Synthesis and Characterization of 40 wt % Ce0.9Pr0.1O2–δ–60 wt % NdxSr1−xFe0.9Cu0.1O3−δ Dual-Phase Membranes for Efficient Oxygen Separation
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Powders and Membranes
2.2. Materials Characterization
2.3. Oxygen Permeation Measurements
3. Results and Discussion
3.1. Structure Characterization and Morphologies
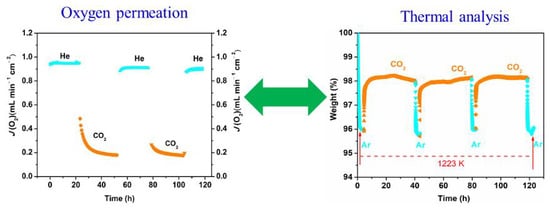
3.2. Materials Tolerance towards Air, CO2 and H2
3.3. Oxygen Permeation Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, T.; Han, N.; Meng, B.; Yang, N.; Zhu, Z.; Liu, S. Enhancing oxygen permeation via the incorporation of silver inside perovskite oxide membranes. Processes 2019, 7, 199. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Yang, W. Critical factors affecting oxygen permeation through dual-phase membranes. In Membrane Science and Technology; Oyama, S.T., Stagg-Williams, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 14, Chapter 12; pp. 275–293. [Google Scholar] [CrossRef]
- Shi, L.; Wang, S.; Lu, T.; He, Y.; Yan, D.; Lan, Q.; Xie, Z.; Wang, H.; Boubeche, M.; Luo, H. Effects of Al content on the oxygen permeability through dual-phase membrane 60Ce0.9Pr0.1O2-δ–40Pr0.6Sr0.4Fe1-x AlxO3-δ. Ceram. Int. 2019, 45, 20033–20039. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Sunarso, J.; Liu, S. Designing CO2-resistant oxygen-selective mixed ionic-electronic conducting membranes: Guidelines, recent advances, and forward directions. Chem. Soc. Rev. 2017, 46, 2941–3005. [Google Scholar] [CrossRef] [PubMed]
- Sunarso, J.; Baumann, S.; Serra, J.M.; Meulenberg, W.A.; Liu, S.; Lin, J.Y.; Diniz da Costa, J.C. Mixed ionic-electronic conducting (MIEC) ceramic-based membranes for oxygen separation. J. Membr. Sci. 2008, 320, 13–41. [Google Scholar] [CrossRef]
- Chen, G.; Tang, B.; Widenmeyer, M.; Wang, L.; Feldhoff, A.; Weidenkaff, A. Novel CO2-tolerant dual-phase Ce0.9Pr0.1O2-δ–La0.5Sr0.5Fe0.9Cu0.1O3-δ membranes with high oxygen permeability. J. Membr. Sci. 2020, 595, 117530. [Google Scholar] [CrossRef]
- Geffroy, P.-M.; Blond, E.; Richet, N.; Chartier, T. Understanding and identifying the oxygen transport mechanisms through a mixed-conductor membrane. Chem. Eng. Sci. 2017, 162, 245–261. [Google Scholar] [CrossRef]
- Widenmeyer, M.; Wiegers, K.-S.; Chen, G.; Yoon, S.; Feldhoff, A.; Weidenkaff, A. Engineering of oxygen pathways for better oxygen permeability in Cr-substituted Ba2In2O5 membranes. J. Membr. Sci. 2020, 595, 117558. [Google Scholar] [CrossRef]
- Efimov, K.; Klande, T.; Juditzki, N.; Feldhoff, A. Ca-Containing CO2-tolerant perovskite materials for oxygen separation. J. Membr. Sci. 2012, 389, 205–215. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H.; Chen, S.; Zhao, H.; Lu, X.; Xu, Q. CO2-Tolerance and oxygen permeability of novel cobalt-free mixed-conductor oxygen-permeable Pr0.6Sr0.4Fe1-xNbxO3-δ membranes. Ceram. Int. 2017, 43, 13791–13799. [Google Scholar] [CrossRef]
- Zhang, Z.; Chena, D.; Dong, F.; Xu, X.; Hao, Y.; Shao, Z. Understanding the doping effect toward the design of CO2-tolerant perovskite membranes with enhanced oxygen permeability. J. Membr. Sci. 2016, 519, 11–21. [Google Scholar] [CrossRef]
- Yi, J.; Schroeder, M.; Martin, M. CO2-tolerant and cobalt-Free SrFe0.8Nb0.2O3-δ perovskite membrane for oxygen separation. Chem. Mater. 2013, 25, 815–817. [Google Scholar] [CrossRef]
