V2-Specific Antibodies in HIV-1 Vaccine Research and Natural Infection: Controllers or Surrogate Markers
Abstract
:1. Introduction
- Can vaccine efficacy and immune correlates be reproduced in other human and non-human primate (NHP) vaccine trials?
- How much did the results depend on the study population, infecting subtypes, and immunogens?
- Were the correlates of reduced risk of infection, and particularly the high levels of V2-specific antibodies (Abs) as the only independent variable, causally linked with protection or were they solely markers for unrelated protective responses?
- How do V2 Abs cooperate with other Abs, other adaptive, and innate immune responses?
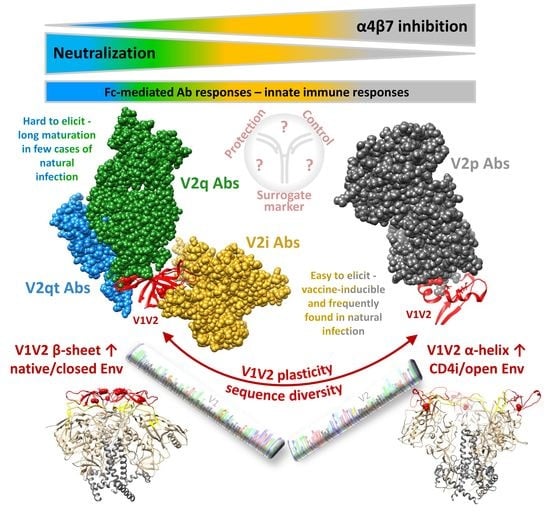
2. The Multifaceted V1V2 Region and Its Epitope-Specific Abs
2.1. Conformational Plasticity of V1V2 and Structural Requirements for the Binding of Different V2 Ab Classes
2.2. High Sequence Diversity in V1V2 and Clade-Specific Differences in Antigenicity and Immunogenicity
2.3. Antiviral Functions Differ According to V2 Ab Class and Epitope Region
2.3.1. Neutralization
2.3.2. V2 Broadly Neutralizing Antibodies
2.3.3. Fc-Mediated Effector Functions and Innate Immunity
3. Human Vaccine Efficacy Trials and the Role of V2 Abs
3.1. RV144 Reassessed in the Context of Other Human Vaccine Trials
3.2. Alternative Correlates of RV144 Vaccine Efficacy with or without the Contribution of V2
3.3. Translation of RV144 Findings into the Development of Future Human Vaccine Trials
4. The Impact of V1V2-Specific Abs in NHP Experiments
4.1. Passive Immunization Experiments
4.2. Vaccine Protection Experiments
5. α4β7-Blocking by V2-Specific Abs
6. V1V2-Specific Immune Responses in Natural Infection
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Global AIDS Update 2018—Miles to Go. 2018. Available online: https://www.unaids.org/sites/default/files/media_asset/miles-to-go_en.pdf (accessed on 27 June 2019).
- Haynes, B.F.; Gilbert, P.B.; McElrath, M.J.; Zolla-Pazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R.; et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012, 366, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, R.C. Protection against HIV Acquisition in the RV144 Trial. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, P.B.; Berger, J.O.; Stablein, D.; Becker, S.; Essex, M.; Hammer, S.M.; Kim, J.H.; Degruttola, V.G. Statistical interpretation of the RV144 HIV vaccine efficacy trial in Thailand: A case study for statistical issues in efficacy trials. J. Infect. Dis. 2011, 203, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Zolla-Pazner, S.; Gilbert, P.B. Revisiting the Correlate of Reduced Hiv Infection Risk in the Rv144 Vaccine Trial. J. Virol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Rolland, M.; Edlefsen, P.T.; Larsen, B.B.; Tovanabutra, S.; Sanders-Buell, E.; Hertz, T.; deCamp, A.C.; Carrico, C.; Menis, S.; Magaret, C.A.; et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 2012, 490, 417–420. [Google Scholar] [CrossRef]
- Corey, L.; Gilbert, P.B.; Tomaras, G.D.; Haynes, B.F.; Pantaleo, G.; Fauci, A.S. Immune correlates of vaccine protection against HIV-1 acquisition. Sci. Transl. Med. 2015, 7, 310rv7. [Google Scholar] [CrossRef]
- Tomaras, G.D.; Plotkin, S.A. Complex immune correlates of protection in HIV-1 vaccine efficacy trials. Immunol. Rev. 2017, 275, 245–261. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Li, H.; Li, L.; Omange, R.W.; Hai, Y.; Luo, M. Current advances in HIV vaccine preclinical studies using Macaque models. Vaccine 2019, 37, 3388–3399. [Google Scholar] [CrossRef]
- Rahman, M.A.; Robert-Guroff, M. Accelerating HIV vaccine development using non-human primate models. Expert Rev. Vaccines 2019, 18, 61–73. [Google Scholar] [CrossRef]
- Manickam, C.; Shah, S.V.; Nohara, J.; Ferrari, G.; Reeves, R.K. Monkeying Around: Using Non-human Primate Models to Study NK Cell Biology in HIV Infections. Front. Immunol. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z. Monkey Models and HIV Vaccine Research. In HIV Vaccines and Cure; Advances in Experimental Medicine and Biology; Zhang, L., Lewin, S., Eds.; Springer: Singapore, 2018; Volume 1075, pp. 97–124. [Google Scholar]
- Rogers, J.; Gibbs, R.A. Comparative primate genomics: Emerging patterns of genome content and dynamics. Nat. Rev. Genet. 2014, 15, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Boesch, A.W.; Osei-Owusu, N.Y.; Crowley, A.R.; Chu, T.H.; Chan, Y.N.; Weiner, J.A.; Bharadwaj, P.; Hards, R.; Adamo, M.E.; Gerber, S.A.; et al. Biophysical and Functional Characterization of Rhesus Macaque IgG Subclasses. Front. Immunol. 2016, 7, 589. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.A.; Jayashankar, L.; Scinicariello, F.; Attanasio, R. Nonhuman primate IgA: Genetic heterogeneity and interactions with CD89. J. Immunol. 2008, 180, 4816–4824. [Google Scholar] [CrossRef] [PubMed]
- Thullier, P.; Chahboun, S.; Pelat, T. A comparison of human and macaque (Macaca mulatta) immunoglobulin germline V regions and its implications for antibody engineering. mAbs 2010, 2, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Thullier, P.; Huish, O.; Pelat, T.; Martin, A.C. The humanness of macaque antibody sequences. J. Mol. Biol. 2010, 396, 1439–1450. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Nanfack, A.; Mayr, L.M.; Soni, S.; Kohutnicki, A.; Agyingi, L.; Wang, X.H.; Tuen, M.; Shao, Y.; et al. Anti-V2 antibody deficiency in individuals infected with HIV-1 in Cameroon. Virology 2019, 529, 57–64. [Google Scholar] [CrossRef]
- Tuen, M.; Bimela, J.S.; Banin, A.N.; Ding, S.; Harkins, G.W.; Weiss, S.; Itri, V.; Durham, A.R.; Porcella, S.F.; Soni, S.; et al. Immune Correlates of Disease Progression in Linked HIV-1 Infection. Front. Immunol. 2019, 10, 1062. [Google Scholar] [CrossRef] [Green Version]
- Van Eeden, C.; Wibmer, C.K.; Scheepers, C.; Richardson, S.I.; Nonyane, M.; Lambson, B.; Mkhize, N.N.; Vijayakumar, B.; Sheng, Z.; Stanfield-Oakley, S.; et al. V2-Directed Vaccine-like Antibodies from HIV-1 Infection Identify an Additional K169-Binding Light Chain Motif with Broad ADCC Activity. Cell Rep. 2018, 25, 3123–3135. [Google Scholar] [CrossRef]
- Wibmer, C.K.; Richardson, S.I.; Yolitz, J.; Cicala, C.; Arthos, J.; Moore, P.L.; Morris, L. Common helical V1V2 conformations of HIV-1 Envelope expose the alpha4beta7 binding site on intact virions. Nat. Commun. 2018, 9, 4489. [Google Scholar] [CrossRef]
- Mayr, L.M.; Cohen, S.; Spurrier, B.; Kong, X.P.; Zolla-Pazner, S. Epitope mapping of conformational V2-specific anti-HIV human monoclonal antibodies reveals an immunodominant site in V2. PLoS ONE 2013, 8, e70859. [Google Scholar] [CrossRef] [PubMed]
- Zolla-Pazner, S.; Alvarez, R.; Kong, X.P.; Weiss, S. Vaccine-induced V1V2-specific antibodies control and or protect against infection with HIV, SIV and SHIV. Curr. Opin. HIV AIDS 2019, 14, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Upadhyay, C.; Zhang, J.; Pan, R.; Zolla-Pazner, S.; Kong, X.P.; Hioe, C.E. Rationally Targeted Mutations at the V1V2 Domain of the HIV-1 Envelope to Augment Virus Neutralization by Anti-V1V2 Monoclonal Antibodies. PLoS ONE 2015, 10, e0141233. [Google Scholar] [CrossRef] [PubMed]
- Spurrier, B.; Sampson, J.; Gorny, M.K.; Zolla-Pazner, S.; Kong, X.P. Functional implications of the binding mode of a human conformation-dependent V2 monoclonal antibody against HIV. J. Virol. 2014, 88, 4100–4112. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, C.; Mayr, L.M.; Zhang, J.; Kumar, R.; Gorny, M.K.; Nadas, A.; Zolla-Pazner, S.; Hioe, C.E. Distinct mechanisms regulate exposure of neutralizing epitopes in the V2 and V3 loops of HIV-1 envelope. J. Virol. 2014, 88, 12853–12865. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Upadhyay, C.; Sharma, A.; Hioe, C.E.; Arora, S.K. Short Communication: Manalpha1-2Man-Binding Anti-HIV Lectins Enhance the Exposure of V2i and V3 Crown Neutralization Epitopes on the V1/V2 and V3 Hypervariable Loops of HIV-1 Envelope. AIDS Res. Hum. Retrovir. 2017, 33, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Doria-Rose, N.A.; Schramm, C.A.; Gorman, J.; Moore, P.L.; Bhiman, J.N.; DeKosky, B.J.; Ernandes, M.J.; Georgiev, I.S.; Kim, H.J.; Pancera, M.; et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 2014, 509, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Landais, E.; Huang, X.; Havenar-Daughton, C.; Murrell, B.; Price, M.A.; Wickramasinghe, L.; Ramos, A.; Bian, C.B.; Simek, M.; Allen, S.; et al. Broadly Neutralizing Antibody Responses in a Large Longitudinal Sub-Saharan HIV Primary Infection Cohort. PLoS Pathog. 2016, 12, e1005369. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.X.; Bonsignori, M.; Alam, S.M.; McLellan, J.S.; Tomaras, G.D.; Moody, M.A.; Kozink, D.M.; Hwang, K.K.; Chen, X.; Tsao, C.Y.; et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 2013, 38, 176–186. [Google Scholar] [CrossRef]
