Development of Polydopamine Forward Osmosis Membranes with Low Reverse Salt Flux
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polydopamine Coating
2.3. Membrane Characterization
2.3.1. Field Emission Scanning Electron Microscopy (FESEM)
2.3.2. X-Ray Photoelectron Spectroscopy
2.3.3. Contact Angle Measurement
2.3.4. Zeta Potential Measurement
2.3.5. Membrane Performance Evaluation
Dead-End Membrane Filtration
Forward Osmosis Test
3. Results and Discussion
3.1. Surface Morphology by FESEM
3.1.1. Effect of Dopamine Concentration
3.1.2. Effect of Coating Temperature
3.2. Surface Chemistry
3.3. Surface Hydrophilicity
3.4. Surface Charge
3.5. Membrane Performance
3.5.1. Dead-End Filtration Performance
Effect of Dopamine Concentration
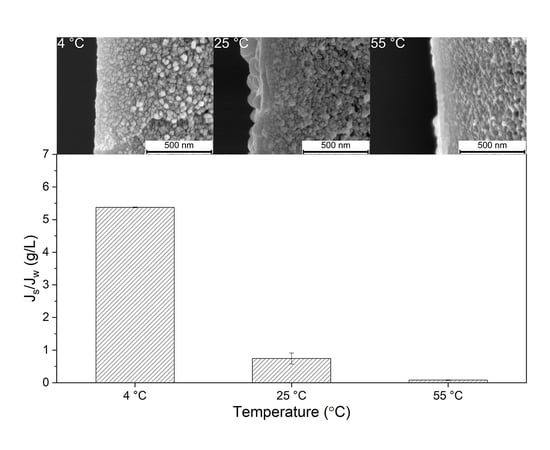
Effect of Coating Temperature
3.5.2. Forward Osmosis Performance
Effect of Dopamine Concentration
Effect of Coating Temperature
Performance Comparison with Commercial FO Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cath, T.; Childress, A.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.; Childress, A.E. Selection of inorganic-based draw solutions for forward osmosis applications. J. Membr. Sci. 2010, 364, 233–241. [Google Scholar] [CrossRef]
- Ibrar, I.; Naji, O.; Sharif, A.; Malekizadeh, A.; Hawari, A.H.; AlAnezi, A.A.; Altaee, A. A review of fouling mechanisms, control strategies and real-time fouling monitoring techniques in forward osmosis. Water 2019, 11, 695. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Hancock, N.T.; Cath, T. Solute coupled diffusion in osmotically driven membrane processes. Environ. Sci. Technol. 2009, 43, 6769–6775. [Google Scholar] [CrossRef] [PubMed]
- Lutchmiah, K.; Verliefde, A.; Roest, K.; Rietveld, L.; Cornelissen, E. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Yen, S.K.; Su, M.; Wang, K.Y.; Chung, T.-S. Study of draw solutes using 2-methylimidazole-based compounds in forward osmosis. J. Membr. Sci. 2010, 364, 242–252. [Google Scholar] [CrossRef]
- Bamaga, O.; Yokochi, A.; Zabara, B.; Babaqi, A. Hybrid FO/RO desalination system: Preliminary assessment of osmotic energy recovery and designs of new FO membrane module configurations. Desalination 2011, 268, 163–169. [Google Scholar] [CrossRef]
- Tan, C.; Ng, H.Y. A novel hybrid forward osmosis-nanofiltration (FO-NF) process for seawater desalination: Draw solution selection and system configuration. Desalin. Water Treat. 2010, 13, 356–361. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Mulcahy, D. Brackish water desalination by a hybrid forward osmosis–nanofiltration system using divalent draw solute. Desalination 2012, 284, 175–181. [Google Scholar] [CrossRef]
- Long, Q.; Jia, Y.; Li, J.; Yang, J.; Liu, F.; Zheng, J.; Biao, Y. Recent advance on draw solutes development in forward osmosis. Processes 2018, 6, 165. [Google Scholar] [CrossRef] [Green Version]
- Setiawan, L.; Wang, R.; Li, K.; Fane, A. Fabrication of novel poly(amide–imide) forward osmosis hollow fiber membranes with a positively charged nanofiltration-like selective layer. J. Membr. Sci. 2011, 369, 196–205. [Google Scholar] [CrossRef]
