Effect of Bridgehead Methyl Substituents on the Gas Permeability of Tröger’s-Base Derived Polymers of Intrinsic Microporosity
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods and Equipment
2.2. Gas Permeation Measurements
3. Results and Discussion
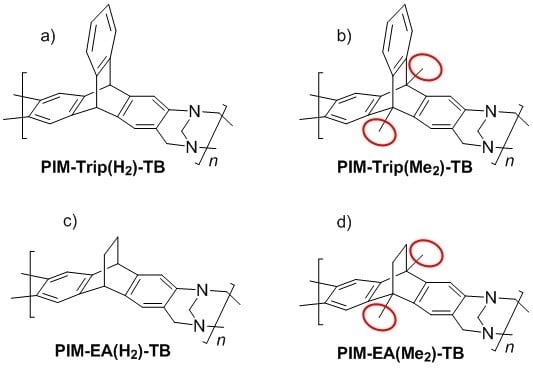
3.1. Polymer Synthesis and Characterisation
3.2. Membrane Preparation and Gas Permeability Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Buonomenna, M.G. Membrane processes for a sustainable industrial growth. RSC Adv. 2013, 3, 5694–5740. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Recent Development of Polymer Electrolyte Membranes for Fuel Cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef] [PubMed]
- Chaoui, N.; Trunk, M.; Dawson, R.; Schmidt, J.; Thomas, A. Trends and challenges for microporous polymers. Chem. Soc. Rev. 2017, 46, 3302–3321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Adewole, J.K.; Ahmad, A.L.; Ismail, S.; Leo, C.P. Current challenges in membrane separation of CO2 from natural gas: A review. Int. J. Greenh. Gas Control 2013, 17, 46–65. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural Gas Processing with Membranes: An Overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef] [Green Version]
- Yampolskii, Y. Polymeric Gas Separation Membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Shao, L.; Low, B.T.; Chung, T.-S.; Greenberg, A.R. Polymeric membranes for the hydrogen economy: Contemporary approaches and prospects for the future. J. Membr. Sci. 2009, 327, 18–31. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Nenoff, T.M. Membranes for Hydrogen Separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Anil, A.G.; James, A.; Patra, A. Multifunctional Porous Organic Polymers: Tuning of Porosity, CO2, and H2 Storage and Visible-Light-Driven Photocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 27669–27678. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Park, H.B.; Dal-Cin, M.M.; Guiver, M.D. Advances in high permeability polymeric membrane materials for CO2 separations. Energy Environ. Sci. 2012, 5, 7306–7322. [Google Scholar] [CrossRef] [Green Version]
- Merkel, T.C.; Zhou, M.; Baker, R.W. Carbon dioxide capture with membranes at an IGCC power plant. J. Membr. Sci. 2012, 389, 441–450. [Google Scholar] [CrossRef]
- Lau, C.H.; Nguyen, P.T.; Hill, M.R.; Thornton, A.W.; Konstas, K.; Doherty, C.M.; Mulder, R.J.; Bourgeois, L.; Liu, A.C.Y.; Sprouster, D.J.; et al. Ending Aging in Super Glassy Polymer Membranes. Angew. Chem. Int. Ed. 2014, 53, 5322–5326. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, S.; Xin, Q.; Li, Y. Perspectives on water-facilitated CO2 capture materials. J. Mater. Chem. A 2017, 5, 6794–6816. [Google Scholar] [CrossRef]
- Roussanaly, S.; Anantharaman, R. Cost-optimal CO2 capture ratio for membrane-based capture from different CO2 sources. Chem. Eng. J. 2017, 327, 618–628. [Google Scholar] [CrossRef] [Green Version]
- Sabetghadam, A.; Liu, X.; Orsi, A.F.; Lozinska, M.M.; Johnson, T.; Jansen, K.M.; Wright, P.A.; Carta, M.; McKeown, N.B.; Kapteijn, F. Towards High Performance Metal–Organic Framework–Microporous Polymer Mixed Matrix Membranes: Addressing Compatibility and Limiting Aging by Polymer Doping. Chem. A Eur. J. 2018, 24, 12796–12800. [Google Scholar] [CrossRef]
