Micellar-Enhanced Ultrafiltration Using a Plant-Derived Surfactant for Dye Separation in Wastewater Treatment
Abstract
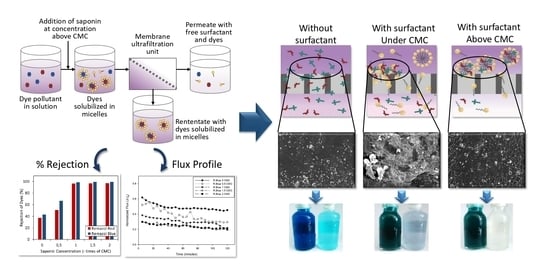
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Profile of Permeate Flux in the Surfactant-Enhanced Ultrafiltration System
3.2. Rejection of Dye and Saponin Residue
3.3. Performance of Surfactant in the MEUF System: Micelle Loading an Equilibrium Distribution Constant
3.4. Model of The Fouling Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Bade, R.; Lee, S. A review of studies on micellar enhanced ultrafiltration for heavy metals removal from wastewater. J. Water Sustain. 2011, 1, 85–102. [Google Scholar] [CrossRef]
- Acero, J.L.; Benitez, F.J.; Real, F.J.; Teva, F. Removal of emerging contaminants from secondary effluents by micellar-enhanced ultrafiltration. Sep. Purif. Technol. 2017, 181, 123–131. [Google Scholar] [CrossRef]
- Zaghbani, N.; Hafiane, A.; Dhahbi, M. Removal of Eriochrome Blue Black R from wastewater using micellar-enhanced ultrafiltration. J. Hazard. Mater. 2009, 168, 1417–1421. [Google Scholar] [CrossRef]
- Tehrani-bagha, A.R.; Singh, R.G.; Holmberg, K. Solubilization of two organic dyes by anionic, cationic and nonionic surfactants. Colloids Surfaces A 2013, 417, 133–139. [Google Scholar] [CrossRef]
- Aryanti, N.; Saraswati, A.; Putra, R.P.; Nafiunisa, A.; Wardhani, D.H. Fouling Mechanism of Micelle Enhanced Ultrafiltration With SDS Surfactant for Indigozol Dye Removal. J. Teknol. 2018, 80, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Peng, L.; Zeng, G.; Li, X.; Zhao, Y.; Liu, L.; Li, F.; Chai, Q. Evaluation of micellar enhanced ultrafiltration for removing methylene blue and cadmium ion simultaneously with mixed surfactants. Sep. Purif. Technol. 2014, 125, 83–89. [Google Scholar] [CrossRef]
- Puasa, S.W.; Ruzitah, M.S.; Kadir, S.A.S.A. An overview of Micellar—Enhanced Ultrafiltration in Wastewater Treatment Process. Int. Conf. Environ. Ind. Innov. 2011, 12, 167–172. [Google Scholar]
- Schwarze, M. Micellar-enhanced ultrafiltration (MEUF)—State of the art. Environ. Sci. Water Res. Technol. 2017, 3, 598–624. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, F.; Zeng, G.; Li, X.; Gu, Y.; Shi, L.; Liu, W.; Shi, Y. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar] [CrossRef]
- Li, X.; Zeng, G.M.; Huang, J.H.; Zhang, D.M.; Shi, L.J.; He, S.B.; Ruan, M. Simultaneous removal of cadmium ions and phenol with MEUF using SDS and mixed surfactants. Desalination 2011, 276, 136–141. [Google Scholar] [CrossRef]
- Samal, K.; Das, C.; Mohanty, K. Application of saponin biosurfactant and its recovery in the MEUF process for removal of methyl violet from wastewater. J. Environ. Manag. 2017, 203, 8–16. [Google Scholar] [CrossRef]
