An On-Board Pure H2 Supply System Based on A Membrane Reactor for A Fuel Cell Vehicle: A Theoretical Study
Abstract
:1. Introduction
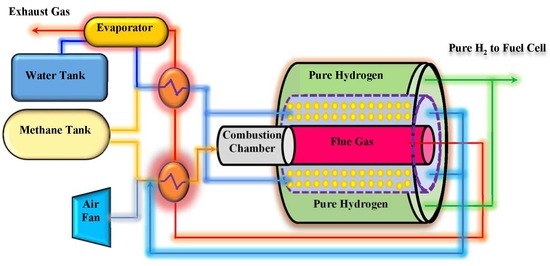
2. On-Board Processor Description
3. Mathematical Modeling
3.1. Combustion Chamber Mass and Energy Balances
3.2. Reformer Mass and Energy Balances
3.2.1. Reaction Side
3.2.2. Membrane Side
3.2.3. Flue Gas Side
3.3. Reaction Kinetics
3.4. Heat Exchangers and Evaporator Modeling
4. Numerical Solution
- The combustion chamber inlet temperature (Tc,in),
- The reformer reaction side inlet temperature (Tr,in),
- The combustion chamber feed composition and flowrate.
5. Results and Discussion
5.1. The Processor Performance
5.2. The Processor Flexibility
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Ac | Cross area of reaction side (m) |
| Cpi | Specific heat of component i (J kmol−1K−1) |
| DSo | External diameter of reaction side (m) |
| E0 | Activation energy of membrane |
| Fi | Molar flow rate of component i |
| Standard Gibbs free energy change of reaction i | |
| ∆Hf,i | Enthalpy change of reaction i |
| ki | Reaction rate constant |
| Ki | Adsorption equilibrium constant |
| Pi | Partial pressure of component i (bar) |
| r0 | Membrane tube radius (m) |
| QPd | Hydrogen permeability |
| rj | Rate of reaction of component j (kmol m−3 s−1) |
| R | Universal gas constant (J kmol−1 K−1) |
| Tcombustion | Reaction side temperature (K) |
| Treact | Reaction side temperature (K) |
| Tshell | Temperature of heating stream (K) |
| Ushell | Overall heat transfer coefficient (W m−1 s−1) |
| Methane conversion | |
| Carbon dioxide conversion | |
| z | Axial direction |
| Membrane thickness | |
| Catalyst effectiveness factor | |
| Bed density (kg m−3) | |
| DME | Dimethyl ether |
| DOE | Department of energy |
| FCV | Fuel cell vehicle |
| GHG | Greenhouse gases |
| ICE | Internal combustion engine |
| MR | Membrane reformer |
| MSR | Methane steam reforming |
| ODE | Ordinary differential equations |
| PEMFC | Proton exchange membrane fuel cell |
| PIS | Process intensification strategy |
References
- Wilberforce, T.; El-Hassan, Z.; Khatib, F.N.; Makky, A.A.; Baroutaji, A.; Carton, J.G.; Olabi, A.G. Developments of electric cars and fuel cell hydrogen electric cars. Int. J. Hydrog. Energ. 2017, 42, 25695–25734. [Google Scholar] [CrossRef] [Green Version]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef] [Green Version]
- Achour, H.; Carton, J.G.; Olabi, A.G. Estimating vehicle emissions from road transport, case study: Dublin City. Appl. Energy 2011, 88, 1957–1964. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Spataru, C. The use of natural gas pipeline network with different energy carriers. Ener. Strategy Rev. 2015, 8, 72–81. [Google Scholar] [CrossRef]
- Qi, A.; Peppley, B.; Karan, K. Integrated fuel processors for fuel cell application: A review. Fuel Process. Technol. 2007, 88, 3–22. [Google Scholar] [CrossRef]
- Boettner, D.D.; Moran, M.J. Proton exchange membrane (PEM) fuel cell-powered vehicle performance using direct-hydrogen fueling and on-board methanol reforming. J. Energy 2004, 29, 2317–2330. [Google Scholar] [CrossRef]
- Wu, W.; Chuang, B.N.; Hwang, J.J.; Lin, G.K.; Yang, S.B. Techno-economic evaluation of a hybrid fuel cell vehicle with on-board MeOH-to-H2 processor. Appl. Energy 2019, 238, 401–412. [Google Scholar] [CrossRef]
- Purnima, P.; Jayanti, S. A high-efficiency, auto-thermal system for onboard hydrogen production for low temperature PEM fuel cells using dual reforming of ethanol. Int. J. Hydrog. Energ. 2016, 41, 13800–13810. [Google Scholar] [CrossRef]
- Zhang, T.; Ou, K.; Jung, S.; Choi, B.; Kim, Y.B. Dynamic analysis of a PEM fuel cell hybrid system with an on-board dimethyl ether (DME) steam reformer (SR). Int. J. Hydrog. Energ. 2018, 43, 13521–13531. [Google Scholar] [CrossRef]
- Karakaya, M.; Avci, A.K. Comparison of compact reformer configurations for on-board fuel processing. Int. J. Hydrog. Energ. 2010, 35, 2305–2316. [Google Scholar] [CrossRef]
- Darwish, N.A.; Hilal, N.; Versteeg, G.; Heesink, B. Feasibility of the direct generation of hydrogen for fuel-cell-powered vehicles by on-board steam reforming of naphtha. Fuel 2004, 83, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Myers, D.B.; Ariff, G.D.; James, B.D. Cost and Performance Comparison of Stationary Hydrogen Fueling Appliances. Task 2 Report 2002. Available online: https://www.nrel.gov/docs/fy02osti/32405b2.pdf (accessed on 13 February 2020).