- Walker, L.M.; Phogat, S.K.; Chan-Hui, P.Y.; Wagner, D.; Phung, P.; Goss, J.L.; Wrin, T.; Simek, M.D.; Fling, S.; Mitcham, J.L.; et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 2009, 326, 285–289. [Google Scholar] [CrossRef]
- Walker, L.M.; Huber, M.; Doores, K.J.; Falkowska, E.; Pejchal, R.; Julien, J.P.; Wang, S.K.; Ramos, A.; Chan-Hui, P.Y.; Moyle, M.; et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 2011, 477, 466–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorny, M.K.; Moore, J.P.; Conley, A.J.; Karwowska, S.; Sodroski, J.; Williams, C.; Burda, S.; Boots, L.J.; Zolla-Pazner, S. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of human immunodeficiency virus type 1. J. Virol. 1994, 68, 8312–8320. [Google Scholar] [PubMed]
- Gorny, M.K.; Pan, R.; Williams, C.; Wang, X.H.; Volsky, B.; O’Neal, T.; Spurrier, B.; Sampson, J.M.; Li, L.; Seaman, M.S.; et al. Functional and immunochemical cross-reactivity of V2-specific monoclonal antibodies from HIV-1-infected individuals. Virology 2012, 427, 198–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyambi, P.N.; Mbah, H.A.; Burda, S.; Williams, C.; Gorny, M.K.; Nadas, A.; Zolla-Pazner, S. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J. Virol. 2000, 74, 7096–7107. [Google Scholar] [CrossRef] [PubMed]
- Pinter, A.; Honnen, W.J.; He, Y.; Gorny, M.K.; Zolla-Pazner, S.; Kayman, S.C. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol. 2004, 78, 5205–5215. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, R.; Pallesen, J.; Allen, J.D.; Song, G.; Zhang, J.; de Val, N.; Gegg, G.; Porter, K.; Su, C.Y.; Pauthner, M.; et al. The Chimpanzee SIV Envelope Trimer: Structure and Deployment as an HIV Vaccine Template. Cell Rep. 2019, 27, 2426–2441.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatatos, L.; Pancera, M.; McGuire, A.T. Germline-targeting immunogens. Immunol. Rev. 2017, 275, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.; Mkhize, N.N. Prospects for passive immunity to prevent HIV infection. PLoS Med. 2017, 14, e1002436. [Google Scholar] [CrossRef]
- O’Connell, R.J.; Kim, J.H.; Excler, J.L. The HIV-1 gp120 V1V2 loop: Structure, function and importance for vaccine development. Expert Rev. Vaccines 2014, 13, 1489–1500. [Google Scholar] [CrossRef]
- Totrov, M. Estimated secondary structure propensities within V1/V2 region of HIV gp120 are an important global antibody neutralization sensitivity determinant. PLoS ONE 2014, 9, e94002. [Google Scholar] [CrossRef]
- Ward, A.B.; Wilson, I.A. The HIV-1 envelope glycoprotein structure: Nailing down a moving target. Immunol. Rev. 2017, 275, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, R.; Voss, J.E.; Liang, C.H.; Briney, B.; McCoy, L.E.; Wu, C.Y.; Wong, C.H.; Poignard, P.; Burton, D.R. Identification of Common Features in Prototype Broadly Neutralizing Antibodies to HIV Envelope V2 Apex to Facilitate Vaccine Design. Immunity 2015, 43, 959–973. [Google Scholar] [CrossRef]
- Wang, H.; Gristick, H.B.; Scharf, L.; West, A.P.; Galimidi, R.P.; Seaman, M.S.; Freund, N.T.; Nussenzweig, M.C.; Bjorkman, P.J. Asymmetric recognition of HIV-1 Envelope trimer by V1V2 loop-targeting antibodies. eLife 2017, 6, e27389. [Google Scholar] [CrossRef] [PubMed]
- Lyumkis, D.; Julien, J.P.; de Val, N.; Cupo, A.; Potter, C.S.; Klasse, P.J.; Burton, D.R.; Sanders, R.W.; Moore, J.P.; Carragher, B.; et al. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 2013, 342, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Gorny, M.K.; Zolla-Pazner, S.; Kong, X.P. The V1V2 Region of HIV-1 gp120 Forms a Five-Stranded Beta Barrel. J. Virol. 2015, 89, 8003–8010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Bartesaghi, A.; Borgnia, M.J.; Sapiro, G.; Subramaniam, S. Molecular architecture of native HIV-1 gp120 trimers. Nature 2008, 455, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Totrov, M.; Li, W.; Sampson, J.M.; Williams, C.; Lu, H.; Wu, X.; Lu, S.; Wang, S.; Zolla-Pazner, S.; et al. Rationally Designed Immunogens Targeting HIV-1 gp120 V1V2 Induce Distinct Conformation-Specific Antibody Responses in Rabbits. J. Virol. 2016, 90, 11007–11019. [Google Scholar] [CrossRef] [Green Version]
- Zolla-Pazner, S.; deCamp, A.; Gilbert, P.B.; Williams, C.; Yates, N.L.; Williams, W.T.; Howington, R.; Fong, Y.; Morris, D.E.; Soderberg, K.A.; et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS ONE 2014, 9, e87572. [Google Scholar] [CrossRef]
- Gottardo, R.; Bailer, R.T.; Korber, B.T.; Gnanakaran, S.; Phillips, J.; Shen, X.; Tomaras, G.D.; Turk, E.; Imholte, G.; Eckler, L.; et al. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS ONE 2013, 8, e75665. [Google Scholar] [CrossRef]
- Ozorowski, G.; Pallesen, J.; de Val, N.; Lyumkis, D.; Cottrell, C.A.; Torres, J.L.; Copps, J.; Stanfield, R.L.; Cupo, A.; Pugach, P.; et al. Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike. Nature 2017, 547, 360–363. [Google Scholar] [CrossRef] [Green Version]
- Gorman, J.; Soto, C.; Yang, M.M.; Davenport, T.M.; Guttman, M.; Bailer, R.T.; Chambers, M.; Chuang, G.Y.; DeKosky, B.J.; Doria-Rose, N.A.; et al. Structures of HIV-1 Env V1V2 with broadly neutralizing antibodies reveal commonalities that enable vaccine design. Nat. Struct. Mol. Biol. 2016, 23, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Andrabi, R.; Su, C.Y.; Yasmeen, A.; Julien, J.P.; Kong, L.; Wu, N.C.; McBride, R.; Sok, D.; Pauthner, M.; et al. A Broadly Neutralizing Antibody Targets the Dynamic HIV Envelope Trimer Apex via a Long, Rigidified, and Anionic beta-Hairpin Structure. Immunity 2017, 46, 690–702. [Google Scholar] [CrossRef] [PubMed]
- McBurney, S.P.; Ross, T.M. Viral sequence diversity: Challenges for AIDS vaccine designs. Expert Rev. Vaccines 2008, 7, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Stefic, K.; Bouvin-Pley, M.; Braibant, M.; Barin, F. Impact of HIV-1 Diversity on Its Sensitivity to Neutralization. Vaccines 2019, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Stewart-Jones, G.B.; Soto, C.; Lemmin, T.; Chuang, G.Y.; Druz, A.; Kong, R.; Thomas, P.V.; Wagh, K.; Zhou, T.; Behrens, A.J.; et al. Trimeric HIV-1-Env Structures Define Glycan Shields from Clades A, B, and G. Cell 2016, 165, 813–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julien, J.P.; Lee, J.H.; Ozorowski, G.; Hua, Y.; Torrents de la Pena, A.; de Taeye, S.W.; Nieusma, T.; Cupo, A.; Yasmeen, A.; Golabek, M.; et al. Design and structure of two HIV-1 clade C SOSIP.664 trimers that increase the arsenal of native-like Env immunogens. Proc. Natl. Acad. Sci. USA 2015, 112, 11947–11952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israel, Z.R.; Gorny, M.K.; Palmer, C.; McKeating, J.A.; Zolla-Pazner, S. Prevalence of a V2 epitope in clade B primary isolates and its recognition by sera from HIV-1-infected individuals. AIDS 1997, 11, 128–130. [Google Scholar]
- Kayman, S.C.; Wu, Z.; Revesz, K.; Chen, H.; Kopelman, R.; Pinter, A. Presentation of native epitopes in the V1/V2 and V3 regions of human immunodeficiency virus type 1 gp120 by fusion glycoproteins containing isolated gp120 domains. J. Virol. 1994, 68, 400–410. [Google Scholar] [Green Version]
- McKeating, J.A.; Shotton, C.; Cordell, J.; Graham, S.; Balfe, P.; Sullivan, N.; Charles, M.; Page, M.; Bolmstedt, A.; Olofsson, S.; et al. Characterization of neutralizing monoclonal antibodies to linear and conformation-dependent epitopes within the first and second variable domains of human immunodeficiency virus type 1 gp120. J. Virol. 1993, 67, 4932–4944. [Google Scholar] [Green Version]
- Moore, J.P.; Sattentau, Q.J.; Yoshiyama, H.; Thali, M.; Charles, M.; Sullivan, N.; Poon, S.W.; Fung, M.S.; Traincard, F.; Pinkus, M.; et al. Probing the structure of the V2 domain of human immunodeficiency virus type 1 surface glycoprotein gp120 with a panel of eight monoclonal antibodies: Human immune response to the V1 and V2 domains. J. Virol. 1993, 67, 6136–6151. [Google Scholar]
- Karasavvas, N.; Billings, E.; Rao, M.; Williams, C.; Zolla-Pazner, S.; Bailer, R.T.; Koup, R.A.; Madnote, S.; Arworn, D.; Shen, X.; et al. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res. Hum. Retrovir. 2012, 28, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- Hioe, C.E.; Kumar, R.; Upadhyay, C.; Jan, M.; Fox, A.; Itri, V.; Peachman, K.K.; Rao, M.; Liu, L.; Lo, N.C.; et al. Modulation of Antibody Responses to the V1V2 and V3 Regions of HIV-1 Envelope by Immune Complex Vaccines. Front. Immunol. 2018, 9, 2441. [Google Scholar] [CrossRef] [PubMed]