- Esposito, E.; Dellamuzia, L.; Moretti, U.; Fuoco, A.; Giorno, L.; Jansen, J.C. Simultaneous production of biomethane and food grade CO2 from biogas: An industrial case study. Energy Environ. Sci. 2019, 12, 281–289. [Google Scholar] [CrossRef]
- Budd, P.M.; McKeown, N.B. Highly permeable polymers for gas separation membranes. Polym. Chem. 2010, 1, 63–68. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-Tuned Intrinsically Ultramicroporous Polymers Redefine the Permeability/Selectivity Upper Bounds of Membrane-Based Air and Hydrogen Separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef] [Green Version]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.-C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef] [Green Version]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. (Cambridge, UK) 2004, 2, 230–231. [Google Scholar] [CrossRef]
- Carta, M.; Bernardo, P.; Clarizia, G.; Jansen, J.C.; McKeown, N.B. Gas Permeability of Hexaphenylbenzene file Based Polymers of Intrinsic Microporosity. Macromolecules 2014, 47, 8320–8327. [Google Scholar] [CrossRef]
- Bezzu, C.G.; Carta, M.; Tonkins, A.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. A Spirobifluorene-Based Polymer of Intrinsic Microporosity with Improved Performance for Gas Separation. Adv. Mater. 2012, 24, 5930–5933. [Google Scholar] [CrossRef]
- Carta, M.; Croad, M.; Malpass-Evans, R.; Jansen, J.C.; Bernardo, P.; Clarizia, G.; Friess, K.; Lanč, M.; McKeown, N.B. Triptycene Induced Enhancement of Membrane Gas Selectivity for Microporous Tröger’s Base Polymers. Adv. Mater. 2014, 26, 3526–3531. [Google Scholar] [CrossRef] [Green Version]
- Fuoco, A.; Comesaña-Gándara, B.; Longo, M.; Esposito, E.; Monteleone, M.; Rose, I.; Bezzu, C.G.; Carta, M.; McKeown, N.B.; Jansen, J.C. Temperature Dependence of Gas Permeation and Diffusion in Triptycene-Based Ultrapermeable Polymers of Intrinsic Microporosity. ACS Appl. Mater. Interfaces 2018, 10, 36475–36482. [Google Scholar] [CrossRef] [Green Version]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Lee, M.; Rose, I.; McKeown, N.B. The synthesis of microporous polymers using Troger’s base formation. Polym. Chem. 2014, 5, 5267–5272. [Google Scholar] [CrossRef] [Green Version]
- Carta, M.; Croad, M.; Jansen, J.C.; Bernardo, P.; Clarizia, G.; McKeown, N.B. Synthesis of cardo-polymers using Troger’s base formation. Polym. Chem. 2014, 5, 5255–5261. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.; Burt, L.A.; Esposito, E.; Jansen, J.C.; Tocci, E.; Rizzuto, C.; Lanč, M.; Carta, M.; McKeown, N.B. A highly rigid and gas selective methanopentacene-based polymer of intrinsic microporosity derived from Tröger’s base polymerization. J. Mater. Chem. A 2018, 6, 5661–5667. [Google Scholar] [CrossRef] [Green Version]
- Rose, I.; Carta, M.; Malpass-Evans, R.; Ferrari, M.