- Huang, J.; Qi, F.; Zeng, G.; Shi, L.; Li, X.; Gu, Y.; Shi, Y. Repeating recovery and reuse of SDS micelles from MEUF retentate containing Cd2+ by acidification UF. Colloids Surfaces A 2017, 520, 361–368. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Abdel, H.; Salman, K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Tarade, K.M.; Singhal, R.S.; Jayram, R.V.; Pandit, A.B. Kinetics of degradation of saponins in soybean flour (Glycine max.) during food processing. J. Food Eng. 2006, 76, 440–445. [Google Scholar] [CrossRef]
- Teng, J.; Shen, L.; Xu, Y.; Chen, Y.; Wu, X.L.; He, Y.; Chen, J.; Lin, H. Effects of molecular weight distribution of soluble microbial products (SMPs) on membrane fouling in a membrane bioreactor (MBR): Novel mechanistic insights. Chemosphere 2020, 248, 126013. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Lin, H.; Zhao, L.; Shen, L.; Li, R.; Xu, Y.; Hong, H.; He, Y. Membrane fouling caused by biological foams in a submerged membrane bioreactor: Mechanism insights. Water Res. 2020, 181, 115932. [Google Scholar] [CrossRef]
- You, X.; Teng, J.; Chen, Y.; Long, Y.; Yu, G.; Shen, L.; Lin, H. New insights into membrane fouling by alginate: Impacts of ionic strength in presence of calcium ions. Chemosphere 2020, 246, 125801. [Google Scholar] [CrossRef]
- Teng, J.; Wu, M.; Chen, J.; Lin, H.; He, Y. Different fouling propensities of loosely and tightly bound extracellular polymeric substances (EPSs) and the related fouling mechanisms in a membrane bioreactor. Chemosphere 2020, 255, 126953. [Google Scholar] [CrossRef]
- Kareru, P.G.; Keriko, J.M.; Gachanja, A.N.; Kenji, G.M. Direct detection of triterpenoid saponins in medical plants. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Melo, R.P.F.; Barros Neto, E.L.; Nunes, S.K.S.; Castro Dantas, T.N.; Dantas Neto, A.A. Removal of Reactive Blue 14 dye using micellar solubilization followed by ionic flocculation of surfactants. Sep. Purif. Technol. 2018, 191, 161–166. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Puasa, S.W.; Zulkali, M.M.D. Micellar-enhanced ultrafiltration for removal of reactive dyes from an aqueous solution. Desalination 2006, 191, 153–161. [Google Scholar] [CrossRef]
- Chang, E.E.; Yang, S.Y.; Huang, C.P.; Liang, C.H.; Chiang, P.C. Assessing the fouling mechanisms of high-pressure nanofiltration membrane using the modified Hermia model and the resistance-in-series model. Sep. Purif. Technol. 2011, 79, 329–336. [Google Scholar] [CrossRef]
- Iritani, E. A Review on Modeling of Pore-Blocking Behaviors of Membranes During Pressurized Membrane Filtration. Dry. Technol. 2013, 31, 146–162. [Google Scholar] [CrossRef]
- Vincent Vela, M.C.; Álvarez Blanco, S.; Lora García, J.; Bergantiños Rodríguez, E. Analysis of membrane pore blocking models adapted to crossflow ultrafiltration in the ultrafiltration of PEG. Chem. Eng. J. 2009, 149, 232–241. [Google Scholar] [CrossRef]
- Víctor-Ortega, M.D.; Martins, R.C.; Gando-Ferreira, L.M.; Quinta-Ferreira, R.M. Recovery of phenolic compounds from wastewaters through micellar enhanced ultrafiltration. Colloids Surfaces A 2017, 531, 18–24. [Google Scholar] [CrossRef]