- Hessell, A.J.; Powell, R.; Jiang, X.; Luo, C.; Weiss, S.; Dussupt, V.; Itri, V.; Fox, A.; Shapiro, M.B.; Pandey, S.; et al. Multimeric Epitope-Scaffold HIV Vaccines Target V1V2 and Differentially Tune Polyfunctional Antibody Responses. Cell Rep. 2019, 28, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.T.; Chamcha, V.; Kesavardhana, S.; Shen, X.; Beaumont, D.; Das, R.; Wyatt, L.S.; LaBranche, C.C.; Stanfield-Oakley, S.; Ferrari, G.; et al. A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karch, C.P.; Bai, H.; Torres, O.B.; Tucker, C.A.; Michael, N.L.; Matyas, G.R.; Rolland, M.; Burkhard, P.; Beck, Z. Design and characterization of a self-assembling protein nanoparticle displaying HIV-1 Env V1V2 loop in a native-like trimeric conformation as vaccine antigen. Nanomedicine 2019, 16, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.I.; Verma, D.; Bailey-Kellogg, C.; Ackerman, M.E. Towards conformational fidelity of a quaternary HIV-1 epitope: Computational design and directed evolution of a minimal V1V2 antigen. Protein Eng. Des. Sel. 2018, 31, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Duffy, R.; Howington, R.; Cope, A.; Sadagopal, S.; Park, H.; Pal, R.; Kwa, S.; Ding, S.; Yang, O.O.; et al. Vaccine-Induced Linear Epitope-Specific Antibodies to Simian Immunodeficiency Virus SIVmac239 Envelope Are Distinct from Those Induced to the Human Immunodeficiency Virus Type 1 Envelope in Nonhuman Primates. J. Virol. 2015, 89, 8643–8650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowder, D.; Thompson, J.; Durst, K.; Hollingsead, H.; Hu, D.Y.; Wei, W.Z.; Xiang, S.H. Characterization of twin-cysteine motif in the V2-loop region of gp120 in primate lentiviruses. Virology 2018, 519, 180–189. [Google Scholar] [CrossRef]
- Cicala, C.; Arthos, J.; Selig, S.M.; Dennis, G., Jr.; Hosack, D.A.; Van Ryk, D.; Spangler, M.L.; Steenbeke, T.D.; Khazanie, P.; Gupta, N.; et al. HIV envelope induces a cascade of cell signals in non-proliferating target cells that favor virus replication. Proc. Natl. Acad. Sci. USA 2002, 99, 9380–9385. [Google Scholar] [CrossRef] [Green Version]
- Guerra, S.; Gonzalez, J.M.; Climent, N.; Reyburn, H.; Lopez-Fernandez, L.A.; Najera, J.L.; Gomez, C.E.; Garcia, F.; Gatell, J.M.; Gallart, T.; et al. Selective induction of host genes by MVA-B, a candidate vaccine against HIV/AIDS. J. Virol. 2010, 84, 8141–8152. [Google Scholar] [CrossRef]
- Vaccari, M.; Gordon, S.N.; Fourati, S.; Schifanella, L.; Liyanage, N.P.; Cameron, M.; Keele, B.F.; Shen, X.; Tomaras, G.D.; Billings, E.; et al. Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat. Med. 2016, 22, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Diedrich, J.K.; Kulp, D.W.; Pauthner, M.; He, L.; Park, S.R.; Sok, D.; Su, C.Y.; Delahunty, C.M.; Menis, S.; et al. Global site-specific N-glycosylation analysis of HIV envelope glycoprotein. Nat. Commun. 2017, 8, 14954. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.S.; Steckbeck, J.D.; Rowles, J.L.; Desrosiers, R.C.; Montelaro, R.C. Removal of N-linked glycosylation sites in the V1 region of simian immunodeficiency virus gp120 results in redirection of B-cell responses to V3. J. Virol. 2004, 78, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.J.; Herschhorn, A.; Haim, H.; Salas, I.; Gu, C.; Sodroski, J.; Gabuzda, D. Loss of a conserved N-linked glycosylation site in the simian immunodeficiency virus envelope glycoprotein V2 region enhances macrophage tropism by increasing CD4-independent cell-to-cell transmission. J. Virol. 2014, 88, 5014–5028. [Google Scholar] [CrossRef] [PubMed]
- Balazs, A.B.; Ouyang, Y.; Hong, C.M.; Chen, J.; Nguyen, S.M.; Rao, D.S.; An, D.S.; Baltimore, D. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat. Med. 2014, 20, 296–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hessell, A.J.; Jaworski, J.P.; Epson, E.; Matsuda, K.; Pandey, S.; Kahl, C.; Reed, J.; Sutton, W.F.; Hammond, K.B.; Cheever, T.A.; et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat. Med. 2016, 22, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Montefiori, D.C.; Karnasuta, C.; Huang, Y.; Ahmed, H.; Gilbert, P.; de Souza, M.S.; McLinden, R.; Tovanabutra, S.; Laurence-Chenine, A.; Sanders-Buell, E.; et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J. Infect. Dis. 2012, 206, 431–441. [Google Scholar] [CrossRef]
- Corti, D.; Langedijk, J.P.; Hinz, A.; Seaman, M.S.; Vanzetta, F.; Fernandez-Rodriguez, B.M.; Silacci, C.; Pinna, D.; Jarrossay, D.; Balla-Jhagjhoorsingh, S.; et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS ONE 2010, 5, e8805. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.H.; Williams, C.; Volsky, B.; Steczko, O.; Seaman, M.S.; Luthra, K.; Nyambi, P.; Nadas, A.; Giudicelli, V.; et al. A broad range of mutations in HIV-1 neutralizing human monoclonal antibodies specific for V2, V3, and the CD4 binding site. Mol. Immunol. 2015, 66, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Excler, J.L.; Ake, J.; Robb, M.L.; Kim, J.H.; Plotkin, S.A. Nonneutralizing functional antibodies: A new “old” paradigm for HIV vaccines. Clin. Vaccine Immunol. 2014, 21, 1023–1036. [Google Scholar] [CrossRef]
- Burton, D.R.; Mascola, J.R. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat. Immunol. 2015, 16, 571–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, L.E.; Burton, D.R. Identification and specificity of broadly neutralizing antibodies against HIV. Immunol. Rev. 2017, 275, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Li, Y.; Michael, N.L.; Robb, M.L.; Rolland, M. The breadth of HIV-1 neutralizing antibodies depends on the conservation of key sites in their epitopes. PLoS Comput. Biol. 2019, 15, e1007056. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R.; Hangartner, L. Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design. Annu. Rev. Immunol. 2016, 34, 635–659. [Google Scholar] [CrossRef] [PubMed]
- Caskey, M.; Klein, F.; Nussenzweig, M.C. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat. Med. 2019, 25, 547–553. [Google Scholar] [CrossRef] [PubMed]
- McCoy, L.E. The expanding array of HIV broadly neutralizing antibodies. Retrovirology 2018, 15, 70. [Google Scholar] [CrossRef]
- Georgiev, I.S.; Doria-Rose, N.A.; Zhou, T.; Kwon, Y.D.; Staupe, R.P.; Moquin, S.; Chuang, G.Y.; Louder, M.K.; Schmidt, S.D.; Altae-Tran, H.R.; et al. Delineating antibody recognition in polyclonal sera from patterns of HIV-1 isolate neutralization. Science 2013, 340, 751–756. [Google Scholar] [CrossRef]
- Sok, D.; van Gils, M.J.; Pauthner, M.; Julien, J.P.; Saye-Francisco, K.L.; Hsueh, J.; Briney, B.; Lee, J.H.; Le, K.M.; Lee, P.S.; et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc. Natl. Acad. Sci. USA 2014, 111, 17624–17629. [Google Scholar] [CrossRef] [Green Version]
- Bonsignori, M.; Hwang, K.K.; Chen, X.; Tsao, C.Y.; Morris, L.; Gray, E.; Marshall, D.J.; Crump, J.A.; Kapiga, S.H.; Sam, N.E.; et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J. Virol. 2011, 85, 9998–10009. [Google Scholar] [CrossRef]
- Doria-Rose, N.A.; Bhiman, J.N.; Roark, R.S.; Schramm, C.A.; Gorman, J.; Chuang, G.Y.; Pancera, M.; Cale, E.M.; Ernandes, M.J.; Louder, M.K.; et al. New Member of the V1V2-Directed CAP256-VRC26 Lineage That Shows Increased Breadth and Exceptional Potency. J. Virol. 2016, 90, 76–91. [Google Scholar] [CrossRef] [Green Version]
- Cale, E.M.; Gorman, J.; Radakovich, N.A.; Crooks, E.T.; Osawa, K.; Tong, T.; Li, J.; Nagarajan, R.; Ozorowski, G.; Ambrozak, D.R.; et al. Virus-like Particles Identify an HIV V1V2 Apex-Binding Neutralizing Antibody that Lacks a Protruding Loop. Immunity 2017, 46, 777–791. [Google Scholar] [CrossRef]
- Andrabi, R.; Su, C.Y.; Liang, C.H.; Shivatare, S.S.; Briney, B.; Voss, J.E.; Nawazi, S.K.; Wu, C.Y.; Wong, C.H.; Burton, D.R. Glycans Function as Anchors for Antibodies and Help Drive HIV Broadly Neutralizing Antibody Development. Immunity 2017, 47, 524–537. [Google Scholar] [CrossRef]
- Landais, E.; Murrell, B.; Briney, B.; Murrell, S.; Rantalainen, K.; Berndsen, Z.T.; Ramos, A.; Wickramasinghe, L.; Smith, M.L.; Eren, K.; et al. HIV Envelope Glycoform Heterogeneity and Localized Diversity Govern the Initiation and Maturation of a V2 Apex Broadly Neutralizing Antibody Lineage. Immunity 2017, 47, 990–1003. [Google Scholar] [CrossRef]
- McCoy, L.E.; Falkowska, E.; Doores, K.J.; Le, K.; Sok, D.; van Gils, M.J.; Euler, Z.; Burger, J.A.; Seaman, M.S.; Sanders, R.W.; et al. Incomplete Neutralization and Deviation from Sigmoidal Neutralization Curves for HIV Broadly Neutralizing Monoclonal Antibodies. PLoS Pathog. 2015, 11, e1005110. [Google Scholar] [CrossRef]
- Doores, K.J.; Burton, D.R. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J. Virol. 2010, 84, 10510–10521. [Google Scholar] [CrossRef]
- De Taeye, S.W.; Go, E.P.; Sliepen, K.; de la Pena, A.T.; Badal, K.; Medina-Ramirez, M.; Lee, W.H.; Desaire, H.; Wilson, I.A.; Moore, J.P.; et al. Stabilization of the V2 loop improves the presentation of V2 loop-associated broadly neutralizing antibody epitopes on HIV-1 envelope trimers. J. Biol. Chem. 2019, 294, 5616–5631. [Google Scholar] [CrossRef]
