Nitric Oxide Production and Fc Receptor-Mediated Phagocytosis as Functional Readouts of Macrophage Activity upon Stimulation with Inactivated Poultry Vaccines In Vitro
Abstract
:1. Introduction
2. Materials and Methods
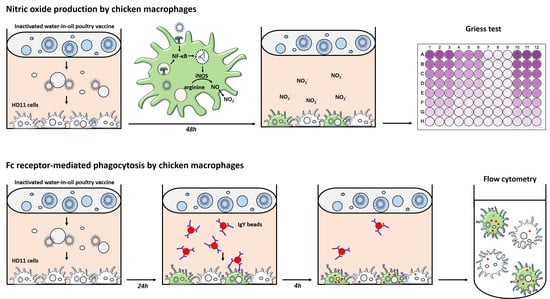
2.1. HD11 Cell Culture and Stimulation
2.2. Griess Assay to Measure Nitric Oxide Production by HD11 Cells
2.3. Phagocytosis of IgY-Opsonized Beads by HD11 Cells
2.3.1. IgY-Opsonization of Fluorescent Beads
2.3.2. Phagocytosis by HD11 Cells
2.3.3. Flow Cytometric Side Scatter to Determine Vaccine Decomposition
2.3.4. Involvement of the Fc Receptor CHIR-AB1 in IgY-Opsonized Bead Uptake by HD11 Cells
2.4. Confocal Microscopy to Assess Internalization of IgY-Opsonized Beads by HD11 Cells
2.5. Statistical Analysis
3. Results
3.1. High Concentrations of Inactivated Poultry Vaccines Induce Nitric Oxide Production by HD11 Cells
3.2. Phagocytosis of IgY-Opsonized Beads by HD11 Cells Is Enhanced upon Stimulation with TLR Agonists
3.3. Allantoic Fluid-Containing Inactivated IBV and NDV Antigens Enhance Phagocytosis by HD11 Cells
3.4. Inactivated IBV and NDV Vaccines Can Enhance Phagocytosis by HD11 Cells
3.5. Fc Receptor CHIR-AB1 Is Responsible for the Enhancement of IgY-Opsonized Bead Uptake by HD11 Cells upon Exposure to Inactivated IBV Antigen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Goddard, R.D.; Nicholas, R.A.J.; Luff, P.R. Serology-based potency test for inactivated Newcastle disease vaccines. Vaccine 1988, 6, 530–532. [Google Scholar] [CrossRef]
- EDQM. European Pharmacopoeia, 10th ed.; European Department for the Quality of Medicines: Strasbourgh, France, 2020. [Google Scholar]
- Schutte, K.; Szczepanska, A.; Halder, M.; Cussler, K.; Sauer, U.G.; Stirling, C.; Uhlrich, S.; Wilk-Zasadna, I.; John, D.; Bopst, M.; et al. Modern science for better quality control of medicinal products “Towards global harmonization of 3Rs in biologicals”: The report of an EPAA workshop. Biologicals 2017, 48, 55–65. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. DIRECTIVE 2010/63/EU on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- Hendriksen, C.; Arciniega, J.L.; Bruckner, L.; Chevalier, M.; Coppens, E.; Descamps, J.; Duchêne, M.; Dusek, D.M.; Halder, M.; Kreeftenberg, H.; et al. The consistency approach for the quality control of vaccines. Biologicals 2008, 36, 73–77. [Google Scholar] [CrossRef]
- Hendriksen, C.F. Replacement, reduction and refinement alternatives to animal use in vaccine potency measurement. Expert Rev. Vaccines 2009, 8, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Bruysters, M.W.P.; Schiffelers, M.J.; Hoonakker, M.; Jungbaeck, C.; Ragan, I.; Rommel, E.; van der Stappen, T.; Viviani, L.; Hessel, E.V.; Akkermans, A.M.; et al. Drivers and barriers in the consistency approach for vaccine batch release testing: Report of an international workshop. In Biologicals; Academic Press: Cambridge, MA, USA, 2017; pp. 1–5. [Google Scholar]
- Claassen, I.; Maas, R.; Oei, H.; Daas, A.; Milne, C. Validation study to evaluate the reproducibility of a candidate in vitro potency assay of newcastle disease vaccines and to establish the suitability of a candidate biological reference preparation. Pharmeuropa Bio 2004, 2004, 1–15. [Google Scholar]
- Maas, P.A.; de Winter, M.P.M.; Venema, S.; Oei, H.L.; Claassen, I.J.T.M. Antigen quantification as in vitro alternative for potency testing of inactivated viral poultry vaccines. Vet. Q. 2000, 4, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Maas, R.; Venema, S.; Kant, A.; Oei, H.; Claassen, I. Quantification of infectious bursal disease viral proteins 2 and 3 in inactivated vaccines as an indicator of serological response and measure of potency. Avian Pathol. 2004, 33, 126–132. [Google Scholar] [CrossRef]