- Du, M.; Liao, K.; Lu, Q.; Shao, Z. Recent advances in the interface engineering of solid-state Li-ion batteries with artificial buffer layers: Challenges, materials, construction, and characterization. Energy Environ. Sci. 2019, 12, 1780–1804. [Google Scholar] [CrossRef]
- Li, R.; Liao, K.; Zhou, W.; Li, X.; Meng, D.; Cai, R.; Shao, Z. Realizing fourfold enhancement in conductivity of perovskite Li0.33La0.557TiO3 electrolyte membrane via a Sr and Ta co-doping strategy. J. Membr. Sci. 2019, 582, 194–202. [Google Scholar] [CrossRef]
- Zou, X.; Lu, Q.; Zhong, Y.; Liao, K.; Zhou, W.; Shao, Z. Flexible, flame-resistant, and dendrite-impermeable gel-polymer electrolyte for Li-O2/Air batteries workable under hurdle conditions. Small 2018, 14, 1801798. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, D.M.A.; Chen, G.; Weidenkaff, A.; Sata, N.; Han, F.; Schiller, G.; Costa, R.; Friedrich, A.K. Synthesis and evaluation of the A-Site deficient perovskite La0.65Sr0.3Cr0.85Ni0.15O3-δ as fuel electrode for high temperature Co-electrolysis enhanced by in situ exsolution of Ni nanoparticles. ECS Trans. 2019, 91, 1751–1760. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for solid oxide fuel cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Xu, X.; Bi, L.; Zhao, X.S. Highly-Conductive proton-conducting electrolyte membranes with a low sintering temperature for solid oxide fuel cells. J. Membr. Sci. 2018, 558, 17–25. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Fang, J.; Jin, X.; Huang, K. Life cycle analysis of a combined CO2 capture and conversion membrane reactor. J. Membr. Sci. 2018, 549, 142–150. [Google Scholar] [CrossRef]
- Plazaola, A.A.; Cruellas, A.; Liu, Y.; Porras, N.B.; Tanaka, D.A.P.; Annaland, M.V.S.; Gallucci, F. Mixed ionic-electronic conducting membranes (MIEC) for their application in membrane reactors: a review. Processes 2019, 7, 128. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Zhang, C.; Chang, X.; Fan, Y.; Xing, W.; Xu, N. Efficient catalytic decomposition of CO2 to CO and O2 over Pd/Mixed-conducting oxide catalyst in an oxygen-permeable membrane reactor. Environ. Sci. Technol. 2008, 42, 3064–3068. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Y.; Ghoniem, A.F. Mixed ionic-electronic conducting (MIEC) membranes for thermochemical reduction of CO2: A review. Prog. Energy Combust. Sci. 2019, 74, 1–30. [Google Scholar] [CrossRef]
- Snoeckx, R.; Bogaerts, A. Plasma technology—A novel solution for CO2 conversion? Chem. Soc. Rev. 2017, 46, 5805–5863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadler, H.; Beggel, F.; Habermehl, M.; Persigehl, B.; Kneer, R.; Modigell, M.; Jeschke, P. Oxyfuel coal combustion by efficient integration of oxygen transport membranes. Int. J. Greenh. Gas Control 2011, 5, 7–15. [Google Scholar] [CrossRef]
- Schiestel, T.; Kilgus, M.; Peter, S.; Caspary, K.; Wang, H.; Caro, J. Hollow fibre perovskite membranes for oxygen separation. J. Membr. Sci. 2005, 258, 1–4. [Google Scholar] [CrossRef]
- Chen, G.; Widenmeyer, M.; Tang, B.; Kaeswurm, L.; Wang, L.; Feldhoff, A.; Weidenkaff, A. A CO and CO 2 tolerating (La0.9Ca0.1)2(Ni0.75Cu0.25)O4+ δ Ruddlesden-Popper membrane for oxygen separation. Front. Chem. Sci. Eng. 2020, 14, 405–414. [Google Scholar] [CrossRef]