- Saren, Q.; Qi, S.; Tang, C.Y. Synthesis and characterization of novel forward osmosis membranes based on layer-by-layer assembly. Environ. Sci. Technol. 2011, 45, 5201–5208. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Boo, C.; Elimelech, M.; Hong, S. Comparison of fouling behavior in forward osmosis (FO) and reverse osmosis (RO). J. Membr. Sci. 2010, 365, 34–39. [Google Scholar] [CrossRef]
- Zou, S.; Qin, M.; He, Z. Tackle reverse solute flux in forward osmosis towards sustainable water recovery: Reduction and perspectives. Water Res. 2019, 149, 362–374. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of polydopamine: A never-ending story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef]
- Delparastan, P.; Malollari, K.G.; Lee, H.; Messersmith, P.B. Direct evidence for the polymeric nature of polydopamine. Angew. Chem. Int. Ed. 2019, 58, 1077–1082. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.-H.; Zhu, L.-P.; Li, X.-L.; Xu, Y.-Y.; Zhou, M.-Y. Surface modification of PE porous membranes based on the strong adhesion of polydopamine and covalent immobilization of heparin. J. Membr. Sci. 2010, 364, 194–202. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Park, H.B.; Ju, H.; Rowe, B.W.; Miller, D.J.; Chun, B.J.; Kin, K.; Freeman, B.D. Influence of polydopamine deposition conditions on pure water flux and foulant adhesion resistance of reverse osmosis, ultrafiltration, and microfiltration membranes. Polymer 2010, 51, 3472–3485. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Zhao, W.; Wei, Q.; Nie, S.; Sun, S.; Zhao, C. The hydrodynamic permeability and surface property of polyethersulfone ultrafiltration membranes with mussel-inspired polydopamine coatings. J. Membr. Sci. 2012, 417, 228–236. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, H.-C.; Liang, H.-Q.; Wan, L.-S.; Xu, Z.-K. Nanofiltration membranes via co-deposition of polydopamine/polyethylenimine followed by cross-linking. J. Membr. Sci. 2015, 476, 50–58. [Google Scholar] [CrossRef]
- Zhou, P.; Deng, Y.; Lyu, B.; Zhang, R.; Zhang, H.; Ma, H.; Lyu, Y.; Wei, S. Rapidly-deposited polydopamine coating via high temperature and vigorous stirring: Formation, characterization and biofunctional evaluation. PLoS ONE 2014, 9, e113087. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef]
- Boo, C.; Elimelech, M.; Hong, S. Fouling control in a forward osmosis process integrating seawater desalination and wastewater reclamation. J. Membr. Sci. 2013, 444, 148–156. [Google Scholar] [CrossRef]
- Schäfer, A.I.; Mauch, R.; Waite, T.D.; Fane, A.G. Charge effects in the fractionation of natural organics using ultrafiltration. Environ. Sci. Technol. 2002, 36, 2572–2580. [Google Scholar] [CrossRef]
- Vecchia, N.F.D.; Luchini, A.; Napolitano, A.; D’Errico, G.; Vitiello, G.; Szekely, N.K.; D’Ischia, M.; Paduano, L. Tris buffer modulates polydopamine growth, aggregation, and paramagnetic properties. Langmuir 2014, 30, 9811–9818. [Google Scholar] [CrossRef] [PubMed]
- Kasemset, S.; Wang, L.; He, Z.; Miller, D.J.; Kirschner, A.; Freeman, B.D.; Sharma, M.M. Influence of polydopamine deposition conditions on hydraulic permeability, sieving coefficients, pore size and pore size distribution for a polysulfone ultrafiltration membrane. J. Membr. Sci. 2017, 522, 100–115. [Google Scholar] [CrossRef] [Green Version]