- Stoel, M.; Pool, J.; de Vries-Idema, J.; Zaaraoui-Boutahar, F.; Bijl, M.; Andeweg, A.C.; Wilschut, J.; Huckriede, A. Innate responses induced by whole inactivated virus or subunit influenza vaccines in cultured dendritic cells correlate with immune responses in vivo. PLoS ONE 2015, 10, e0125228. [Google Scholar] [CrossRef] [Green Version]
- Hoonakker, M.E.; Verhagen, L.M.; Hendriksen, C.F.M.; van Els, C.A.C.M.; Vandebriel, R.J.; Sloots, A.; Han, W.G.H. In vitro innate immune cell based models to assess whole cell Bordetella pertussis vaccine quality: A proof of principle. Biologicals 2015, 43, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Marichal, T.; Ohata, K.; Bedoret, D.; Mesnil, C.; Sabatel, C.; Kobiyama, K.; Lekeux, P.; Coban, C.; Akira, S.; Ishii, K.J.; et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 2011, 17, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Momota, M.; Kuroda, E.; Kusakabe, T.; Kobari, S.; Makisaka, K.; Ohno, Y.; Suzuki, Y.; Nakagawa, F.; Lee, M.S.J.; et al. DAMP-inducing adjuvant and PAMP adjuvants parallelly enhance protective type-2 and type-1 immune responses to influenza split vaccination. Front. Immunol. 2018, 9, 02619. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Philbin, V.J.; Smith, A.L. Expression patterns of chicken Toll-like receptor mRNA in tissues, immune cell subsets and cell lines. Vet. Immunol. Immunopathol. 2005, 104, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Crippen, T.L. The selective inhibition of nitric oxide production in the avian macrophage cell line HD11. Vet. Immunol. Immunopathol. 2006, 109, 127–137. [Google Scholar] [CrossRef]
- He, H.; Genovese, K.J.; Nisbet, D.J.; Kogut, M.H. Synergy of CpG oligodeoxynucleotide and double-stranded RNA (poly I:C) on nitric oxide induction in chicken peripheral blood monocytes. Mol. Immunol. 2007, 44, 3234–3242. [Google Scholar] [CrossRef]
- Peroval, M.Y.; Boyd, A.C.; Young, J.R.; Smith, A.L.; Muller, M. A Critical Role for MAPK Signalling Pathways in the Transcriptional Regulation of Toll Like Receptors. PLoS ONE 2013, 8, e51243. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.F.; Deng, M.C.; Tseng, L.P.; Jiang, P.R.; Jan, T.R.; Hsieh, F.I.; Liu, D.Z. Adjuvant effect of liposome in chicken result from induction of nitric oxide. Biomed. Mater. 2011, 6, 015011. [Google Scholar] [CrossRef]
- van Dijk, A.; van Eldik, M.; Veldhuizen, E.J.A.; Tjeerdsma-van Bokhoven, H.L.M.; de Zoete, M.R.; Bikker, F.J.; Haagsman, H.P. Immunomodulatory and Anti-Inflammatory Activities of Chicken Cathelicidin-2 Derived Peptides. PLoS ONE 2016, 11, e0147919. [Google Scholar] [CrossRef]
- He, H.; Genovese, K.J.; Kogut, M.H. Modulation of chicken macrophage effector function by T(H)1/T(H)2 cytokines. Cytokine 2011, 53, 363–369. [Google Scholar] [CrossRef]
- Haq, K.; Elawadli, I.; Parvizi, P.; Mallick, A.I.; Behboudi, S.; Sharif, S. Interferon-γ influences immunity elicited by vaccines against very virulent Marek’s disease virus. Antivir. Res. 2011, 90, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Sekellick, M.J.; Lowenthal, J.W.; O’Neil, T.E.; Marcus, P.I. Chicken interferon types I and II enhance synergistically the antiviral state and nitric oxide secretion. J. Interf. Cytokine Res. 1998, 18, 407–414. [Google Scholar] [CrossRef]
- He, H.; Genovese, K.J.; Swaggerty, C.L.; Nisbet, D.J.; Kogut, M.H. A Comparative Study on Invasion, Survival, Modulation of Oxidative Burst, and Nitric Oxide Responses of Macrophages (HD11), and Systemic Infection in Chickens by Prevalent Poultry Salmonella Serovars. Foodborne Pathog. Dis. 2012, 9, 1104–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Ding, Z.; Liu, X.; Chen, Y.; Li, J.; Tao, Z.; Fei, Y.; Xue, C.; Qian, J.; Wang, X.; et al. Enhanced replication of virulent Newcastle disease virus in chicken macrophages is due to polarized activation of cells by inhibition of TLR7. Front. Immunol. 2018, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Wu, C.C.; Lin, T.L. Role of chicken melanoma differentiation-associated gene 5 in induction and activation of innate and adaptive immune responses to infectious bursal disease virus in cultured macrophages. Arch. Virol. 2015, 160, 3021–3035. [Google Scholar] [CrossRef]