- Sliepen, K.; Han, B.W.; Bontjer, I.; Mooij, P.; Garces, F.; Behrens, A.J.; Rantalainen, K.; Kumar, S.; Sarkar, A.; Brouwer, P.J.M.; et al. Structure and immunogenicity of a stabilized HIV-1 envelope trimer based on a group-M consensus sequence. Nat. Commun. 2019, 10, 2355. [Google Scholar] [CrossRef]
- Torrents de la Pena, A.; Sanders, R.W. Stabilizing HIV-1 envelope glycoprotein trimers to induce neutralizing antibodies. Retrovirology 2018, 15, 63. [Google Scholar] [CrossRef]
- Barouch, D.H.; Stephenson, K.E.; Borducchi, E.N.; Smith, K.; Stanley, K.; McNally, A.G.; Liu, J.; Abbink, P.; Maxfield, L.F.; Seaman, M.S.; et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 2013, 155, 531–539. [Google Scholar] [CrossRef]
- Musich, T.; Li, L.; Liu, L.; Zolla-Pazner, S.; Robert-Guroff, M.; Gorny, M.K. Monoclonal Antibodies Specific for the V2, V3, CD4-Binding Site, and gp41 of HIV-1 Mediate Phagocytosis in a Dose-Dependent Manner. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Chung, A.W.; Crispin, M.; Pritchard, L.; Robinson, H.; Gorny, M.K.; Yu, X.; Bailey-Kellogg, C.; Ackerman, M.E.; Scanlan, C.; Zolla-Pazner, S.; et al. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. AIDS 2014, 28, 2523–2530. [Google Scholar] [CrossRef] [Green Version]
- Gach, J.S.; Bouzin, M.; Wong, M.P.; Chromikova, V.; Gorlani, A.; Yu, K.T.; Sharma, B.; Gratton, E.; Forthal, D.N. Human immunodeficiency virus type-1 (HIV-1) evades antibody-dependent phagocytosis. PLoS Pathog. 2017, 13, e1006793. [Google Scholar] [CrossRef]
- Bradley, T.; Pollara, J.; Santra, S.; Vandergrift, N.; Pittala, S.; Bailey-Kellogg, C.; Shen, X.; Parks, R.; Goodman, D.; Eaton, A.; et al. Pentavalent HIV-1 vaccine protects against simian-human immunodeficiency virus challenge. Nat. Commun. 2017, 8, 15711. [Google Scholar] [CrossRef]
- Mayr, L.M.; Decoville, T.; Schmidt, S.; Laumond, G.; Klingler, J.; Ducloy, C.; Bahram, S.; Zolla-Pazner, S.; Moog, C. Non-neutralizing Antibodies Targeting the V1V2 Domain of HIV Exhibit Strong Antibody-Dependent Cell-mediated Cytotoxic Activity. Sci. Rep. 2017, 7, 12655. [Google Scholar] [CrossRef]
- Forthal, D.N.; Finzi, A. Antibody-dependent cellular cytotoxicity in HIV infection. AIDS 2018, 32, 2439–2451. [Google Scholar] [CrossRef]
- Zolla-Pazner, S.; deCamp, A.C.; Cardozo, T.; Karasavvas, N.; Gottardo, R.; Williams, C.; Morris, D.E.; Tomaras, G.; Rao, M.; Billings, E.; et al. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS ONE 2013, 8, e53629. [Google Scholar] [CrossRef]
- Wiehe, K.; Easterhoff, D.; Luo, K.; Nicely, N.I.; Bradley, T.; Jaeger, F.H.; Dennison, S.M.; Zhang, R.; Lloyd, K.E.; Stolarchuk, C.; et al. Antibody light-chain-restricted recognition of the site of immune pressure in the RV144 HIV-1 vaccine trial is phylogenetically conserved. Immunity 2014, 41, 909–918. [Google Scholar] [CrossRef]
- Alter, G.; Barouch, D. Immune Correlate-Guided HIV Vaccine Design. Cell Host Microbe 2018, 24, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Ackerman, M.E.; Mikhailova, A.; Brown, E.P.; Dowell, K.G.; Walker, B.D.; Bailey-Kellogg, C.; Suscovich, T.J.; Alter, G. Polyfunctional HIV-Specific Antibody Responses Are Associated with Spontaneous HIV Control. PLoS Pathog. 2016, 12, e1005315. [Google Scholar] [CrossRef]
- Anand, S.P.; Prevost, J.; Baril, S.; Richard, J.; Medjahed, H.; Chapleau, J.P.; Tolbert, W.D.; Kirk, S.; Smith, A.B., 3rd; Wines, B.D.; et al. Two Families of Env Antibodies Efficiently Engage Fc-Gamma Receptors and Eliminate HIV-1-Infected Cells. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [Green Version]
- Calenda, G.; Frank, I.; Arrode-Bruses, G.; Pegu, A.; Wang, K.; Arthos, J.; Cicala, C.; Rogers, K.A.; Shirreff, L.; Grasperge, B.; et al. Delayed vaginal SHIV infection in VRC01 and anti-alpha4beta7 treated rhesus macaques. PLoS Pathog. 2019, 15, e1007776. [Google Scholar] [CrossRef]
- Pollara, J.; Bonsignori, M.; Moody, M.A.; Liu, P.; Alam, S.M.; Hwang, K.K.; Gurley, T.C.; Kozink, D.M.; Armand, L.C.; Marshall, D.J.; et al. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J. Virol. 2014, 88, 7715–7726. [Google Scholar] [CrossRef]
- Perez, L.G.; Martinez, D.R.; deCamp, A.C.; Pinter, A.; Berman, P.W.; Francis, D.; Sinangil, F.; Lee, C.; Greene, K.; Gao, H.; et al. V1V2-specific complement activating serum IgG as a correlate of reduced HIV-1 infection risk in RV144. PLoS ONE 2017, 12, e0180720. [Google Scholar] [CrossRef]
- Buchbinder, S.P.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, P.B.; Lama, J.R.; Marmor, M.; Del Rio, C.; et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008, 372, 1881–1893. [Google Scholar] [CrossRef]
- Gray, G.E.; Allen, M.; Moodie, Z.; Churchyard, G.; Bekker, L.G.; Nchabeleng, M.; Mlisana, K.; Metch, B.; de Bruyn, G.; Latka, M.H.; et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: A double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect. Dis. 2011, 11, 507–515. [Google Scholar] [CrossRef]
- Flynn, N.M.; Forthal, D.N.; Harro, C.D.; Judson, F.N.; Mayer, K.H.; Para, M.F.; rgp120 HIV Vaccine Study Group. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 2005, 191, 654–665. [Google Scholar] [CrossRef]
- Pitisuttithum, P.; Gilbert, P.; Gurwith, M.; Heyward, W.; Martin, M.; van Griensven, F.; Hu, D.; Tappero, J.W.; Choopanya, K.; Bangkok Vaccine Evaluation Group. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 2006, 194, 1661–1671. [Google Scholar] [CrossRef]
- Hammer, S.M.; Sobieszczyk, M.E.; Janes, H.; Karuna, S.T.; Mulligan, M.J.; Grove, D.; Koblin, B.A.; Buchbinder, S.P.; Keefer, M.C.; Tomaras, G.D.; et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N. Engl. J. Med. 2013, 369, 2083–2092. [Google Scholar] [CrossRef]
- Tomaras, G.D.; Ferrari, G.; Shen, X.; Alam, S.M.; Liao, H.X.; Pollara, J.; Bonsignori, M.; Moody, M.A.; Fong, Y.; Chen, X.; et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc. Natl. Acad. Sci. USA 2013, 110, 9019–9024. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, P.; Williams, C.; Shapiro, M.B.; Sinangil, F.; Higgins, K.; Nadas, A.; Totrov, M.; Kong, X.P.; Fiore-Gartland, A.J.; Haigwood, N.L.; et al. Functional Antibody Response Against V1V2 and V3 of HIV gp120 in the VAX003 and VAX004 Vaccine Trials. Sci. Rep. 2018, 8, 542. [Google Scholar] [CrossRef]
- Chung, A.W.; Ghebremichael, M.; Robinson, H.; Brown, E.; Choi, I.; Lane, S.; Dugast, A.S.; Schoen, M.K.; Rolland, M.; Suscovich, T.J.; et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci. Transl. Med. 2014, 6, 228ra38. [Google Scholar] [CrossRef] [PubMed]
- Karnasuta, C.; Akapirat, S.; Madnote, S.; Savadsuk, H.; Puangkaew, J.; Rittiroongrad, S.; Rerks-Ngarm, S.; Nitayaphan, S.; Pitisuttithum, P.; Kaewkungwal, J.; et al. Comparison of Antibody Responses Induced by RV144, VAX003, and VAX004 Vaccination Regimens. AIDS Res. Hum. Retrovir. 2017, 33, 410–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, N.L.; Liao, H.X.; Fong, Y.; de Camp, A.; Vandergrift, N.A.; Williams, W.T.; Alam, S.M.; Ferrari, G.; Yang, Z.Y.; Seaton, K.E.; et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci. Transl. Med. 2014, 6, 228ra39. [Google Scholar] [CrossRef] [PubMed]
- Kam, Y.W.; Simarmata, D.; Chow, A.; Her, Z.; Teng, T.S.; Ong, E.K.; Renia, L.; Leo, Y.S.; Ng, L.F. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J. Infect. Dis. 2012, 205, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Roussilhon, C.; Oeuvray, C.; Muller-Graf, C.; Tall, A.; Rogier, C.; Trape, J.F.; Theisen, M.; Balde, A.; Perignon, J.L.; Druilhe, P. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 2007, 4, e320. [Google Scholar] [CrossRef] [PubMed]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Excler, J.L.; Nitayaphan, S.; Kaewkungwal, J.; Premsri, N.; Kunasol, P.; Karasavvas, N.; Schuetz, A.; Ngauy, V.; et al. Randomized, Double-Blind Evaluation of Late Boost Strategies for HIV-Uninfected Vaccine Recipients in the RV144 HIV Vaccine Efficacy Trial. J. Infect. Dis. 2017, 215, 1255–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prevost, J.; Zoubchenok, D.; Richard, J.; Veillette, M.; Pacheco, B.; Coutu, M.; Brassard, N.; Parsons, M.S.; Ruxrungtham, K.; Bunupuradah, T.; et al. Influence of the Envelope gp120 Phe 43 Cavity on HIV-1 Sensitivity to Antibody-Dependent Cell-Mediated Cytotoxicity Responses. J. Virol. 2017, 91, e02452-16. [Google Scholar] [CrossRef]
- Alam, S.M.; Liao, H.X.; Tomaras, G.D.; Bonsignori, M.; Tsao, C.Y.; Hwang, K.K.; Chen, H.; Lloyd, K.E.; Bowman, C.; Sutherland, L.; et al. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J. Virol. 2013, 87, 1554–1568. [Google Scholar] [CrossRef]
- Teigler, J.E.; Phogat, S.; Franchini, G.; Hirsch, V.M.; Michael, N.L.; Barouch, D.H. The canarypox virus vector ALVAC induces distinct cytokine responses compared to the vaccinia virus-based vectors MVA and NYVAC in rhesus monkeys. J. Virol. 2014, 88, 1809–1814. [Google Scholar] [CrossRef]