- Buck, F.; Kistner, I.; Rösler, C.; Schulz, A.; Walker, M.; Tovar, G.E.M.; Schiestel, T.; Tovar, G. Einsatz von perowskitischen Hohlfasermembranen in einem Mikrowellenplasma. Chem. Ing. Tech. 2019, 91, 1117–1122. [Google Scholar] [CrossRef]
- Chen, G.; Buck, F.; Kistner, I.; Widenmeyer, M.; Schiestel, T.; Schulz, A.; Walker, M.; Weidenkaff, A. A novel plasma-assisted hollow fiber membrane concept for efficiently separating oxygen from CO in a CO2 plasma. Chem. Eng. J. 2020, 392, 123699. [Google Scholar] [CrossRef]
- Chen, G.; Britun, N.; Godfroid, T.; Delplancke-Ogletree, M.; Snyders, R. Role of plasma catalysis in the microwave plasma-assisted conversion of CO2. In Green Chemical Processing and Synthesis; Karame, I., Srour, H., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Georgieva, V.; Godfroid, T.; Snyders, R.; Delplancke-Ogletree, M.-P. Plasma assisted catalytic decomposition of CO2. Appl. Catal. B Environ. 2016, 190, 115–124. [Google Scholar] [CrossRef]
- Chen, G.; Godfroid, T.; Georgieva, V.; Britun, N.; Delplancke-Ogletree, M.-P.; Snyders, R. Plasma-catalytic conversion of CO2 and CO2/H2O in a surface-wave sustained microwave discharge. Appl. Catal. B Environ. 2017, 214, 114–125. [Google Scholar] [CrossRef]
- Chen, G.; Wang, L.; Godfroid, T.; Snyders, R. Progress in plasma-assisted catalysis for carbon dioxide reduction. In Plasma Chemistry and Gas Conversion; Britun, N., Silva, T., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Kerstiens, T.; Markus, T. Oxygen permeability and phase stability of Ba0.5Sr0.5Co0.8Fe0.2O3-δ perovskite at intermediate temperatures. J. Membr. Sci. 2013, 438, 83–89. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.P.; Son, S.H. Oxygen permeation and stability of Ba0.5Sr0.5Co0.8Fe0.2O3-δ membrane according to trace elements and oxygen partial pressure in synthetic air. Energy Procedia 2009, 1, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Alaee, M.A.; Movahednia, M.M.; Mohammadi, T. Correction to effect of Ba content on oxygen permeation performance of BaxSr1-xCo0.8Fe0.2O3-δ (x = 0.2, 0.5, and 0.8) Perovskite-Type Membrane. J. Chem. Eng. Data 2018, 63, 857. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Yang, W. Microstructural and interfacial designs of oxygen-permeable membranes for oxygen separation and reaction-separation coupling. Adv. Mater. 2019, 31, e1902547. [Google Scholar] [CrossRef]
- Li, K.; Zhao, H.; Lü, Y.; Ma, Y.; Du, Z.; Zhang, Z. High CO2 tolerance oxygen permeation membranes BaFe0.95-xCa0.05TixO3-δ. J. Membr. Sci. 2018, 550, 302–312. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, H.; Jiang, B.; Lu, X.; Ding, W. Effects of tantalum content on the structure stability and oxygen permeability of BaCo0.7Fe0.3-xTaxO3-δ ceramic membrane. Int. J. Hydrogen Energy 2013, 38, 11090–11096. [Google Scholar] [CrossRef]
- Chen, G.; Liu, W.; Widenmeyer, M.; Ying, P.; Dou, M.; Xie, W.; Bubeck, C.; Wang, L.; Fyta, M.; Feldhoff, A.; et al. High flux and CO2-resistance of La0.6Ca0.4Co1-xFexO3-δ oxygen-transporting membranes. J. Membr. Sci. 2019, 590, 117082. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, H.; Chang, X.; Du, X.; Li, K.; Ma, Y.; Yi, S.; Du, Z.; Zheng, K.; Świerczek, K. Novel cobalt-free BaFe1-xGdxO3-δ perovskite membranes for oxygen separation. J. Mater. Chem. A 2016, 4, 10454–10466. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Martynczuk, J.; Efimov, K.; Harvey, A.S.; Infortuna, A.; Kocher, P.; Gauckler, L.J. Oxygen-vacancy-related structural phase transition of Ba0.8Sr0.2Co0.8 Fe0.2O3-δ. Chem. Mater. 2011, 23, 3169–3175. [Google Scholar] [CrossRef]
