosome parasite in the absence of repeated praziquantel treatment. Therefore, tailoring vaccine development to individuals is required, albeit impractical. Additionally, factors that affect the response of each antigen/adjuvant are also dependent, to some extent, on the animal model employed. For example, BALB/c and BL/6 mice are considered high responders to the vaccine made from irradiated
S. mansoni cercariae with fewer worms observed after challenge infection than occurs with moderate responders, such as CBA mice. However, infected CBA mice show a stronger splenic proliferation response and a lesser suppressor T cell response once a schistosome infection becomes patent than do high responder mice. From this, the selection of mouse strain used in the vaccine/challenge model is an important aspect to consider in the experimental design and for critical interpretation/comparison of results [
38]. Translational research from mouse to humans are complicated by disease severity and immune response variations in outbred populations and only a small number of studies have tried to compare these. To add to this, correlation of the mouse to human model is also under studied [
4,
110,
111,
112,
113]. Here, detailed analysis, and genetic and immunological studies in mice and humans are required to identify a suitable model for schistosome vaccine development. Moreover, it is not clear what effect genetic susceptibility of individuals to make a Th1 response would have on their ability to subsequently develop resistance to infection, given that the latter seems to be Th2-responses-mediated in the endemic setting [
4,
110,
111,
112,
113].
Traditionally, CFA is used when a candidate antigen is first being assessed as a vaccine and although it has been backbone of immunological adjuvants in research for decades, it is still not suitable for human applications. Therefore, once efficacy has been established with CFA, other adjuvants should be explored to formulate a vaccine antigen. Less conventional or less widely used approaches have been explored as adjuvants for schistosome vaccines, including cholera toxin, BCG, liposomes, and others (refer to
Table 3,
Table 4,
Table 5,
Table 6,
Table 7 and
Table 8 for details).
Several reasons have led to the widespread use of traditional adjuvants including cost, difficulty to access new adjuvants, formulation ease and characterisation. On the other hand, adjuvants are not approved as a product alone but in combination with a vaccine or formulation defining a determined combination of antigen(s) plus adjuvant(s) and each combination requires full product development, restraining the progression of those adjuvants for new vaccine applications. Fortunately, over the past few years, new rules to promote the use of new adjuvants have been initiated [
6].
It is important to know how adjuvants work in order to determine their role in vaccine formulation and to design new adjuvants. Recent advances in parasitic disease and immune response pathways aid this. For instance, although Alum has been around for more than 80 years, only recently has insight into its mechanism of action been described. These steps have also promoted the discovery of new adjuvants [
6]. Improved knowledge of toll-like and pattern recognition receptors and the link between innate and adaptive immunity has enabled a new generation of synthetic adjuvants overcoming some of the concerns with toxicity, potency, and manufacturing problems [
6]. Olds
et al., attempted to identify and correlate immune responses between 10 schistosomiasis vaccine candidates and to investigate their association with resistance
vs. susceptibility to re-infection in human participants from Egypt [
44]. Highly specific humoral and cellular immune reactions in response to the 10 antigens correlated, both prospectively and retrospectively, with detailed epidemiological data covering a 66-month period. Each antigen produced a unique immune response profile but no clear “winner(s)” was recognised. However, a marker for both resistance and susceptibility to re-infection was identified for each molecule indicating which types of responses to aim for in vaccination and which ones to avoid. Insights gained from this approach will be useful for antigen selection and ultimately for vaccine formulation prior to clinical trials in humans [
44].
The bar to achieve protective efficacy in humans was set in the 1990s at a consistent induction of 40% protection or better by the World Health Organisation (WHO), and although this is a modest goal, it was not reached with the six most promising schistosomiasis vaccine candidates (Sm28GST, IrV5, Sm14, paramyosin, TPI, and Sm23) at the time [
114]. This highlighted the need for standardised and effective adjuvant formulations.
All of this information is relevant in deciding how best to formulate and deliver a vaccine for schistosomiasis. Skewing an immune response towards Th1 is currently seen as the most promising way to obtain protection, and this can be achieved using Th1-driving adjuvants. Recent focus on the use of cytokines (e.g., IL-12 and IL-18) as adjuvants has been met with mixed results [
8]. Lung stage antigen administered with IL-12 was shown to be a powerful inducer of a Th1 immune response, boosting protection levels to 90%. However, IL-12 failed to protect when administered with Sm28GST antigen. Furthermore, cytokines as adjuvants may not be very feasible with the high production cost and the logistics of the distribution and administration of the vaccine [
8]. Here, formulations that minimise the need for refrigeration and avoid the use of needles are highly desirable. Adjuvants such as unmethylated CpG ODNs are also attractive, showing promise for experimental vaccines against other parasites by targeting the TLRs. Additionally, if a mixed Th1/Th2 response is desired, combination adjuvants such as alum-CpG seem to be an appropriate way forward. Finally, Lewis X-based carbohydrates isolated from schistosome eggs offer a promising antigen without the need for an additional adjuvant [
4].
Vaccine-challenge experiments modifying the delivery of the vaccine and testing the different adjuvant formulation will enable a better assessment of an adjuvant’s role in inducing protective immunity. Furthermore, combination adjuvants are promising next generation adjuvants offering tailored immune responses to new antigen targets [
115]. In addition, the efficacy of anti-schistosome vaccines could be successfully enhanced by combining new combinations of existing adjuvants with novel ones developed based on emerging immunological targets.