- Bonsignori, M.; Pollara, J.; Moody, M.A.; Alpert, M.D.; Chen, X.; Hwang, K.K.; Gilbert, P.B.; Huang, Y.; Gurley, T.C.; Kozink, D.M.; et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J. Virol. 2012, 86, 11521–11532. [Google Scholar] [CrossRef]
- Hessell, A.J.; Hangartner, L.; Hunter, M.; Havenith, C.E.; Beurskens, F.J.; Bakker, J.M.; Lanigan, C.M.; Landucci, G.; Forthal, D.N.; Parren, P.W.; et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 2007, 449, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Gilbert, P.B.; Tomaras, G.D.; Kijak, G.; Ferrari, G.; Thomas, R.; Pyo, C.W.; Zolla-Pazner, S.; Montefiori, D.; Liao, H.X.; et al. FCGR2C polymorphisms associate with HIV-1 vaccine protection in RV144 trial. J. Clin. Investig. 2014, 124, 3879–3890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassauniere, R.; Tiemessen, C.T. Variability at the FCGR locus: Characterization in Black South Africans and evidence for ethnic variation in and out of Africa. Genes Immun. 2016, 17, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Finak, G.; Ushey, K.; Seshadri, C.; Hawn, T.R.; Frahm, N.; Scriba, T.J.; Mahomed, H.; Hanekom, W.; Bart, P.A.; et al. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat. Biotechnol. 2015, 33, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Tomaka, F.L.; Wegmann, F.; Stieh, D.J.; Alter, G.; Robb, M.L.; Michael, N.L.; Peter, L.; Nkolola, J.P.; Borducchi, E.N.; et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 2018, 392, 232–243. [Google Scholar] [CrossRef]
- Bekker, L.G.; Moodie, Z.; Grunenberg, N.; Laher, F.; Tomaras, G.D.; Cohen, K.W.; Allen, M.; Malahleha, M.; Mngadi, K.; Daniels, B.; et al. Subtype C ALVAC-HIV and bivalent subtype C gp120/MF59 HIV-1 vaccine in low-risk, HIV-uninfected, South African adults: A phase 1/2 trial. Lancet HIV 2018, 5, e366–e378. [Google Scholar] [CrossRef]
- Gray, G.E.; Andersen-Nissen, E.; Grunenberg, N.; Huang, Y.; Roux, S.; Laher, F.; Innes, C.; Gu, N.; DiazGranados, C.; Phogat, S.; et al. HVTN 097: Evaluation of the RV144 Vaccine Regimen in HIV Uninfected South African Adults. AIDS Res. Hum. Retrovir. 2014, 30, A33–A34. [Google Scholar] [CrossRef]
- Shen, X.; Moodie, Z.; McMillan, S.; Goodman, D.; Yates, N.L.; Spreng, R.; Grunenberg, N.; Gilbert, P.; Laher, F.; Bekker, L.G.; et al. V1V2 IgG and Antibody Fc Effector Functions in a Subtype C ALVAC-HIV and Bivalent Subtype C gp120/MF59 HIV-1 Vaccine Trial in South Africa. In Proceedings of the HIVR4P 2018, Madrid, Spain, 21–25 October 2018. [Google Scholar]
- Rademeyer, C.; Korber, B.; Seaman, M.S.; Giorgi, E.E.; Thebus, R.; Robles, A.; Sheward, D.J.; Wagh, K.; Garrity, J.; Carey, B.R.; et al. Features of Recently Transmitted HIV-1 Clade C Viruses that Impact Antibody Recognition: Implications for Active and Passive Immunization. PLoS Pathog. 2016, 12, e1005742. [Google Scholar] [CrossRef]
- Richardson, S.I.; Gray, E.S.; Mkhize, N.N.; Sheward, D.J.; Lambson, B.E.; Wibmer, C.K.; Masson, L.; Werner, L.; Garrett, N.; Passmore, J.A.; et al. South African HIV-1 subtype C transmitted variants with a specific V2 motif show higher dependence on alpha4beta7 for replication. Retrovirology 2015, 12, 54. [Google Scholar] [CrossRef]
- Andrus, L.; Prince, A.M.; Bernal, I.; McCormack, P.; Lee, D.H.; Gorny, M.K.; Zolla-Pazner, S. Passive immunization with a human immunodeficiency virus type 1-neutralizing monoclonal antibody in Hu-PBL-SCID mice: Isolation of a neutralization escape variant. J. Infect. Dis. 1998, 177, 889–897. [Google Scholar] [CrossRef]
- Emini, E.A.; Schleif, W.A.; Nunberg, J.H.; Conley, A.J.; Eda, Y.; Tokiyoshi, S.; Putney, S.D.; Matsushita, S.; Cobb, K.E.; Jett, C.M.; et al. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature 1992, 355, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Prince, A.M.; Reesink, H.; Pascual, D.; Horowitz, B.; Hewlett, I.; Murthy, K.K.; Cobb, K.E.; Eichberg, J.W. Prevention of HIV infection by passive immunization with HIV immunoglobulin. AIDS Res. Hum. Retrovir. 1991, 7, 971–973. [Google Scholar] [CrossRef] [PubMed]
- Haigwood, N.L.; Montefiori, D.C.; Sutton, W.F.; McClure, J.; Watson, A.J.; Voss, G.; Hirsch, V.M.; Richardson, B.A.; Letvin, N.L.; Hu, S.L.; et al. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. J. Virol. 2004, 78, 5983–5995. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.W.; Liska, V.; Hofmann-Lehmann, R.; Vlasak, J.; Xu, W.; Ayehunie, S.; Cavacini, L.A.; Posner, M.R.; Katinger, H.; Stiegler, G.; et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 2000, 6, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Mascola, J.R.; Stiegler, G.; VanCott, T.C.; Katinger, H.; Carpenter, C.B.; Hanson, C.E.; Beary, H.; Hayes, D.; Frankel, S.S.; Birx, D.L.; et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 2000, 6, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Hessell, A.J.; Shapiro, M.B.; Powell, R.; Malherbe, D.C.; McBurney, S.P.; Pandey, S.; Cheever, T.; Sutton, W.F.; Kahl, C.; Park, B.; et al. Reduced Cell-Associated DNA and Improved Viral Control in Macaques following Passive Transfer of a Single Anti-V2 Monoclonal Antibody and Repeated Simian/Human Immunodeficiency Virus Challenges. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Moog, C.; Dereuddre-Bosquet, N.; Teillaud, J.L.; Biedma, M.E.; Holl, V.; Van Ham, G.; Heyndrickx, L.; Van Dorsselaer, A.; Katinger, D.; Vcelar, B.; et al. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2014, 7, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R.; Hessell, A.J.; Keele, B.F.; Klasse, P.J.; Ketas, T.A.; Moldt, B.; Dunlop, D.C.; Poignard, P.; Doyle, L.A.; Cavacini, L.; et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl. Acad. Sci. USA 2011, 108, 11181–11186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santra, S.; Tomaras, G.D.; Warrier, R.; Nicely, N.I.; Liao, H.X.; Pollara, J.; Liu, P.; Alam, S.M.; Zhang, R.; Cocklin, S.L.; et al. Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques. PLoS Pathog. 2015, 11, e1005042. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.R.; Kattenhorn, L.M.; Kondur, H.R.; von Schaewen, M.; Dorfman, T.; Chiang, J.J.; Haworth, K.G.; Decker, J.M.; Alpert, M.D.; Bailey, C.C.; et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 2015, 519, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, R.; Nishimura, Y.; Pegu, A.; Nason, M.C.; Klein, F.; Gazumyan, A.; Golijanin, J.; Buckler-White, A.; Sadjadpour, R.; Wang, K.; et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 2016, 533, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hessell, A.J.; Poignard, P.; Hunter, M.; Hangartner, L.; Tehrani, D.M.; Bleeker, W.K.; Parren, P.W.; Marx, P.A.; Burton, D.R. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 2009, 15, 951–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hessell, A.J.; Rakasz, E.G.; Poignard, P.; Hangartner, L.; Landucci, G.; Forthal, D.N.; Koff, W.C.; Watkins, D.I.; Burton, D.R. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009, 5, e1000433. [Google Scholar] [CrossRef] [PubMed]
- Hessell, A.J.; Rakasz, E.G.; Tehrani, D.M.; Huber, M.; Weisgrau, K.L.; Landucci, G.; Forthal, D.N.; Koff, W.C.; Poignard, P.; Watkins, D.I.; et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 2010, 84, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Moldt, B.; Rakasz, E.G.; Schultz, N.; Chan-Hui, P.Y.; Swiderek, K.; Weisgrau, K.L.; Piaskowski, S.M.; Bergman, Z.; Watkins, D.I.; Poignard, P.; et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 18921–18925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pegu, A.; Yang, Z.Y.; Boyington, J.C.; Wu, L.; Ko, S.Y.; Schmidt, S.D.; McKee, K.; Kong, W.P.; Shi, W.; Chen, X.; et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci. Transl. Med. 2014, 6, 243ra88. [Google Scholar] [CrossRef]
- Shingai, M.; Donau, O.K.; Plishka, R.J.; Buckler-White, A.; Mascola, J.R.; Nabel, G.J.; Nason, M.C.; Montefiori, D.; Moldt, B.; Poignard, P.; et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J. Exp. Med. 2014, 211, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Pegu, A.; Rao, E.; Doria-Rose, N.; Beninga, J.; McKee, K.; Lord, D.M.; Wei, R.R.; Deng, G.; Louder, M.; et al. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science 2017, 358, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Julg, B.; Tartaglia, L.J.; Keele, B.F.; Wagh, K.; Pegu, A.; Sok, D.; Abbink, P.; Schmidt, S.D.; Wang, K.; Chen, X.; et al. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Pauthner, M.G.; Nkolola, J.P.; Havenar-Daughton, C.; Murrell, B.; Reiss, S.M.; Bastidas, R.; Prevost, J.; Nedellec, R.; von Bredow, B.; Abbink, P.; et al. Vaccine-Induced Protection from Homologous Tier 2 SHIV Challenge in Nonhuman Primates Depends on Serum-Neutralizing Antibody Titers. Immunity 2019, 50, 241–252. [Google Scholar] [CrossRef]
