Organic-Inorganic Artificial Ion Channel Polyvinylidene Fluoride Membranes for Controllable Selectivity Transport of Alkali Metal Cations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
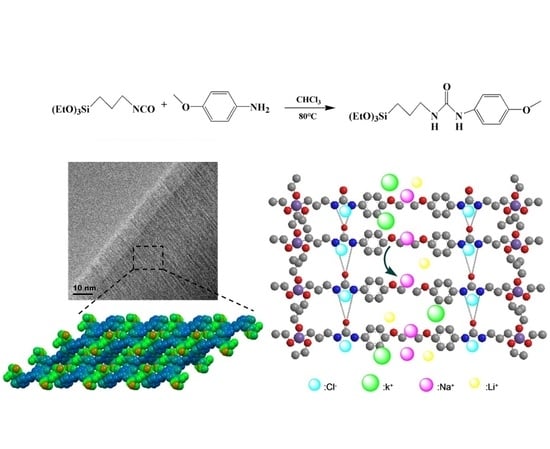
2.2. Preparation of Hybrid Membranes with Different Hybrid Organic–Inorganic Materials
2.3. Preparation of Hybrid Membranes with Different Ratios of H2
2.4. Characterization of Hybrid Membranes
2.4.1. Observation of the Morphology of the Hybrid Membranes
2.4.2. Dialysis Transport Procedure
3. Results and Discussions
3.1. Microstructure and Morphology of Hybrid Membranes
3.2. Dialysis of the Transport Properties of the Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beginn, U.; Zipp, G.; Möller, M. Functional Membranes Containing Ion-Selective Matrix-Fixed Supramolecular Channels. Adv. Mater. 2000, 12, 510–513. [Google Scholar] [CrossRef]
- Gillers, A.; Barboiu, M. Highly Selective Artificial K+ Channels: An Example of Selectivity-Induced Transmembrane Potential. J. Am. Chem. Soc. 2016, 138, 426–432. [Google Scholar] [CrossRef]
- Acar, E.T.; Buchsbaum, S.F.; Combs, C.; Fornasiero, F.; Siwy, Z.S. Biomimetic potassium-selective nanopores. Sci. Adv. 2019, 5, eaav2568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, A.; Kroenlein, K.; Riccardi, D.; Smolyanitsky, A. Highly mechanosensitive ion channels from graphene-embedded crown ethers. Nat. Mater. 2019, 18, 76–81. [Google Scholar] [CrossRef]
- Bong, D.T.; Clark, T.D.; Granja, J.R.; Ghadiri, M.R. Self-Assembling Organic Nanotubes. Angew. Chem. Int. Ed. Engl. 2001, 40, 998–1011. [Google Scholar] [CrossRef]
- Sidorov, V.; Kotch, F.W.; Abdrakhmanova, G.; Mizani, R.; Fettinger, J.C.; Davis, J.T. Ion channel formation from a calix[4]arene amide that binds HCl. J. Am. Chem. Soc. 2002, 124, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.L.; Zhang, J.J.; Meng, F.N.; Cao, C.; Yao, Y.Y.; Willner, I.; Tian, H.; Long, Y.T. A Stimuli-responsive nanopore based on a photoresponsive host-Guest system. Sci. Rep. 2013, 3, 1662. [Google Scholar] [CrossRef]
- Bayrakci, M.; Yigiter, S. Synthesis of tetra-substituted calix [4] arene ionophores and their recognition studies toward toxic arsenate anions. Tetrahedron 2013, 69, 3218–3224. [Google Scholar] [CrossRef]
- Moreau, J.J.E.; Vellutini, L.; Man, M.W.C.; Bied, C. Shape-controlled bridged silsesquioxanes: Hollow tubes and spheres. Chem. Eur. J. 2003, 9, 1594–1599. [Google Scholar] [CrossRef]
- Moreau, J.J.E.; Vellutini, L.; Man, M.W.C.; Bied, C.; Dieudonné, P.; Bantignies, J.L.; Sauvajol, J.L. Lamellar bridged silsesquioxanes: Self-assembly through a combination of hydrogen bonding and hydrophobic interactions. Chem. Eur. J. 2005, 11, 1527–1537. [Google Scholar] [CrossRef]
- Michau, M.; Caraballo, R.; Arnal-Hérault, C.; Barboiu, M. Alkali cation-π aromatic conduction pathways in self-organized hybrid membranes. J. Membr. Sci. 2008, 321, 22–30. [Google Scholar] [CrossRef]
- Hariprasad, R.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. SPVdF-HFP/SGO nanohybrid proton exchange membrane for the applications of direct methanol fuel cells. J. Dispers. Sci. Technol. 2019, 1–13. [Google Scholar] [CrossRef]
- Kim, A.R.; Gabunada, J.C.; Yoo, D.J. Sulfonated fluorinated block copolymer containing naphthalene unit/sulfonated polyvinylidene-co-hexafluoropropylene/functionalized silicon dioxide ternary composite membrane for low-humidity fuel cell applications. Colloid Polym. Sci. 2018, 296, 1891–1903. [Google Scholar] [CrossRef]