- Guironnet, L.; Geffroy, P.-M.; Tessier-Doyen, N.; Boulle, A.; Richet, N.; Chartier, T. The surface roughness effect on electrochemical properties of La0.5Sr0.5Fe0.7Ga0.3O3-δ perovskite for oxygen transport membranes. J. Membr. Sci. 2019, 588, 117199. [Google Scholar] [CrossRef]
- Li, T.; Kamhangdatepon, T.; Wang, B.; Hartley, U.W.; Li, K.; Khamhangdatepon, T. New bio-inspired design for high-performance and highly robust La0.6Sr0.4Co0.2Fe0.8O3-δ membranes for oxygen permeation. J. Membr. Sci. 2019, 578, 203–208. [Google Scholar] [CrossRef]
- Garcia-Fayos, J.; Ruhl, R.; Navarrete, L.; Serra, J.M.; Bouwmeester, H.J.M. Enhancing oxygen permeation through Fe2NiO4–Ce0.8Tb0.2O2-δ composite membranes using porous layers activated with Pr6O11 nanoparticles. J. Mater. Chem. A 2018, 6, 1201–1209. [Google Scholar] [CrossRef]
- Magnone, E.; Chae, J.; Park, J.H. Synthesis and oxygen permeation properties of Ce0.8Sm0.2O2-d—Sm1-xSrxCu0.2Fe0.8O3-d dual-phase ceramic membranes: effect of strontium contents and Pd coating layer. Ceram. Int. 2018, 44, 12948–12956. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, Y.; Gao, J.; Chen, C. Oxygen permeability of asymmetric membrane of functional La0.8 Sr0.2Cr0.5Fe0.5O3-δ(LSCrF)–Zr0.8Y0.2O2-δ(YSZ) supported on porous YSZ. Ceram. Int. 2014, 40, 799–803. [Google Scholar] [CrossRef]
- Shi, L.; Wang, S.; Lu, T.; He, Y.; Yan, N.; Lan, Q.; Xie, Z.; Wang, H.; Li, M.-R.; Caro, J.; et al. High CO2-tolerance oxygen permeation dual-phase membranes Ce0.9Pr0.1O2-δ–Pr0.6Sr0.4Fe0.8Al0.2O3-δ. J. Alloy. Compd. 2019, 806, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Klande, T.; Cao, Z.; Liang, F.; Wang, H.; Caro, J. A CO2-Stable reduction-tolerant Nd-containing dual phase membrane for oxyfuel CO2 capture. J. Mater. Chem. A 2014, 2, 7780–7787. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Shi, L.; Wu, F.; Xie, W.; Wang, S.; Yan, N.; Liu, P.; Li, M.-R.; Caro, J.; Luo, H. A novel dual phase membrane 40 wt % Nd0.6Sr0.4CoO3-δ–60 wt % Ce0.9Nd0.1O2-δ: design, synthesis and properties. J. Mater. Chem. A 2018, 6, 84–92. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, H.; Efimov, K.; Caro, J.; Wang, H. Influence of the preparation methods on the microstructure and oxygen permeability of a CO2-stable dual phase membrane. AIChE J. 2010, 57, 2738–2745. [Google Scholar] [CrossRef]
- Partovi, K.; Rüscher, C.H.; Steinbach, F.; Caro, J. Enhanced oxygen permeability of novel Cu-containing CO2-tolerant dual-phase membranes. J. Membr. Sci. 2016, 503, 158–165. [Google Scholar] [CrossRef]
- Liang, F.; Luo, H.; Partovi, K.; Ravkina, O.; Cao, Z.; Liu, Y.; Caro, J. A novel CO2-stable dual phase membrane with high oxygen permeability. Chem. Commun. 2014, 50, 2451–2454. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Steinbach, F.; Chen, C.; Feldhoff, A. An Approach to enhance the CO2 tolerance of fluorite-perovskite dual-phase oxygen-transporting membrane. Chem. Mater. 2015, 27, 7820–7826. [Google Scholar] [CrossRef]