- Burton, S.L.; Kilgore, K.M.; Smith, S.A.; Reddy, S.; Hunter, E.; Robinson, H.L.; Silvestri, G.; Amara, R.R.; Derdeyn, C.A. Breakthrough of SIV strain smE660 challenge in SIV strain mac239-vaccinated rhesus macaques despite potent autologous neutralizing antibody responses. Proc. Natl. Acad. Sci. USA 2015, 112, 10780–10785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruel, T.; Guivel-Benhassine, F.; Amraoui, S.; Malbec, M.; Richard, L.; Bourdic, K.; Donahue, D.A.; Lorin, V.; Casartelli, N.; Noel, N.; et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat. Commun. 2016, 7, 10844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruel, T.; Guivel-Benhassine, F.; Lorin, V.; Lortat-Jacob, H.; Baleux, F.; Bourdic, K.; Noel, N.; Lambotte, O.; Mouquet, H.; Schwartz, O. Lack of ADCC Breadth of Human Nonneutralizing Anti-HIV-1 Antibodies. J. Virol. 2017, 91, e02440-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Korom, M.; Truong, R.; Chan, D.; Huang, S.H.; Kovacs, C.C.; Benko, E.; Safrit, J.T.; Lee, J.; Garban, H.; et al. Susceptibility to Neutralization by Broadly Neutralizing Antibodies Generally Correlates with Infected Cell Binding for a Panel of Clade B HIV Reactivated from Latent Reservoirs. J. Virol. 2018, 92, e00895-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Bredow, B.; Arias, J.F.; Heyer, L.N.; Moldt, B.; Le, K.; Robinson, J.E.; Zolla-Pazner, S.; Burton, D.R.; Evans, D.T. Comparison of Antibody-Dependent Cell-Mediated Cytotoxicity and Virus Neutralization by HIV-1 Env-Specific Monoclonal Antibodies. J. Virol. 2016, 90, 6127–6139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, M.S.; Lee, W.S.; Kristensen, A.B.; Amarasena, T.; Khoury, G.; Wheatley, A.K.; Reynaldi, A.; Wines, B.D.; Hogarth, P.M.; Davenport, M.P.; et al. Fc-dependent functions are redundant to efficacy of anti-HIV antibody PGT121 in macaques. J. Clin. Investig. 2019, 129, 182–191. [Google Scholar] [CrossRef] [PubMed]
- von Bredow, B.; Andrabi, R.; Grunst, M.; Grandea, A.G., 3rd; Le, K.; Song, G.; Berndsen, Z.T.; Porter, K.; Pallesen, J.; Ward, A.B.; et al. Differences in the Binding Affinity of an HIV-1 V2 Apex-Specific Antibody for the SIVsmm/mac Envelope Glycoprotein Uncouple Antibody-Dependent Cellular Cytotoxicity from Neutralization. MBio 2019, 10, e01255-19. [Google Scholar] [CrossRef]
- Malherbe, D.C.; Mendy, J.; Vang, L.; Barnette, P.T.; Reed, J.; Lakhashe, S.K.; Owuor, J.; Gach, J.S.; Legasse, A.W.; Axthelm, M.K.; et al. Combination Adenovirus and Protein Vaccines Prevent Infection or Reduce Viral Burden after Heterologous Clade C Simian-Human Immunodeficiency Virus Mucosal Challenge. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Hessell, A.J.; Malherbe, D.; Barnette, P.; Pandey, S.; Sutton, W.; Li, L.; Tuen, M.; Gach, J.S.; Forthal, D.N.; Duerr, R.; et al. Tight Control of SHIV Challenge in Macaques with Vaccine-Induced Neutralizing and Non-Neutralizing Anti-V2 Antibodies. In Proceedings of the Keystone Symposia, HIV Vaccines (X7), Whistler, BC, Canada, 24–28 March 2019. [Google Scholar]
- Jones, A.T.; Shen, X.; Walter, K.L.; LaBranche, C.C.; Wyatt, L.S.; Tomaras, G.D.; Montefiori, D.C.; Moss, B.; Barouch, D.H.; Clements, J.D.; et al. HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge. Nat. Commun. 2019, 10, 798. [Google Scholar] [CrossRef]
- Styles, T.M.; Gangadhara, S.; Reddy, P.B.J.; Hicks, S.; LaBranche, C.C.; Montefiori, D.C.; Derdeyn, C.A.; Kozlowski, P.A.; Velu, V.; Amara, R.R. HIV C.1086 Envelope Gp140 Protein Boosts Following DNA/MVA Vaccination Fail to Enhance Heterologous Anti-V1V2 Antibody Response and Protection Against Clade C SHIV Challenge. J. Virol. 2019, JVI.00934-19. [Google Scholar] [CrossRef]
- Barouch, D.H.; Liu, J.; Li, H.; Maxfield, L.F.; Abbink, P.; Lynch, D.M.; Iampietro, M.J.; SanMiguel, A.; Seaman, M.S.; Ferrari, G.; et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 2012, 482, 89–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, S.N.; Doster, M.N.; Kines, R.C.; Keele, B.F.; Brocca-Cofano, E.; Guan, Y.; Pegu, P.; Liyanage, N.P.; Vaccari, M.; Cuburu, N.; et al. Antibody to the gp120 V1/V2 loops and CD4+ and CD8+ T cell responses in protection from SIVmac251 vaginal acquisition and persistent viremia. J. Immunol. 2014, 193, 6172–6183. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.N.; Liyanage, N.P.; Doster, M.N.; Vaccari, M.; Vargas-Inchaustegui, D.A.; Pegu, P.; Schifanella, L.; Shen, X.; Tomaras, G.D.; Rao, M.; et al. Boosting of ALVAC-SIV Vaccine-Primed Macaques with the CD4-SIVgp120 Fusion Protein Elicits Antibodies to V2 Associated with a Decreased Risk of SIVmac251 Acquisition. J. Immunol. 2016, 197, 2726–2737. [Google Scholar] [CrossRef] [PubMed]
- Pegu, P.; Vaccari, M.; Gordon, S.; Keele, B.F.; Doster, M.; Guan, Y.; Ferrari, G.; Pal, R.; Ferrari, M.G.; Whitney, S.; et al. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J. Virol. 2013, 87, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- Roederer, M.; Keele, B.F.; Schmidt, S.D.; Mason, R.D.; Welles, H.C.; Fischer, W.; Labranche, C.; Foulds, K.E.; Louder, M.K.; Yang, Z.Y.; et al. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature 2014, 505, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Ramirez-Salazar, E.G.; Doueiri, R.; Valentin, A.; Rosati, M.; Hu, X.; Keele, B.F.; Shen, X.; Tomaras, G.D.; Ferrari, G.; et al. Control of Heterologous Simian Immunodeficiency Virus SIVsmE660 Infection by DNA and Protein Coimmunization Regimens Combined with Different Toll-Like-Receptor-4-Based Adjuvants in Macaques. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, M.; Fourati, S.; Gordon, S.N.; Brown, D.R.; Bissa, M.; Schifanella, L.; Silva de Castro, I.; Doster, M.N.; Galli, V.; Omsland, M.; et al. HIV vaccine candidate activation of hypoxia and the inflammasome in CD14(+) monocytes is associated with a decreased risk of SIVmac251 acquisition. Nat. Med. 2018, 24, 847–856. [Google Scholar] [CrossRef]
- Kwa, S.; Sadagopal, S.; Shen, X.; Hong, J.J.; Gangadhara, S.; Basu, R.; Victor, B.; Iyer, S.S.; LaBranche, C.C.; Montefiori, D.C.; et al. CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus (SIV) vaccine enhances protection against neutralization-resistant mucosal SIV infection. J. Virol. 2015, 89, 4690–4695. [Google Scholar] [CrossRef]
- Bohl, C.; Bowder, D.; Thompson, J.; Abrahamyan, L.; Gonzalez-Ramirez, S.; Mao, Y.; Sodroski, J.; Wood, C.; Xiang, S.H. A twin-cysteine motif in the V2 region of gp120 is associated with SIV envelope trimer stabilization. PLoS ONE 2013, 8, e69406. [Google Scholar] [CrossRef]
- Hunt, P.W.; Sinclair, E.; Rodriguez, B.; Shive, C.; Clagett, B.; Funderburg, N.; Robinson, J.; Huang, Y.; Epling, L.; Martin, J.N.; et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J. Infect. Dis. 2014, 210, 1228–1238. [Google Scholar] [CrossRef]
- Mehandru, S.; Poles, M.A.; Tenner-Racz, K.; Horowitz, A.; Hurley, A.; Hogan, C.; Boden, D.; Racz, P.; Markowitz, M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 2004, 200, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Jahn, H.U.; Schmidt, W.; Riecken, E.O.; Zeitz, M.; Ullrich, R. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut 1995, 37, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Veazey, R.S.; DeMaria, M.; Chalifoux, L.V.; Shvetz, D.E.; Pauley, D.R.; Knight, H.L.; Rosenzweig, M.; Johnson, R.P.; Desrosiers, R.C.; Lackner, A.A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998, 280, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Berlin, C.; Berg, E.L.; Briskin, M.J.; Andrew, D.P.; Kilshaw, P.J.; Holzmann, B.; Weissman, I.L.; Hamann, A.; Butcher, E.C. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 1993, 74, 185–195. [Google Scholar] [CrossRef]
- Cicala, C.; Martinelli, E.; McNally, J.P.; Goode, D.J.; Gopaul, R.; Hiatt, J.; Jelicic, K.; Kottilil, S.; Macleod, K.; O’Shea, A.; et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. USA 2009, 106, 20877–20882. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.; Wang, X.; Piatak, M.; Lifson, J.; Roederer, M.; Veazey, R.; Mattapallil, J.J. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009, 2, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Arthos, J.; Cicala, C.; Martinelli, E.; Macleod, K.; Van Ryk, D.; Wei, D.; Xiao, Z.; Veenstra, T.D.; Conrad, T.P.; Lempicki, R.A.; et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 2008, 9, 301–309. [Google Scholar] [CrossRef]
- Peachman, K.K.; Karasavvas, N.; Chenine, A.L.; McLinden, R.; Rerks-Ngarm, S.; Jaranit, K.; Nitayaphan, S.; Pitisuttithum, P.; Tovanabutra, S.; Zolla-Pazner, S.; et al. Identification of New Regions in HIV-1 gp120 Variable 2 and 3 Loops that Bind to alpha4beta7 Integrin Receptor. PLoS ONE 2015, 10, e0143895. [Google Scholar] [CrossRef]