-C.; Bernardo, P.; Clarizia, G.; Jansen, J.C.; McKeown, N.B. Highly permeable benzotriptycene-based polymer of intrinsic microporosity. ACS Macro Lett. 2015, 4, 912–915. [Google Scholar] [CrossRef]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An Efficient Polymer Molecular Sieve for Membrane Gas Separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.; Scorzafave, V.; Clarizia, G.; Tocci, E.; Jansen, J.C.; Borgogno, A.; Malpass-Evans, R.; McKeown, N.B.; Carta, M.; Tasselli, F. Thin film composite membranes based on a polymer of intrinsic microporosity derived from Tröger’s base: A combined experimental and computational investigation of the role of residual casting solvent. J. Membr. Sci. 2019, 569, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Genduso, G.; Wang, Y.; Ghanem, B.S.; Pinnau, I. Permeation, sorption, and diffusion of CO2-CH4 mixtures in polymers of intrinsic microporosity: The effect of intrachain rigidity on plasticization resistance. J. Membr. Sci. 2019, 584, 100–109. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975; p. 414. [Google Scholar]
- Fraga, S.C.; Monteleone, M.; Lanč, M.; Esposito, E.; Fuoco, A.; Giorno, L.; Pilnáček, K.; Friess, K.; Carta, M.; McKeown, N.B.; et al. A novel time lag method for the analysis of mixed gas diffusion in polymeric membranes by on-line mass spectrometry: Method development and validation. J. Membr. Sci. 2018, 561, 39–58. [Google Scholar] [CrossRef]
- Benito, J.; Sánchez-Laínez, J.; Zornoza, B.; Martín, S.; Carta, M.; Malpass-Evans, R.; Téllez, C.; McKeown, N.B.; Coronas, J.; Gascón, I. Ultrathin Composite Polymeric Membranes for CO2/N2 Separation with Minimum Thickness and High CO2 Permeance. ChemSusChem 2017, 10, 4014–4017. [Google Scholar] [CrossRef] [Green Version]
- Tocci, E.; De Lorenzo, L.; Bernardo, P.; Clarizia, G.; Bazzarelli, F.; McKeown, N.B.; Carta, M.; Malpass-Evans, R.; Friess, K.; Pilnacek, K.; et al. Molecular Modeling and Gas Permeation Properties of a Polymer of Intrinsic Microporosity Composed of Ethanoanthracene and Troger’s Base Units. Macromolecules 2014, 47, 7900–7916. [Google Scholar] [CrossRef]
- Cristol, S.J.; Hause, N.L. Mechanisms of Elimination Reactions. V. Preparation and Elimination Reactions of cis- and trans-11,12-Dichloro-9,10-dihydro-9,10-ethanoanthracene1,2, 3. J. Am. Chem. Soc. 1952, 74, 2193–2197. [Google Scholar] [CrossRef]
- Naghipur, A.; Reszka, K.; Sapse, A.M.; Lown, J.W. Formation of benzoxathiete under mild conditions and its valence tautomerism in solution to monothio-o-benzoquinone: An experimental and quantum chemical study. J. Am. Chem. Soc. 1989, 111, 258–268. [Google Scholar] [CrossRef]
- Lanč, M.; Pilnáček, K.; Mason, C.R.; Budd, P.M.; Rogan, Y.; Malpass-Evans, R.; Carta, M.; Gándara, B.C.; McKeown, N.B.; Jansen, J.C.; et al. Gas sorption in polymers of intrinsic microporosity: The difference between solubility coefficients determined via time-lag and direct sorption experiments. J. Membr. Sci. 2019, 570–571, 522–536. [Google Scholar] [CrossRef]
- Fuoco, A.; Rizzuto, C.; Tocci, E.; Monteleone, M.; Esposito, E.; Budd, P.M.; Carta, M.; Comesaña-Gándara, B.; McKeown, N.B.; Jansen, J.C. The origin of size-selective gas transport through polymers of intrinsic microporosity. J. Mater. Chem. A 2019, 7, 20121–20126. [Google Scholar] [CrossRef]
- Teplyakov, V.; Meares, P. Correlation aspects of the selective gas permeabilities of polymeric materials and membranes. Gas Sep. Purif. 1990, 4, 66–74. [Google Scholar] [CrossRef]







| Polymer | BET Surface Area (N2 at 77 K) (m2g−1) | Total Pore Volume at (P/P0) = 0.9814 (cm3 g−1) | Mw × 103 (g mol−1) | PDI | CO2 Uptake (273 K, 1 bar) cc g−1 (mmol g−1) | Ref. |
|---|---|---|---|---|---|---|
| PIM-EA(Me2)-TB | 1028 | 0.75 | 156 | 3.8 | 79.3 (3.54) | [35] |