- Boodhoo, K.; Harvey, A. Process intensification for green chemistry. Chem. Listy. 2013, 107, 665–669. [Google Scholar]
- Drioli, E.; Barbieri, G.; Brunetti, A. Membrane Engineering for the Treatment of Gases: Vol. 2, Gas-Separation Issues Combined with Membrane Reactors; RSC: Cambridge, UK, 2017; pp. 1–366. ISBN ISBN 978-1-78262-875-0. [Google Scholar] [CrossRef]
- Bagnato, G.; Iulianelli, A.; Sanna, A.; Basile, A. Glycerol production and transformation: A critical review with particular emphasis on glycerol reforming reaction for producing hydrogen in conventional and membrane reactors. Membranes 2017, 7, 17. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Wilcox, J.; Basile, A. Advances on methane steam reforming to produce hydrogen through membrane reactors technology: A review. Catal. Rev. Sci. Eng. 2016, 58, 1–35. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M.; Kong, H.; Hao, Y. Thermodynamic analysis on mid/low temperature solar methane steam reforming with hydrogen permeation membrane reactors. Appl. Therm. Eng. 2019, 152, 925–936. [Google Scholar] [CrossRef]
- Yan, Y.-F.; Zhang, L.; Li, L.-X.; Tang, Q. Progress in catalytic membrane reactors for high purity hydrogen production. J. Inorg. Mat. 2011, 26, 1233–1243. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Ren. Sust. En. Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Yun, S.; Oyama, S.T. Correlations in palladium membranes for hydrogen separation: A review. J. Membr. Sci. 2011, 375, 28. [Google Scholar] [CrossRef]
- Caravella, A.; Scura, F.; Barbieri, G.; Drioli, E. Sieverts law empirical exponent for Pd-based membranes: Critical analysis in pure H2 permeation. J. Phys. Chem. B 2010, 114, 6033–6047. [Google Scholar] [CrossRef]
- Iaquaniello, G.; Giacobbe, F.; Morico, B.; Cosenza, S.; Farace, A. Membrane reforming in converting natural gas to hydrogen: Production costs, Part II. Int. J. Hydrog. Energ. 2008, 33, 6559–6601. [Google Scholar] [CrossRef]
- Lu, N.; Xie, D. Novel membrane reactor concepts for hydrogen production from hydrocarbons: A review. Int. J. Chem. Reactor Eng. 2016, 14, 1–31. [Google Scholar] [CrossRef]
- Yücel, O.; Alaittin, H.M. Comprehensive study of steam reforming of methane in membrane reactors. J. Energy. Resour. Technol. 2016, 138, 052204. [Google Scholar] [CrossRef]
- Jørgensen, S.L.; Nielsen, P.E.H.; Lehrmann, P. Steam reforming of methane in a membrane reactor. Catal. Today 1995, 25, 303–307. [Google Scholar] [CrossRef]
- Oyama, S.T.; Hacarlioglu, P. The boundary between simple and complex descriptions of membrane reactors: The transition between 1-D and 2-D analysis. Int. J. Hydrog. Energ. 2009, 337, 188–199. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Samimia, F.; Babapoor, A.; Tohidian, T.; Mohebi, S. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review. Chem. Eng. Proc. Proc. Intens. 2017, 121, 24–49. [Google Scholar] [CrossRef]
- Perez, P.; Cornaglia, C.A.; Mendes, A.; Madeira, L.M.; Tosti, S. Surface effects and CO/CO2 influence in the H2 permeation through a Pd-Ag membrane: A comprehensive model. Int. J. Hydrog. Energ. 2015, 40, 6566–6572. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, H. Palladium-based membranes for hydrogen separation. Platin. Met. Rev. 2012, 56, 117–123. [Google Scholar]
- Plazaola, A.A.; Pacheco, T.A.D.; Van, S.A.M.; Gallucci, F. Recent advances in Pd-based membranes for membrane reactors. Membranes 2017, 22, 51. [Google Scholar]
- Iulianelli, A.; Liguori, S.; Vita, A.; Italiano, C.; Fabiano, C.; Huang, Y.; Basile, A. The oncoming energy vector: Hydrogen produced in Pd-composite membrane reactor via bioethanol reforming over Ni/CeO2 catalyst. Catal. Today 2016, 259, 368–375. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. Amer. Inst. Chem. Eng. J. 1989, 35, 88–103. [Google Scholar] [CrossRef]
- Smith, J.M. Chemical Engineering Kinetics; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Acampora, L.; Marra, F.S. Investigation by thermodynamic properties of methane combustion mechanisms under harmonic oscillations in perfectly stirred reactor. Chem. Eng. Trans. 2017, 57, 1459–1464. [Google Scholar]
- Shampine, L.F.; Reichelt, M.W. The Matlab ODE suite. SIAM. J. Sci. Comput. 1977, 18, 1–12. [Google Scholar]
- Toyota Mirai Hydrogen Fuel Cell Electric Vehicle. Available online: https://h2.live/en/wasserstoffautos/toyota-mirai (accessed on 9 April 2020).