- Tassaneetrithep, B.; Tivon, D.; Swetnam, J.; Karasavvas, N.; Michael, N.L.; Kim, J.H.; Marovich, M.; Cardozo, T. Cryptic determinant of alpha4beta7 binding in the V2 loop of HIV-1 gp120. PLoS ONE 2014, 9, e108446. [Google Scholar] [CrossRef]
- Plotnik, D.; Guo, W.; Cleveland, B.; von Haller, P.; Eng, J.K.; Guttman, M.; Lee, K.K.; Arthos, J.; Hu, S.L. Extracellular Matrix Proteins Mediate HIV-1 gp120 Interactions with alpha4beta7. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Perez, L.G.; Chen, H.; Liao, H.X.; Montefiori, D.C. Envelope glycoprotein binding to the integrin alpha4beta7 is not a general property of most HIV-1 strains. J. Virol. 2014, 88, 10767–10777. [Google Scholar] [CrossRef] [PubMed]
- Chand, S.; Messina, E.L.; AlSalmi, W.; Ananthaswamy, N.; Gao, G.; Uritskiy, G.; Padilla-Sanchez, V.; Mahalingam, M.; Peachman, K.K.; Robb, M.L.; et al. Glycosylation and oligomeric state of envelope protein might influence HIV-1 virion capture by alpha4beta7 integrin. Virology 2017, 508, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.; Cicala, C.; Van Ryk, D.; Block, K.E.; Jelicic, K.; McNally, J.P.; Ogundare, O.; Pascuccio, M.; Patel, N.; Wei, D.; et al. The genotype of early-transmitting HIV gp120s promotes alpha (4) beta(7)-reactivity, revealing alpha (4) beta(7) +/CD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 2011, 7, e1001301. [Google Scholar] [CrossRef] [PubMed]
- Sivro, A.; Schuetz, A.; Sheward, D.; Joag, V.; Yegorov, S.; Liebenberg, L.J.; Yende-Zuma, N.; Stalker, A.; Mwatelah, R.S.; Selhorst, P.; et al. Integrin alpha4beta7 expression on peripheral blood CD4(+) T cells predicts HIV acquisition and disease progression outcomes. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Veglia, F.; Goode, D.; Guerra-Perez, N.; Aravantinou, M.; Arthos, J.; Piatak, M., Jr.; Lifson, J.D.; Blanchard, J.; Gettie, A.; et al. The frequency of alpha(4)beta(7)(high) memory CD4(+) T cells correlates with susceptibility to rectal simian immunodeficiency virus infection. J. Acquir. Immune Defic. Syndr. 2013, 64, 325–331. [Google Scholar] [CrossRef]
- Ding, J.; Tasker, C.; Lespinasse, P.; Dai, J.; Fitzgerald-Bocarsly, P.; Lu, W.; Heller, D.; Chang, T.L. Integrin alpha4beta7 Expression Increases HIV Susceptibility in Activated Cervical CD4+ T Cells by an HIV Attachment-Independent Mechanism. J. Acquir. Immune Defic. Syndr. 2015, 69, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, G.R.; Fonseca, D.P.; O’Rourke, S.M.; Vollrath, A.L.; Berman, P.W. Monoclonal antibodies to the V2 domain of MN-rgp120: Fine mapping of epitopes and inhibition of alpha4beta7 binding. PLoS ONE 2012, 7, e39045. [Google Scholar] [CrossRef]
- Lertjuthaporn, S.; Cicala, C.; Van Ryk, D.; Liu, M.; Yolitz, J.; Wei, D.; Nawaz, F.; Doyle, A.; Horowitch, B.; Park, C.; et al. Select gp120 V2 domain specific antibodies derived from HIV and SIV infection and vaccination inhibit gp120 binding to alpha4beta7. PLoS Pathog. 2018, 14, e1007278. [Google Scholar] [CrossRef]
- Arthos, J.; Cicala, C.; Nawaz, F.; Byrareddy, S.N.; Villinger, F.; Santangelo, P.J.; Ansari, A.A.; Fauci, A.S. The Role of Integrin alpha4beta7 in HIV Pathogenesis and Treatment. Curr. HIV/AIDS Rep. 2018, 15, 127–135. [Google Scholar] [CrossRef]
- Uzzan, M.; Tokuyama, M.; Rosenstein, A.K.; Tomescu, C.; SahBandar, I.N.; Ko, H.M.; Leyre, L.; Chokola, A.; Kaplan-Lewis, E.; Rodriguez, G.; et al. Anti-alpha4beta7 therapy targets lymphoid aggregates in the gastrointestinal tract of HIV-1-infected individuals. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Ansari, A.A.; Reimann, K.A.; Mayne, A.E.; Takahashi, Y.; Stephenson, S.T.; Wang, R.J.; Wang, X.Y.; Li, J.C.; Price, A.A.; Little, D.M.; et al. Blocking of alpha 4 beta 7 Gut-Homing Integrin during Acute Infection Leads to Decreased Plasma and Gastrointestinal Tissue Viral Loads in Simian Immunodeficiency Virus-Infected Rhesus Macaques. J. Immunol. 2011, 186, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Byrareddy, S.N.; Kallam, B.; Arthos, J.; Cicala, C.; Nawaz, F.; Hiatt, J.; Kersh, E.N.; McNicholl, J.M.; Hanson, D.; Reimann, K.A.; et al. Targeting alpha(4)beta(7) integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat. Med. 2014, 20, 1397–1400. [Google Scholar] [CrossRef]
- Byrareddy, S.N.; Arthos, J.; Cicala, C.; Villinger, F.; Ortiz, K.T.; Little, D.; Sidell, N.; Kane, M.A.; Yu, J.; Jones, J.W.; et al. Sustained virologic control in SIV+ macaques after antiretroviral and alpha4beta7 antibody therapy. Science 2016, 354, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Wu, T.; To, K.K.W.; Cheung, K.W.; Lui, K.O.; Niu, M.; Lam, K.S.; Wang, C.C.; Li, J.; Wang, H.; et al. Vedolizumab-mediated integrin alpha4beta7 blockade does not control HIV-1SF162 rebound after cART interruption in humanized mice. AIDS 2019, 33, F1–F12. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, N.; Mason, R.; Song, K.; Gorman, J.; Welles, H.; Arthos, J.; Cicala, C.; Foulds, K.; Kwong, P.; Lifson, J.; et al. SIV Escapes from Non-neutralizing Antibodies Blocking α4β7 Integrin Binding. In Proceedings of the Keystone Symposia, HIV Vaccines, Whistler, BC, Canada, 24–28 March 2019. [Google Scholar]
- McGuinty, M.; Angel, J.; Kumar, A.; Sy, R.; Murthy, S.; Kilby, D.; Tremblay, N.; Lavoie, E.; Schinkel, S.B.; Byrareddy, S.N.; et al. Seeking suppression in havarti: Viremia and T cells after vedolizumab and analytical treatment interruption in HIV/ART. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, WA, USA, 4–7 March 2019. [Google Scholar]
- Girard, A.; Jelicic, K.; Van Ryk, D.; Rochereau, N.; Cicala, C.; Arthos, J.; Noailly, B.; Genin, C.; Verrier, B.; Laurant, S.; et al. Neutralizing and Targeting Properties of a New Set of alpha4beta7-Specific Antibodies Are Influenced by Their Isotype. J. Acquir. Immune Defic. Syndr. 2017, 75, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Hait, S.H.; Soares, E.A.; Sprinz, E.; Arthos, J.; Machado, E.S.; Soares, M.A. Worldwide Genetic Features of HIV-1 Env alpha4beta7 Binding Motif: The Local Dissemination Impact of the LDI Tripeptide. J. Acquir. Immune Defic. Syndr. 2015, 70, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.R.; Mayr, L.; Nanfack, A.J.; Banin, A.N.; Tuen, M.; Pan, R.; Jiang, X.; Kong, X.P.; Kirkpatrick, A.R.; Bruno, D.; et al. Contrasting antibody responses to intrasubtype superinfection with CRF02_AG. PLoS ONE 2017, 12, e0173705. [Google Scholar] [CrossRef] [PubMed]
- Redd, A.D.; Quinn, T.C.; Tobian, A.A. Frequency and implications of HIV superinfection. Lancet Infect. Dis. 2013, 13, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Moore, P.L.; Gray, E.S.; Sheward, D.; Madiga, M.; Ranchobe, N.; Lai, Z.; Honnen, W.J.; Nonyane, M.; Tumba, N.; Hermanus, T.; et al. Potent and broad neutralization of HIV-1 subtype C by plasma antibodies targeting a quaternary epitope including residues in the V2 loop. J. Virol. 2011, 85, 3128–3141. [Google Scholar] [CrossRef]
- Bhiman, J.N.; Anthony, C.; Doria-Rose, N.A.; Karimanzira, O.; Schramm, C.A.; Khoza, T.; Kitchin, D.; Botha, G.; Gorman, J.; Garrett, N.J.; et al. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat. Med. 2015, 21, 1332–1336. [Google Scholar] [CrossRef]
- Voss, J.E.; Andrabi, R.; McCoy, L.E.; de Val, N.; Fuller, R.P.; Messmer, T.; Su, C.Y.; Sok, D.; Khan, S.N.; Garces, F.; et al. Elicitation of Neutralizing Antibodies Targeting the V2 Apex of the HIV Envelope Trimer in a Wild-Type Animal Model. Cell Rep. 2017, 21, 222–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerman, M.E.; Barouch, D.H.; Alter, G. Systems serology for evaluation of HIV vaccine trials. Immunol. Rev. 2017, 275, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.W.; Kumar, M.P.; Arnold, K.B.; Yu, W.H.; Schoen, M.K.; Dunphy, L.J.; Suscovich, T.J.; Frahm, N.; Linde, C.; Mahan, A.E.; et al. Dissecting Polyclonal Vaccine-Induced Humoral Immunity against HIV Using Systems Serology. Cell 2015, 163, 988–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, N.L.; de Camp, A.C.; Korber, B.T.; Liao, H.X.; Irene, C.; Pinter, A.; Peacock, J.; Harris, L.J.; Sawant, S.; Hraber, P.; et al. HIV-1 Envelope Glycoproteins from Diverse Clades Differentiate Antibody Responses and Durability among Vaccinees. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, R.L.R.; Totrov, M.; Itri, V.; Liu, X.; Fox, A.; Zolla-Pazner, S. Plasticity and Epitope Exposure of the HIV-1 Envelope Trimer. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Curlin, M.E.; Zioni, R.; Hawes, S.E.; Liu, Y.; Deng, W.; Gottlieb, G.S.; Zhu, T.; Mullins, J.I. HIV-1 envelope subregion length variation during disease progression. PLoS Pathog. 2010, 6, e1001228. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, M.J.; Bunnik, E.M.; Boeser-Nunnink, B.D.; Burger, J.A.; Terlouw-Klein, M.; Verwer, N.; Schuitemaker, H. Longer V1V2 region with increased number of potential N-linked glycosylation sites in the HIV-1 envelope glycoprotein protects against HIV-specific neutralizing antibodies. J. Virol. 2011, 85, 6986–6995. [Google Scholar] [CrossRef]