- Rezaei, H.; Ashtiani, F.Z.; Fouladitajar, A. Fouling behavior and performance of microfiltration membranes for whey treatment in steady and unsteady-state conditions. Braz. J. Chem. Eng. 2014, 31, 503–518. [Google Scholar] [CrossRef] [Green Version]
- Bielska, M.; Sobczyńska, A.; Prochaska, K. Dye-surfactant interaction in aqueous solutions. Dye. Pigment. 2009, 80, 201–205. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.D.P.; Bueno, M.; Parada-Alfonso, F.; Cifuentes, A.; Ibáñez, E. Hansen solubility parameters for selection of green extraction solvents. TrAC Trends Anal. Chem. 2019, 118, 227–237. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Varughese, S.; Deshpande, A.P. Micellar characterisation of saponin from Sopindus mukorossi. Tenside Surfactants Deterg. 2006, 43, 262–268. [Google Scholar] [CrossRef]
- Tortora, F.; Innocenzi, V.; Mazziotti di Celso, G.; Vegliò, F.; Capocelli, M.; Piemonte, V.; Prisciandaro, M. Application of micellar-enhanced ultrafiltration in the pre-treatment of seawater for boron removal. Desalination 2018, 428, 21–28. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, G.M.; Huang, J.H.; Fang, Y.Y.; Xu, K.; Qu, Y.H.; Yang, C.P.; Li, J.B. Removal of zinc ions from aqueous solution using micellar-enhanced ultrafiltration at low surfactant concentrations. Water SA 2007, 33, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Bielska, M.; Szymanowski, J. Removal of methylene blue from waste water using micellar enhanced ultrafiltration. Water Res. 2006, 40, 1027–1033. [Google Scholar] [CrossRef]
- Córdova, A.; Astudillo, C.; Guerrero, C.; Vera, C.; Illanes, A. Assessment of the fouling mechanisms of an ultrafiltration membrane bioreactor during synthesis of galacto-oligosaccharides: Effect of the operational variables. Desalination 2016, 393, 79–89. [Google Scholar] [CrossRef]
- Grzegorzek, M.; Majewska-Nowak, K. The use of micellar-enhanced ultrafiltration (MEUF) for fluoride removal from aqueous solutions. Sep. Purif. Technol. 2018, 195, 1–11. [Google Scholar] [CrossRef]
- Petcu, A.R.; Rogozea, E.A.; Lazar, C.A.; Olteanu, N.L.; Meghea, A.; Mihaly, M. Specific interactions within micelle microenvironment in different charged dye/surfactant systems. Arab. J. Chem. 2016, 9, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Antony, A.; Leslie, G.; Le-Clech, P. Real-time monitoring of scale formation in reverse osmosis using electrical impedance spectroscopy. J. Memb. Sci. 2014, 453, 320–327. [Google Scholar] [CrossRef]
- Khayet, M. Fouling and Scaling in Desalination. DES 2016, 393, 1. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chong, T.H.; Fane, A.G. Colloidal interactions and fouling of NF and RO membranes: A review. Adv. Colloid Interface Sci. 2011, 164, 126–143. [Google Scholar] [CrossRef]










| Model of Blocking Mechanism | Linearized Equation | Physical Concept | Eq. Number |
|---|---|---|---|
| Complete Blocking | Formation of surface deposit | (7) | |
| Standard Blocking | Pore blocking and surface deposit | (8) | |
| Intermediate Blocking | Pore constriction | (9) | |
| Gel/Cake Formation | Pore blocking | (10) |