- Gili, A.; Bischoff, B.; Simon, U.; Schmidt, F.; Kober, D.; Görke, O.; Bekheet, M.F.; Gurlo, A. Ceria-based dual-phase membranes for high-temperature carbon dioxide separation: effect of iron doping and pore generation with MgO template. Membranes 2019, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, W.; Liang, F.; Cao, Z.; Steinbach, F.; Feldhoff, A. A mixed ionic and electronic conducting dual-phase membrane with high oxygen permeability. Angew. Chem. Int. Ed. 2015, 54, 4847–4850. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, H.; Yan, G.; Zhao, H.; Lu, X.; Wang, P. Effects of B-site Nb doping on the CO2 resistance and rate-controlling step of Ce0.8Gd0.2O2-δ—Pr0.6Sr0.4Co0.5Fe0.5O3-δ dual-phase membranes. J. Mater. Sci. 2018, 53, 11962–11976. [Google Scholar] [CrossRef]
- Luo, H.; Feldhoff, A.; Wang, H.; Efimov, K.; Jiang, H.; Caro, J. CO2-Stable and cobalt-free dual-phase membrane for oxygen separation. Angew. Chem. Int. Ed. 2010, 50, 759–763. [Google Scholar] [CrossRef]
- Du, Z.; Ma, Y.; Zhao, H.; Li, K.; Lu, Y. High CO2-tolerant and cobalt-free dual-phase membranes for pure oxygen separation. J. Membr. Sci. 2019, 574, 243–251. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, H.; Cong, Y.; Yang, W. Novel dual-phase membranes for CO2 capture via an oxyfuel route. Chem. Commun. 2012, 48, 251–253. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Chew, J.; Liu, S.; Zhu, X.; Sunarso, J. Rate determining step in SDC-SSAF dual-phase oxygen permeation membrane. J. Membr. Sci. 2019, 573, 628–638. [Google Scholar] [CrossRef]
- Nan, N.; Wei, Q.; Tian, H.; Zhang, S.; Zhu, Z.; Liu, J.; Liu, S. Highly stable dual-phase membrane based on Ce0.9Gd0.1O2-δ–La2NiO4-δ for oxygen permeation under pure CO2 atmosphere. Energy Technol. 2019, 7, 1800701. [Google Scholar] [CrossRef]
- Garcia-Fayos, J.; Balaguer, M.; Baumann, S.; Serra, J.M. Dual-phase membrane based on LaCo0.2Ni0.4Fe0.4 O3-x-Ce0.8Gd0.2O2-x composition for oxygen permeation under CO2/SO2-rich gas environments. J. Membr. Sci. 2018, 548, 117–124. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, W.; Chen, Y.; Chena, D.; Chen, J.; Liu, S.; Jin, W.; Shao, Z. Novel approach for developing dual-phase ceramic membranes for oxygen separation through beneficial phase reaction. ACS Appl. Mater. Interfaces 2015, 7, 22918–22926. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, L.; Xie, Z.; He, Y.; Yan, D.; Li, M.-R.; Caro, J.; Luo, H. High-flux dual-phase percolation membrane for oxygen separation. J. Eur. Ceram. Soc. 2019, 39, 4882–4890. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Zhang, N.; Xiong, X.; Lu, X.; Zhao, H.; Li, S.; Zhou, Z. Synthesis, Oxygen permeation, and CO2-tolerance properties of Ce0.8Gd0.2O2-δ—Ba0.95La0.05Fe1-xNbxO3-δ dual-phase membranes. ACS Sustain. Chem. Eng. 2015, 3, 1982–1992. [Google Scholar] [CrossRef]
- Zhang, G.; Bao, X.; Zeng, F.; Minh, N.Q.; Wang, S. Preparation and characterization of Ce0.8Sm0.2O1.9—La0.8 Sr0.2Cr0.5Fe0.5O3-d dual-phase membranes for oxygen permeation. J. Materiomics 2020, 6, 224–231. [Google Scholar] [CrossRef]
- Omar, S.; Wachsman, E.D.; Jones, J.L.; Nino, J.C. Crystal structure-ionic conductivity relationships in doped ceria systems. J. Am. Ceram. Soc. 2009, 92, 2674–2681. [Google Scholar] [CrossRef]