- Qi, X.; Liu, C.; Li, R.; Zhang, H.; Xu, X.; Wang, J. Modulation of the innate immune-related genes expression in H9N2 avian influenza virus-infected chicken macrophage-like cells (HD11) in response to Escherichia coli LPS stimulation. Res. Vet. Sci. 2017, 111, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Viertlboeck, B.C.; Schweinsberg, S.; Hanczaruk, M.A.; Schmitt, R.; Du Pasquier, L.; Herberg, F.W.; Göbel, T.W. The chicken leukocyte receptor complex encodes a primordial, activating, high-affinity IgY Fc receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 11718–11723. [Google Scholar] [CrossRef] [Green Version]
- de Geus, E.D.; Jansen, C.A.; Vervelde, L. Uptake of particulate antigens in a nonmammalian lung: Phenotypic and functional characterization of avian respiratory phagocytes using bacterial or viral antigens. J. Immunol. 2012, 188, 4516–4526. [Google Scholar] [CrossRef] [Green Version]
- Naghizadeh, M.; Wattrang, E.; Kjærup, R.B.; Bakke, M.; Shih, S.; Dalgaard, T.S. In vitro phagocytosis of opsonized latex beads by HD11 cells as a method to assess the general opsonization potential of chicken serum. Avian Pathol. 2018, 47, 479–488. [Google Scholar] [CrossRef]
- Grabowska, J.; Lopez-Venegas, M.A.; Affandi, A.J.; Den Haan, J.M.M. CD169+ macrophages capture and dendritic cells instruct: The interplay of the gatekeeper and the general of the immune system. Front. Immunol. 2018, 9, 2472. [Google Scholar] [CrossRef] [Green Version]
- de Geus, E.D.; Vervelde, L. Regulation of macrophage and dendritic cell function by pathogens and through immunomodulation in the avian mucosa. Dev. Comp. Immunol. 2013, 41, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Canthaboo, C.; Xing, D.; Wei, X.Q.; Corbel, M.J. Investigation of role of nitric oxide in protection from Bordetella pertussis respiratory challenge. Infect. Immun. 2002, 70, 679–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quattrocchi, V.; Langellotti, C.; Pappalardo, J.S.; Olivera, V.; Di Giacomo, S.; van Rooijen, N.; Mongini, C.; Waldner, C.; Zamorano, P.I. Role of macrophages in early protective immune responses induced by two vaccines against foot and mouth disease. Antivir. Res. 2011, 92, 262–270. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, C.J.; Mullarkey, C.E.; Hamilton, J.R.; Wong, C.K.; Leon, P.E.; Uccellini, M.B.; Chromikova, V.; Henry, C.; Hoffman, K.W.; et al. Alveolar macrophages are critical for broadly-reactive antibody-mediated protection against influenza A virus in mice. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Beug, H.; von Kirchbach, A.; Döderlein, G.; Conscience, J.-F.; Graf, T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell 1979, 18, 375–390. [Google Scholar] [CrossRef]
- Ariaans, M.P.; Matthijs, M.G.R.; van Haarlem, D.; van de Haar, P.; van Eck, J.H.H.; Hensen, E.J.; Vervelde, L. The role of phagocytic cells in enhanced susceptibility of broilers to colibacillosis after Infectious Bronchitis Virus infection. Vet. Immunol. Immunopathol. 2008, 123, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Stringer, B.; Imrich, A.; Kobzik, L. Flow cytometric assay of lung macrophage uptake of environmental particulates. Cytometry 1995, 20, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Stils, H.F. Adjuvants and Antibody Production: Dispelling the Myths Associated with Freund’s Complete and Other Adjuvants. ILAR J. 2005, 46, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Philbin, V.J.; Iqbal, M.; Boyd, Y.; Goodchild, M.J.; Beal, R.K.; Bumstead, N.; Young, J.; Smith, A.L. Identification and characterization of a functional, alternatively spliced Toll-like receptor 7 (TLR7) and genomic disruption of TLR8 in chickens. Immunology 2005, 114, 507–521. [Google Scholar] [CrossRef]