- McGuire, E.P.; Fong, Y.; Toote, C.; Cunningham, C.K.; McFarland, E.J.; Borkowsky, W.; Barnett, S.; Itell, H.L.; Kumar, A.; Gray, G.; et al. HIV-Exposed Infants Vaccinated with an MF59/Recombinant gp120 Vaccine Have Higher-Magnitude Anti-V1V2 IgG Responses than Adults Immunized with the Same Vaccine. J. Virol. 2017, 92. [Google Scholar] [CrossRef]
- Permar, S.R.; Fong, Y.Y.; Vandergrift, N.; Fouda, G.G.; Gilbert, P.; Parks, R.; Jaeger, F.H.; Pollara, J.; Martelli, A.; Liebl, B.E.; et al. Maternal HIV-1 envelope-specific antibody responses and reduced risk of perinatal transmission. J. Clin. Investig. 2015, 125, 270–2706. [Google Scholar] [CrossRef]
- Trinh, H.V.; Gohain, N.; Pham, P.T.; Hamlin, C.; Song, H.; Sanders-Buell, E.; Bose, M.; Eller, L.A.; Jain, S.; Uritskiy, G.; et al. Humoral Response to the HIV-1 Envelope V2 Region in a Thai Early Acute Infection Cohort. Cells 2019, 8. [Google Scholar] [CrossRef]
- Munro, J.B.; Gorman, J.; Ma, X.; Zhou, Z.; Arthos, J.; Burton, D.R.; Koff, W.C.; Courter, J.R.; Smith, A.B., 3rd; Kwong, P.D.; et al. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science 2014, 346, 759–763. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Ma, X.; Castillo-Menendez, L.R.; Gorman, J.; Alsahafi, N.; Ermel, U.; Terry, D.S.; Chambers, M.; Peng, D.; Zhang, B.; et al. Associating HIV-1 envelope glycoprotein structures with states on the virus observed by smFRET. Nature 2019, 568, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Prevost, J.; Richard, J.; Ding, S.; Pacheco, B.; Charlebois, R.; Hahn, B.H.; Kaufmann, D.E.; Finzi, A. Envelope glycoproteins sampling states 2/3 are susceptible to ADCC by sera from HIV-1-infected individuals. Virology 2018, 515, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.S.; Madiga, M.C.; Hermanus, T.; Moore, P.L.; Wibmer, C.K.; Tumba, N.L.; Werner, L.; Mlisana, K.; Sibeko, S.; Williamson, C.; et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 2011, 85, 4828–4840. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.P.; Carrington, M. Immunogenetics of HIV disease. Immunol. Rev. 2013, 254, 245–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolland, M.; Gilbert, P. Evaluating immune correlates in HIV type 1 vaccine efficacy trials: What RV144 may provide. AIDS Res. Hum. Retrovir. 2012, 28, 400–404. [Google Scholar] [CrossRef] [PubMed]



| V2 Ab Class/Effector Functions 1 | V2qt | V2q | V2i | V2p |
|---|---|---|---|---|
| Neutralization | +++ | +++ | +(+) | (+) |
| viral capture | +++ | +++ | +(+) | + |
| ADCC | + | + | + | + |
| ADCP | + | + | + | n.a. |
| α4β7 inhibition | − | − | (+) | ++ |
| # | Trial | Vaccine | Year | Location/Clades/Target Population | Study Number | Ref | Result/Protection | Immune Correlates | V2 Ab Response |
|---|---|---|---|---|---|---|---|---|---|
| I | VAX003 | AIDSVAX B/01_AE Gp120 | 2003 | Thailand IDU | 2546 | [118] | No efficacy | - | Yes, RV144-like, peak Ab responses after 3–4 immunizations, but waning after 5–7 immunizations; relative enhancement of IgG2 and IgG4 responses with limited antiviral functionality |
| II | VAX004 | AIDSVAX B/B Gp120 | 2003 | USA, Canada, Puerto Rico, Netherlands MSM | 5417 | [119] | No efficacy | - | Yes, lower frequency and titers compared to VAX003 and RV144 |
| III | STEP | MRK-Ad5 B gag, pol, nef (T-cell based) | 2007 | North America, the Caribbean, South America, Australia MSM | 3000 | [116] | No efficacy; Immunizations halted; potential for increased risk of HIV infection among Ad5-seropositive, uncircumcised men. | - | No (no Env in vaccine) |
| IV | Phambili | MRK-Ad5 B gag, pol, nef (T-cell based) | 2007 | South Africa heterosexual | 801 (3000 were planned) | [117] | Immunizations halted after eight months based on STEP trial result. No efficacy at this point | - | No (no Env in vaccine) |
| V | Thai Prime-Boost/ RV 144 | ALVAC-HIV (vCP1521) 01_AE (TH023), AIDSVAX gp120/alum B/01_AE (MN/A244) | 2009 | Thailand Heterosexual, high risk | 16,402 | [2,3,7] | Yes, 31.2% efficacy after 3.5 y (60.5% after 1 y) | High titers of V2i and V2p Abs, ADCC in combination with low Env-specific IgA in plasma, viruses with K169 and mismatch at I181 | Yes, strong IgG1 and IgG3 responses associated with polyfunctional responses; strong immunogenicity of 01_AE strain A244 |
| VI | HVTN 505 | DNA gag, pol, nef, env A/B/C, rAd5 gag-pol B, env A/B/C | 2013 | USA MSM | 2500 | [120] | No efficacy; Immunizations halted; no prevention of HIV infection nor reduction of viral load among vaccine recipients who became HIV infected. | - | Low titers and frequency |
| VII | HVTN702 (The Uhambo Study) | RV144-like, ALVAC-HIV (vCP2438) C (96ZM651), bivalent gp120/MF59 C (TV1 and 1086) | Ongoing, 2016–2021 | South Africa adults | 5400 | https://clinicaltrials.gov/ct2/show/NCT02968849 | |||
| VIII | HVTN 705/HPX2008 (The Imbokodo Study) | Ad26 Mosaic (4x) HIV (gag, pol, env), gp140/alum protein C | Ongoing, 2017–2022 | South Africa women | 2600 | https://clinicaltrials.gov/ct2/show/NCT03060629 | |||
| # | Author | Immunization | Challenge | % Protection | Immune Correlates | V2 Antigens Tested | V2 Abs Correlation |
|---|---|---|---|---|---|---|---|
| HIV Env | |||||||
| 1 | Barouch DH Cell, 2013 | Ad/MVA (mosaic) | SHIVSF162P3 | 3 chall. 45% 6 chall. 18% | Env Abs Neutral SF162, ADCP | V2 peptides V1V2-gp70 | NO |
| 2 | Bradley T Nat Comm, 2017 | ALVAC/Pentavalent (B and AE clade) | SHIV1157 (clade C) | 55% | Cell-bound Env Abs, ADCC MIP-1b in NK cells | V2 peptides | NO |
| 3 | Barouch DH Lancet 2018 | Ad26, gp140 mosaic | SHIVSF162P3 | 67% | Env Abs T-cell response | V1V2-gp70 | NO |
| 4 | Malherbe DC J. Virol., 2018 | Replicating SAd7 Non-replicating Ad4 (1086, clade C) | SHIV157ipEL | Sad—40% Ad4—30% | V2 Abs (SAd7) | V1V2 recombinant (1086.C, JRFL, AE244, 14/00/4) | YES—SAd7 NO—Ad4 |
| 5 | Hessell A/Gorny MK (Keystone abstract 2019) | DNA gp160, AE gp120, clade AE, B | SHIVBaL.P4 | 55% | SHIV capture Abs Neutral. HIV-SF162 | V1V2 scaffolds V2 peptides (CaseA2, AE244, 1086, ZM109) | NO |
| 6 | Hessell A/Gorny MK (Keystone abstract 2019) | DNA V1V2, AE V1V2 scaffolds, AE, B | SHIVBaL.P4 | 45% | Not determined yet | V1V2 scaffolds V2 peptides (CaseA2, AE244, 1086, ZM109) | NO |
| SIV Env | |||||||
| 7 | Barouch DH Nature, 2012 | Ad/poxvirus SIVsmE543 | SIVmac251 grown in human cells | 80% | Env Abs | V2 peptide | YES |
| 8 | Roederer M Nature 2014 | DNA/Ad5 SIVmac239 | SIVmac660 | Vaccine efficacy: 69% (mac239) | Env Abs (C3, CD4bs) Neutralization | V1V2mac239 | YES |
| 9 | Singh S. J. Virol. 2018 | DNA, gp120 SIVmac251 | SIVsmE660 | 0% | Neutral. SIVsm660 T cells response | V1V2-gp70 SIVmac251, smE660 | YES Mucosal V2 Abs |
| 10 | Pegu P. J. Virol. 2013 | ALVAC, gp120 SIVmac251 | SIVmac251 | 27% (3 of 11) | Env Abs avidity | V2 peptides SIVmac251 | YES |
| 11 | Gordon SN. J. Immunol. 2014 | HPV, ALVAC, gp120 SIVmac251 | SIVmac251 | 25% | Env-T cells | V1V2 mini protein SIVmac239 | YES |
| 12 | Gordon SN J. Immunol. 2016 | ALVAC, gp120 SIVmac251 | SIVmac251 | 44% | Only V2 Abs | V1V2-gp70 V2 peptides SIVmac251, smE543 | YES mucosal V2 Abs-Yes serum V2 Abs-No |
| 13 | Kwa S J. Virol. 2015 | CD40L DNA MVA SIVmac239 | SIVmac251 | 50% | V2p Abs, gp41 Abs, V1 Abs, gut CD8 T cells | V2 peptides | YES Serum V2p Abs |
| 14 | Vaccari M. Nat Med, 2016 | ALVAC, gp120 SIVmac251 (alum, MF59) | SIVmac251 | 44% (alum) 0% (MF59) | Mucosal NKp44+IL17 (alum) | V1V2-gp70 V2 peptides SIVmac239, 251, smE660 | YES (Alum, mucosal V2) NO (MF59, mucosal V2 increased risk) |
| 15 | Vaccari M Nat Med, 2018 | ALVAC, DNA, Ad26 +gp120 SIVmac251, smE660 | SIVmac251 | 52% DNA and ALVAC | Activation CD14 monocytes | V2 peptides SIVmac251, smE543 | YES |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duerr, R.; Gorny, M.K. V2-Specific Antibodies in HIV-1 Vaccine Research and Natural Infection: Controllers or Surrogate Markers. Vaccines 2019, 7, 82. https://doi.org/10.3390/vaccines7030082
Duerr R, Gorny MK. V2-Specific Antibodies in HIV-1 Vaccine Research and Natural Infection: Controllers or Surrogate Markers. Vaccines. 2019; 7(3):82. https://doi.org/10.3390/vaccines7030082
Chicago/Turabian StyleDuerr, Ralf, and Miroslaw K. Gorny. 2019. "V2-Specific Antibodies in HIV-1 Vaccine Research and Natural Infection: Controllers or Surrogate Markers" Vaccines 7, no. 3: 82. https://doi.org/10.3390/vaccines7030082
APA StyleDuerr, R., & Gorny, M. K. (2019). V2-Specific Antibodies in HIV-1 Vaccine Research and Natural Infection: Controllers or Surrogate Markers. Vaccines, 7(3), 82. https://doi.org/10.3390/vaccines7030082