- Bocher, L.; Robert, R.; Aguirre, M.H.; Malo, S.; Hébert, S.; Maignan, A.; Weidenkaff, A. Thermoelectric and magnetic properties of perovskite-type manganate phases synthesised by an Ultrasonic Spray Combustion (USC) method. J. Sol. State Sci. 2008, 10, 496–501. [Google Scholar] [CrossRef]
- Stevenson, J.W.; Armstrong, T.R.; Carneim, R.D.; Pederson, L.R.; Weber, W.J. Electrochemical properties of mixed conducting perovskites La1-xMxCo1-yFeyO3-δ (M = Sr, Ba, Ca). J. Electrochem. Soc. 1996, 143, 2722–2729. [Google Scholar] [CrossRef]
- Elshof, J.E.T.; Bouwmeester, H.J.; Verweij, H. Oxygen transport through La1-xSrxFeO3-δ membranes II. Permeation in air/CO, CO2 gradients. Solid State Ionics 1996, 89, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Khromushin, I.V.; Aksenova, T.; Zhotabaev, Z. Mechanism of gas-solid exchange processes for some perovskites. Solid State Ionics 2003, 162, 37–40. [Google Scholar] [CrossRef]













| Sample | J(O2) (air/He) (mL min−1 cm−2) | J(O2) (air/CO2) (mL min−1 cm−2) | d (mm) | T (K) | J(O2) (air/CO2) Stability (h) | Material cost (EUR/g) | Ref. |
|---|---|---|---|---|---|---|---|
| CPO-NSFCO | 0.97 | 0.32 | 0.6 | 1223 | 70 | 1.76 | This work (Figure S2) |
| CPO-NSFCO | 0.94 | 0.61 | 0.6 | 1223 | 5 | 1.76 | This work (Figure 8) |
| CPO-NSFCO | 1.02 | -- | 0.6 | 1223 | 10 | 1.76 | This work (Figure 9) |
| CPO-NSFCO | 0.94 | 0.2 | 0.65 | 1223 | 125 | 1.76 | This work (Figure 10) |
| CNO-NSFO | 0.26 | 0.21 | 0.6 | 1223 | 120 | 1.70 | [49] |
| CNO-NSCO | 0.65 | 0.55 | 0.6 | 1223 | 150 | 1.89 | [50] |
| CSO-SSCFO | 1.01 | 0.7 | 0.6 | 1223 | 50 | 2.81 | [52] |
| CPO-PSFCO | 0.84 | 0.7 | 0.6 | 1223 | 400 | 2.23 | [53] |
| CGO-PSCFO | 0.6 | 0.45 | 0.5 | 1173 | -- | -- | [55] |
| CGCO-LCFO | 0.87 | 0.7 | 0.5 | 1223 | -- | -- | [56] |
| LCO-30LSFO | 0.32 | 0.19 | 0.6 | 1173 | 100 | -- | [59] |
| LCCO-30LSFO | 0.45 | 0.27 | 0.6 | 1173 | 100 | -- | [59] |
| CSO-SCMCO | 0.4 | 0.34 | 0.5 | 1173 | 75 | -- | [60] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Zhao, Z.; Widenmeyer, M.; Yan, R.; Wang, L.; Feldhoff, A.; Weidenkaff, A. Synthesis and Characterization of 40 wt % Ce0.9Pr0.1O2–δ–60 wt % NdxSr1−xFe0.9Cu0.1O3−δ Dual-Phase Membranes for Efficient Oxygen Separation. Membranes 2020, 10, 183. https://doi.org/10.3390/membranes10080183
Chen G, Zhao Z, Widenmeyer M, Yan R, Wang L, Feldhoff A, Weidenkaff A. Synthesis and Characterization of 40 wt % Ce0.9Pr0.1O2–δ–60 wt % NdxSr1−xFe0.9Cu0.1O3−δ Dual-Phase Membranes for Efficient Oxygen Separation. Membranes. 2020; 10(8):183. https://doi.org/10.3390/membranes10080183
Chicago/Turabian StyleChen, Guoxing, Zhijun Zhao, Marc Widenmeyer, Ruijuan Yan, Ling Wang, Armin Feldhoff, and Anke Weidenkaff. 2020. "Synthesis and Characterization of 40 wt % Ce0.9Pr0.1O2–δ–60 wt % NdxSr1−xFe0.9Cu0.1O3−δ Dual-Phase Membranes for Efficient Oxygen Separation" Membranes 10, no. 8: 183. https://doi.org/10.3390/membranes10080183
APA StyleChen, G., Zhao, Z., Widenmeyer, M., Yan, R., Wang, L., Feldhoff, A., & Weidenkaff, A. (2020). Synthesis and Characterization of 40 wt % Ce0.9Pr0.1O2–δ–60 wt % NdxSr1−xFe0.9Cu0.1O3−δ Dual-Phase Membranes for Efficient Oxygen Separation. Membranes, 10(8), 183. https://doi.org/10.3390/membranes10080183