- Susanto, H.; Ulbricht, M. Characteristics, performance and stability of polyethersulfone ultrafiltration membranes prepared by phase separation method using different macromolecular additives. J. Membr. Sci. 2009, 327, 125–135. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Ahmad, A.; Ooi, B. Superhydrophilic (superwetting) surfaces: A review on fabrication and application. J. Ind. Eng. Chem. 2017, 47, 19–40. [Google Scholar] [CrossRef]
- Feng, X.J.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Marmur, A. Equilibrium contact angles: Theory and measurement. Colloids Surf. A Physicochem. Eng. Asp. 1996, 116, 55–61. [Google Scholar] [CrossRef]
- Luxbacher, T. The Zeta Potential for Solid Surface Analysis, 1st ed.; Anton Paar GmbH: Graz, Austria, 2014; ISBN 978-3-200-03553-9. [Google Scholar]
- Liu, Q.; Yu, B.; Ye, W.; Zhou, F. Highly selective uptake and release of charged molecules by pH-responsive polydopamine microcapsules. Macromol. Biosci. 2011, 11, 1227–1234. [Google Scholar] [CrossRef]
- Liebscher, J. Chemistry of polydopamine-scope, variation, and limitation. Eur. J. Org. Chem. 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- Tanganov, B.B. About sizes of the hydrated salt ions—The components of sea water. Eur. J. Nat. Hist. 2013, 1, 36–37. [Google Scholar]
- Phuntsho, S.; Sahebi, S.; Majeed, T.; Lotfi, F.; Kim, J.E.; Shon, H.K. Assessing the major factors affecting the performances of forward osmosis and its implications on the desalination process. Chem. Eng. J. 2013, 231, 484–496. [Google Scholar] [CrossRef]
- Holloway, R.W.; Maltos, R.; Vanneste, J.; Cath, T. Mixed draw solutions for improved forward osmosis performance. J. Membr. Sci. 2015, 491, 121–131. [Google Scholar] [CrossRef] [Green Version]










| Dopamine Concentration (g/L) | Temperature (°C) | C 1s (%) | N 1s (%) | S 2p (%) | N/C | N/S |
|---|---|---|---|---|---|---|
| Pristine PES | - | 74.99 | 2.02 | 6.23 | 0.03 | 0.32 |
| 0.5 | 25 | 72.71 | 8.15 | 0.13 | 0.11 | 62.69 |
| 2.0 | 25 | 72.55 | 7.34 | 0.05 | 0.10 | 146.80 |
| 3.0 | 25 | 72.19 | 7.43 | 0.08 | 0.10 | 92.88 |
| 2.0 | 4 | 72.98 | 8.11 | 0.86 | 0.11 | 9.43 |
| 2.0 | 55 | 71.45 | 8.63 | 0.07 | 0.12 | 123.29 |
| Membrane | Water Flux (L/m2·h) | Reverse Salt Flux (g/m2·h) | Js/Jw Ratio (g/L) | Reference |
|---|---|---|---|---|
| PDA coated at 55 °C | 4.05 ± 0.20 | 0.34 ± 0.03 | 0.08 ± 0.01 | This work |
| CTA FO | 5.54 | 1.20 | 0.21 | [2] |
| CTA FO | ~ 4.33 | ~ 1.50 | ~ 0.33 | [41] |
| TFC FO | ~ 7.00 | ~ 0.13 | ~ 0.02 | [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oymaci, P.; Nijmeijer, K.; Borneman, Z. Development of Polydopamine Forward Osmosis Membranes with Low Reverse Salt Flux. Membranes 2020, 10, 94. https://doi.org/10.3390/membranes10050094
Oymaci P, Nijmeijer K, Borneman Z. Development of Polydopamine Forward Osmosis Membranes with Low Reverse Salt Flux. Membranes. 2020; 10(5):94. https://doi.org/10.3390/membranes10050094
Chicago/Turabian StyleOymaci, Pelin, Kitty Nijmeijer, and Zandrie Borneman. 2020. "Development of Polydopamine Forward Osmosis Membranes with Low Reverse Salt Flux" Membranes 10, no. 5: 94. https://doi.org/10.3390/membranes10050094
APA StyleOymaci, P., Nijmeijer, K., & Borneman, Z. (2020). Development of Polydopamine Forward Osmosis Membranes with Low Reverse Salt Flux. Membranes, 10(5), 94. https://doi.org/10.3390/membranes10050094