- Jokar, S.M.; Parvasi, P.; Basile, A. The evaluation of methane mixed reforming reaction in an industrial membrane reformer for hydrogen production. Int. J. Hydrog. Energ. 2018, 43, 15321–15329. [Google Scholar] [CrossRef]
- Greenhouse Gas Emissions from a Typical Passenger Vehicle, U.S. Environmental Protection Agency (EPA) Report. 2018. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P100U8YT.pdf (accessed on 9 April 2020).
- Basile, A.; Campanari, S.; Manzolini, G.; Iulianelli, A.; Longo, T.; Liguori, S.; De, F.M.; Piemonte, V. Methane steam reforming in a Pd-Ag membrane reformer: An experimental study on reaction pressure influence at middle temperature. Int. J. Hydrog. Energ. 2011, 36, 1531–1539. [Google Scholar] [CrossRef]
- Brown, L.F. A comparative study of fuels for on-board hydrogen production for fuel-cell-powered automobiles. Int. J. Hydrog. Energ. 2001, 26, 381–397. [Google Scholar] [CrossRef]










| T (°C) | CO | CO2 | ||
|---|---|---|---|---|
| α | Ki | α | Ki | |
| 450 | 0.12 | 1 × 10−4 | 0.62 | 4.93 × 10−6 |
| 400 | 0.20 | 1.03 × 10−4 | 0.77 | 6.30 × 10−6 |
| 300 | 0.77 | 1.34 × 10−4 | 0.80 | 1.17 × 10−5 |
| 250 | 0.78 | 3.35 × 10−4 | 0.90 | 1.50 × 10−5 |
| (26) | |
| (27) | |
| (28) | |
| (29) | |
| (30) | |
| (31) | |
| (32) | |
| (33) |
| Parameter | Value |
|---|---|
| Maximum output | 155 hp |
| H2 consumption (combined cycle) | 0.76 kg H2/100 km |
| Cruising range | 500 km |
| H2 tank capacity | 5 kg |
| Parameter | Value |
|---|---|
| Tube length in pure hydrogen producer (cm) | 50 |
| Tube length in combustion chamber (cm) | 10 |
| Cross area of the tubes (cm2) | 5.4 |
| Number of tubes | 25 |
| Pressure of methane and steam in pure H2 producer inlet (bar) | 10 |
| Temperature of methane in pure H2 producer inlet (°C) | 500 |
| Pressure of methane and steam in combustion chamber inlet (bar) | 1 |
| Temperature of methane in combustion chamber inlet (°C) | 25 |
| Outer reaction side diameter in pure H2 producer | 1” |
| Outer shell side diameter in pure H2 producer (cm) | 3 |
| Catalytic bed density (kg/m3) | 780 |
| Outer tube side diameter in combustion chamber (cm) | 0.5 |
| Membrane thickness (µm) | 50 |
| Material | Consumption Value (kg/kg pure H2) |
|---|---|
| Methane | 50 |
| Water | 70 |
| Air | 435 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvasi, P.; Mohammad Jokar, S.; Basile, A.; Iulianelli, A. An On-Board Pure H2 Supply System Based on A Membrane Reactor for A Fuel Cell Vehicle: A Theoretical Study. Membranes 2020, 10, 159. https://doi.org/10.3390/membranes10070159
Parvasi P, Mohammad Jokar S, Basile A, Iulianelli A. An On-Board Pure H2 Supply System Based on A Membrane Reactor for A Fuel Cell Vehicle: A Theoretical Study. Membranes. 2020; 10(7):159. https://doi.org/10.3390/membranes10070159
Chicago/Turabian StyleParvasi, Payam, Seyyed Mohammad Jokar, Angelo Basile, and Adolfo Iulianelli. 2020. "An On-Board Pure H2 Supply System Based on A Membrane Reactor for A Fuel Cell Vehicle: A Theoretical Study" Membranes 10, no. 7: 159. https://doi.org/10.3390/membranes10070159
APA StyleParvasi, P., Mohammad Jokar, S., Basile, A., & Iulianelli, A. (2020). An On-Board Pure H2 Supply System Based on A Membrane Reactor for A Fuel Cell Vehicle: A Theoretical Study. Membranes, 10(7), 159. https://doi.org/10.3390/membranes10070159