- Tian, Y.; Jin, S.; Jin, Y.; Ma, W.; Lin, Y.; Li, L. Preparation of polyvinylidene fluoride/modified attapulgite composite ultrafiltration membrane. Polym. Adv. Technol. 2020, 1–7. [Google Scholar] [CrossRef]
- Wang, D.L.; Li, K.; Teo, W.K. Preparation and characterization of polyvinylidene fluoride (PVDF) hollow fiber membranes. J. Membr. Sci. 1999, 163, 211–220. [Google Scholar] [CrossRef]
- Gu, M.H.; Zhang, J.; Wang, X.L.; Tao, H.J.; Ge, L.T. Formation of poly(vinylidene fluoride) (PVDF) membranes via thermally induced phase separation. Desalination 2006, 192, 160–167. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Tang, X.X.; Li, W.; Liu, H.N.; Zhang, J.; Wang, H.; Li, J.X. EVOH in situ fibrillation and its effect of strengthening, toughening and hydrophilic modification on PVDF hollow fiber microfiltration membrane via TIPS process. J. Membr. Sci. 2019, 54, 5971–5987. [Google Scholar] [CrossRef]
- Wang, L.H.; Zhang, X.X.; Yun, Y.B. Preparation of hybrid ion channel membrane for recognizing and transporting sodium ion. Desalin. Water Treat. 2011, 34, 234–238. [Google Scholar] [CrossRef] [Green Version]
- Nasr, G.; Barboiu, M.; Ono, T.; Fujii, S.; Lehn, J.M. Dynamic polymer membranes displaying tunable transport properties on constitutional exchange. J. Membr. Sci. 2008, 321, 8–14. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wang, L.H.; Yun, Y.B. Construction of Self-Organized Hybrid Ion Channel Membrane and Cationic Transport Mechanism. Acta Chim. Sin. 2012, 70, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Cabarcos, O.M.; Weinheimer, C.J.; Lisy, J.M. Competitive solvation of K+ by benzene and water: Cation-π interactions and π-hydrogen bonds. J. Chem. Phys. 1998, 108, 5151–5154. [Google Scholar] [CrossRef]
- Cabarcos, O.M.; Weinheimer, C.J.; Lisy, J.M. Size selectivity by cation-π interactions: Solvation of K+ and Na+ by benzene and water. J. Chem. Phys. 1999, 110, 8429–8435. [Google Scholar] [CrossRef]











| Materials | M1 | M2 | M3 |
|---|---|---|---|
| PVDF(15%)/g | 1.5 | 1.5 | 1.5 |
| DMAC/g | 8.4 | 8 | 7.5 |
| H2/g | 0.1(1%) | 0.5(5%) | 1(10%) |
| HCl/mL | 0.02 | 0.04 | 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Jin, S.; Zhang, X.; Wang, L.; Lin, Y.; Jin, Y.; Li, L. Organic-Inorganic Artificial Ion Channel Polyvinylidene Fluoride Membranes for Controllable Selectivity Transport of Alkali Metal Cations. Membranes 2020, 10, 174. https://doi.org/10.3390/membranes10080174
Tian Y, Jin S, Zhang X, Wang L, Lin Y, Jin Y, Li L. Organic-Inorganic Artificial Ion Channel Polyvinylidene Fluoride Membranes for Controllable Selectivity Transport of Alkali Metal Cations. Membranes. 2020; 10(8):174. https://doi.org/10.3390/membranes10080174
Chicago/Turabian StyleTian, Ye, Shaohua Jin, Xinxin Zhang, Lihua Wang, Yakai Lin, Yutao Jin, and Lijie Li. 2020. "Organic-Inorganic Artificial Ion Channel Polyvinylidene Fluoride Membranes for Controllable Selectivity Transport of Alkali Metal Cations" Membranes 10, no. 8: 174. https://doi.org/10.3390/membranes10080174
APA StyleTian, Y., Jin, S., Zhang, X., Wang, L., Lin, Y., Jin, Y., & Li, L. (2020). Organic-Inorganic Artificial Ion Channel Polyvinylidene Fluoride Membranes for Controllable Selectivity Transport of Alkali Metal Cations. Membranes, 10(8), 174. https://doi.org/10.3390/membranes10080174