| Surfactant Concentration | Dye Concentration on Permeate (ppm) | Saponin Concentration on Permeate (ppm) | ||
|---|---|---|---|---|
| Remazol Red RB | Remazol Blue Tq | Remazol Red RB | Remazol Blue Tq | |
| 0 times CMC | 187.73 | 170.75 | 0 | 0 |
| 0.5 times CMC | 148.58 | 100.03 | 2246.25 | 2160.63 |
| 1 times CMC | 10.34 | 3.83 | 471.75 | 535.25 |
| 1.5 times CMC | 9.52 | 2.02 | 573.38 | 619 |
| 2 times CMC | 8.93 | 1.74 | 611.89 | 639 |
| Saponin Concentration | Micelle Loading (Lm) (mM/mM) | Equilibrium Distribution Constant (Kd) (mM/mM) | ||
|---|---|---|---|---|
| Remazol Red RB | Remazol Blue TQ | Remazol Red RB | Remazol Blue TQ | |
| 0.5 times CMC | 0.002 | 0.006 | 2.079 | 4.187 |
| 1 times CMC | 0.068 | 0.055 | 43.263 | 134.556 |
| 1.5 times CMC | 0.041 | 0.047 | 47.068 | 256.336 |
| 2 times CMC | 0.034 | 0.043 | 50.249 | 297.960 |
| Remazol Dye | CMC | Complete Blocking (n = 2) | Intermediate Blocking (n = 1) | Standard Blocking (n = 3/2) | Cake Formation (n = 0) | ||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | Kc | R2 | Ki | R2 | Ks | R2 | Kfc | ||
| Red RB | 0 (UF) | 0.923 | −0.004 | 0.924 | 1 × 10−4 | 0.952 | 1 × 10−4 | 0.921 | 3 × 10−7 |
| 0.5 | 0.878 | −0.003 | 0.887 | 1 × 10−4 | 0.888 | 2 × 10−4 | 0.912 | 1 × 10−6 | |
| 1 | 0.887 | −0.004 | 0.855 | 4 × 10−4 | 0.868 | 4 × 10−4 | 0.870 | 1 × 10−5 | |
| 1.5 | 0.876 | −0.004 | 0.871 | 3 × 10−4 | 0.872 | 3 × 10−4 | 0.829 | 4 × 10−6 | |
| 2 | 0.901 | −0.011 | 0.814 | 3.5 × 10−3 | 0.888 | 2.2 × 10−5 | 0.690 | 7 × 10−4 | |
| Blue TQ | 0 (UF) | 0.869 | −0.002 | 0.870 | 9 × 10−5 | 0.897 | 9 × 10−5 | 0.863 | 3 × 10−7 |
| 0.5 | 0.910 | −0.006 | 0.918 | 3 × 10−4 | 0.918 | 3 × 10−4 | 0.925 | 3 × 10−6 | |
| 1 | 0.876 | −0.004 | 0.849 | 4 × 10−4 | 0.857 | 4 × 10−4 | 0.776 | 2 × 10−5 | |
| 1.5 | 0.914 | −0.004 | 0.900 | 3 × 10−4 | 0.912 | 3 × 10−4 | 0.896 | 6 × 10−6 | |
| 2 | 0.975 | −0.003 | 0.948 | 2 × 10−4 | 0.948 | 2 × 10−4 | 0.942 | 4 × 10−6 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aryanti, N.; Nafiunisa, A.; Kusworo, T.D.; Wardhani, D.H. Micellar-Enhanced Ultrafiltration Using a Plant-Derived Surfactant for Dye Separation in Wastewater Treatment. Membranes 2020, 10, 220. https://doi.org/10.3390/membranes10090220
Aryanti N, Nafiunisa A, Kusworo TD, Wardhani DH. Micellar-Enhanced Ultrafiltration Using a Plant-Derived Surfactant for Dye Separation in Wastewater Treatment. Membranes. 2020; 10(9):220. https://doi.org/10.3390/membranes10090220
Chicago/Turabian StyleAryanti, Nita, Aininu Nafiunisa, Tutuk Djoko Kusworo, and Dyah Hesti Wardhani. 2020. "Micellar-Enhanced Ultrafiltration Using a Plant-Derived Surfactant for Dye Separation in Wastewater Treatment" Membranes 10, no. 9: 220. https://doi.org/10.3390/membranes10090220
APA StyleAryanti, N., Nafiunisa, A., Kusworo, T. D., & Wardhani, D. H. (2020). Micellar-Enhanced Ultrafiltration Using a Plant-Derived Surfactant for Dye Separation in Wastewater Treatment. Membranes, 10(9), 220. https://doi.org/10.3390/membranes10090220