| PIM-Trip(Me2)-TB | 926 | 0.65 | 118 | 2.7 | 87.0 (3.88) | This work |
| PIM-EA(H2)-TB | 845 | 0.62 | 62 | 2.3 | 71.8 (3.20) | [36,40] |
| PIM-Trip(H2)-TB | 899 | 0.55 | 50 | 2.4 | 90.4 (4.03) | [29] |
| PIM | Transport Parameters | Gas Type | |||||
|---|---|---|---|---|---|---|---|
| N2 | O2 | CO2 | CH4 | H2 | He | ||
| Trip(Me2)-TB | Px [Barrer] | 255 | 1002 | 3718 | 347 | 5446 | 2178 |
| (aged) a) | (96) | (500) | (1880) | (156) | (2625) | (1134) | |
| Dx [10−12 m2 s−1] | 25 | 106 | 24 | 8 | 4393 | 7580 | |
| (aged) a) | (12.7) | (63.6) | (17.8) | (4.8) | (2487) | (4424) | |
| Sx [cm3 cm−3 bar−1] | 7.53 | 7.12 | 117 | 34.2 | 0.93 | 0.22 | |
| (aged) a) | (5.69) | (5.90) | (79) | (24.3) | (0.79) | (0.19) | |
| EA(Me2)-TB [35] | Px [Barrer] | 525 | 2150 | 7140 | 699 | 7760 | 2570 |
| (aged) b) | (188) | (933) | (2644) | (219) | (4442) | (1630) | |
| Dx [10−12 m2 s−1] | 99.5 | 318 | 87 | 36 | >7000 | >10000 | |
| (aged) b) | (22.9) | (104) | (35.2) | (6.9) | (4000) | (7700) | |
| Sx [cm3 cm−3 bar−1] | 3.96 | 5.07 | 61.5 | 14.56 | 0.83 | 0.19 | |
| (aged) b) | (6.16) | (6.73) | (56.3) | 23.81 | (0.83) | (0.16) | |
| EA(H2)-TB | Px [Barrer] | 358 | 1673 | 6097 | 458 | 6088 | 1938 |
| (aged) c) | (188) | (902) | (2999) | (196) | (4066) | (1367) | |
| Dx [10−12 m2 s−1] | 47.6 | 216 | 66.4 | 15.1 | 5635 | 7822 | |
| (aged) c) | (26.5) | (121) | (29.7) | (5.5) | (4074) | (6269) | |
| Sx [cm3 cm−3 bar−1] | 5.64 | 5.81 | 68.87 | 22.75 | 0.81 | 0.19 | |
| (aged) c) | (5.32) | (5.60) | (75.74) | (26.73) | (0.75) | (0.16) | |
| Trip(H2)-TB [29] | Px [Barrer] | 629 | 2718 | 9709 | 905 | 8039 | 2500 |
| (aged) d) | (189) | (1073) | (3951) | (218) | (4740) | (1585) | |
| Dx [10−12 m2 s−1] | 135 | 462 | 111 | 48.9 | 7800 | >10000 | |
| (aged) d) | (28.5) | (148) | (34.6) | (7.5) | (4920) | (7738) | |
| Sx [cm3 cm−3 bar−1] | 3.49 | 4.41 | 65.61 | 13.88 | 0.77 | 0.18 | |
| (aged) d) | (4.97) | (5.43) | (85.6) | (21.75) | (0.72) | (0.15) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malpass-Evans, R.; Rose, I.; Fuoco, A.; Bernardo, P.; Clarizia, G.; McKeown, N.B.; Jansen, J.C.; Carta, M. Effect of Bridgehead Methyl Substituents on the Gas Permeability of Tröger’s-Base Derived Polymers of Intrinsic Microporosity. Membranes 2020, 10, 62. https://doi.org/10.3390/membranes10040062
Malpass-Evans R, Rose I, Fuoco A, Bernardo P, Clarizia G, McKeown NB, Jansen JC, Carta M. Effect of Bridgehead Methyl Substituents on the Gas Permeability of Tröger’s-Base Derived Polymers of Intrinsic Microporosity. Membranes. 2020; 10(4):62. https://doi.org/10.3390/membranes10040062
Chicago/Turabian StyleMalpass-Evans, Richard, Ian Rose, Alessio Fuoco, Paola Bernardo, Gabriele Clarizia, Neil B. McKeown, Johannes C. Jansen, and Mariolino Carta. 2020. "Effect of Bridgehead Methyl Substituents on the Gas Permeability of Tröger’s-Base Derived Polymers of Intrinsic Microporosity" Membranes 10, no. 4: 62. https://doi.org/10.3390/membranes10040062
APA StyleMalpass-Evans, R., Rose, I., Fuoco, A., Bernardo, P., Clarizia, G., McKeown, N. B., Jansen, J. C., & Carta, M. (2020). Effect of Bridgehead Methyl Substituents on the Gas Permeability of Tröger’s-Base Derived Polymers of Intrinsic Microporosity. Membranes, 10(4), 62. https://doi.org/10.3390/membranes10040062