- Brownlie, R.; Zhu, J.; Allan, B.; Mutwiri, G.K.; Babiuk, L.A.; Potter, A.; Griebel, P. Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Mol. Immunol. 2009, 46, 3163–3170. [Google Scholar] [CrossRef]
- Herrera-Rodriguez, J.; Signorazzi, A.; Holtrop, M.; de Vries-Idema, J.; Huckriede, A. Inactivated or damaged? Comparing the effect of inactivation methods on influenza virions to optimize vaccine production. Vaccine 2019, 37, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Geeraedts, F.; Goutagny, N.; Hornung, V.; Severa, M.; de Haan, A.; Pool, J.; Wilschut, J.; Fitzgerald, K.A.; Huckriede, A. Superior Immunogenicity of Inactivated Whole Virus H5N1 Influenza Vaccine is Primarily Controlled by Toll-like Receptor Signalling. PLoS Pathog. 2008, 4, e1000138. [Google Scholar] [CrossRef] [PubMed]
- Bolin, G.; Burggren, W.W. Metanephric kidney development in the chicken embryo: Glomerular numbers, characteristics and perfusion. Comp. Biochem. Physiol A Mol. Integr. Physiol. 2013, 166, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Marrack, P.; McKee, A.S.; Munks, M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009, 9, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depelsenaire, A.C.I.; Meliga, S.C.; McNeilly, C.L.; Pearson, F.E.; Coffey, J.W.; Haigh, O.L.; Flaim, C.J.; Frazer, I.H.; Kendall, M.A.F. Colocalization of cell death with antigen deposition in skin enhances vaccine immunogenicity. J. Investig. Dermatol. 2014, 134, 2361–2370. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-W.; Shen, S.-S. Enhanced antigen delivery via cell death induced by the vaccine adjuvants. Vaccine 2007, 25, 7763–7772. [Google Scholar] [CrossRef]
- Behrens, M.D.; Wagner, W.M.; Krco, C.J.; Erskine, C.L.; Kalli, K.R.; Krempski, J.; Gad, E.A.; Disis, M.L.; Knutson, K.L. The endogenous danger signal, crystalline uric acid, signals for enhanced antibody immunity. Blood 2008, 111, 1472–1479. [Google Scholar] [CrossRef] [Green Version]
- Droual, R.; Bickford, A.A.; Charlton, B.R.; Kuney, D.R. Investigation of problems associated with intramuscular breast injection of oil-adjuvanted killed vaccines in chickens. Avian Dis. 1990, 34, 473–478. [Google Scholar] [CrossRef]
- Yamanaka, M.; Okabe, T.; Nakai, M.; Goto, N. Local pathological reactions and immune response of chickens to ISA-70 and other adjuvants containing Newcastle disease virus antigen. Avian Dis. 1993, 37, 459–466. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Biggelaar, R.H.G.A.; van Eden, W.; Rutten, V.P.M.G.; Jansen, C.A. Nitric Oxide Production and Fc Receptor-Mediated Phagocytosis as Functional Readouts of Macrophage Activity upon Stimulation with Inactivated Poultry Vaccines In Vitro. Vaccines 2020, 8, 332. https://doi.org/10.3390/vaccines8020332
van den Biggelaar RHGA, van Eden W, Rutten VPMG, Jansen CA. Nitric Oxide Production and Fc Receptor-Mediated Phagocytosis as Functional Readouts of Macrophage Activity upon Stimulation with Inactivated Poultry Vaccines In Vitro. Vaccines. 2020; 8(2):332. https://doi.org/10.3390/vaccines8020332
Chicago/Turabian Stylevan den Biggelaar, Robin H.G.A., Willem van Eden, Victor P.M.G. Rutten, and Christine A. Jansen. 2020. "Nitric Oxide Production and Fc Receptor-Mediated Phagocytosis as Functional Readouts of Macrophage Activity upon Stimulation with Inactivated Poultry Vaccines In Vitro" Vaccines 8, no. 2: 332. https://doi.org/10.3390/vaccines8020332
APA Stylevan den Biggelaar, R. H. G. A., van Eden, W., Rutten, V. P. M. G., & Jansen, C. A. (2020). Nitric Oxide Production and Fc Receptor-Mediated Phagocytosis as Functional Readouts of Macrophage Activity upon Stimulation with Inactivated Poultry Vaccines In Vitro. Vaccines, 8(2), 332. https://doi.org/10.3390/vaccines8020332