Is the Bacterial Cellulose Membrane Feasible for Osteopromotive Property?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development of the Bacterial Cellulose Membrane
2.2. Samples
2.3. Experimental Surgery
2.4. Histological/Histometric Analysis
2.5. Immunohistochemical Analysis
2.6. Statistical Analysis
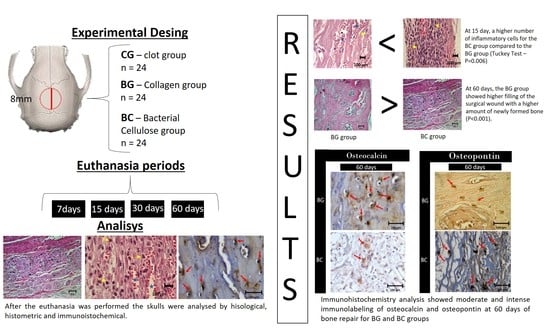
3. Results
3.1. Morphological Evaluation (Microscopic)
3.2. Histometric Analysis
3.2.1. Inflammatory Cells and Membrane
3.2.2. Newly Formed Bone
3.3. Immunohistochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seeman, E. Reduced bone formation and increased bone resorption: Rational targets for the treatment of osteoporosis. Osteoporos. Int. 2003, 14, 2–8. [Google Scholar] [CrossRef]
- Ingle, J.N.; Chlebowski, R.T.; Gralow, J.; Yee, G.C.; Janjan, N.A.; Cauley, J.A.; Blumenstein, B.A.; Albain, K.S.; Lipton, A.; Brown, S. American Society of Clinical Oncology. Update on the role of bisphosphonates and bone health issues in women with breast cancer. J. Clin. Oncol. 2003, 21, 4042–4057. [Google Scholar]
- Wallace, S.S.; Froum, S.J.; Cho, S.C.; Elian, N.; Monteiro, D.; Kim, B.S.; Tarnow, D.P. Sinus augmentation utilizing anorganic bovine bone (Bio-Oss) with absorbable and nonabsorbable membranes placed over the lateral window: Histomorphometric and clinical analyses. Int. J. Periodontics Restor. Dent. 2005, 25, 551–559. [Google Scholar]
- Sims, N.A.; Dupont, S.; Krust, A.; Clement-Lacroix, P.; Minet, D.; Resche-Rigon, M.; Gaillard-Kelly, M.; Baron, R. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 2002, 30, 18–25. [Google Scholar] [CrossRef]
- Faria, P.E.; Okamoto, R.; Bonilha-Neto, R.M.; Xavier, S.P.; Santos, A.C.; Salata, L.A. Immunohistochemical, tomographic and histological study on onlay iliac grafts remodeling. Clin. Oral. Implant. Res. 2008, 19, 393–401. [Google Scholar] [CrossRef]
- Alvarez, O.M.; Patel, M.; Booker, J.; Markowitz, L. Effectiveness of a biocellulose wound dressing for the treatment of chronic venous leg ulcers: Results of a single center randomized study involving 24 patients. Wounds-A Compend. Clin. Res. Pract. 2004, 16, 224–233. [Google Scholar]
- Kowalska-Ludwicka, K.; Cala, J.; Grobelski, B.; Sygut, D.; Jesionek-Kupnicka, D.; Kolodziejczyk, M.; Bielecki, S.; Pasieka, Z. Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch. Med. Sci. 2013, 9, 527–534. [Google Scholar] [CrossRef]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. A 2006, 76, 431–438. [Google Scholar] [CrossRef]
- Salata, L.A.; Hatton, P.V.; Devlin, A.J.; Craig, G.T.; Brook, I.M. In vitro and in vivo evaluation of e-PTFE and alkali-cellulose membranes for guided bone regeneration. Clin. Oral. Implant. Res. 2001, 12, 62–68. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Yang, F.; Shao, Y.; Zhang, X.; Dai, K. Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1034–1041. [Google Scholar] [CrossRef]
- Gruss, J.S.; Antonyshyn, O.; Phillips, J.H. Early definitive bone and soft-tissue reconstruction of major gunshot wounds of the face. Plast. Reconstr. Surg. 1991, 87, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Danieletto-Zanna, C.F.; Bizelli, V.F.; Ramires, G.A.D.A.; Francatti, T.M.; de Carvalho, P.S.P.; Bassi, A.P.F. Osteopromotion Capacity of Bovine Cortical Membranes in Critical Defects of Rat Calvaria: Histological and Immunohistochemical Analysis. Int. J. Biomater. 2020, 2020, 6426702. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, N.M. The use of collagen membranes to guide regeneration of new connective tissue attachment in dogs. J. Periodontol. 1988, 59, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Von Arx, T.; Buser, D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: A clinical study with 42 patients. Clin. Oral. Implant. Res. 2006, 17, 359–366. [Google Scholar] [CrossRef]
- Wang, H.L.; O’Neal, R.B.; Thomas, C.L.; Shyr, Y.; MacNeil, R.L. Evaluation of an absorbable collagen membrane in treating Class II furcation defects. J. Periodontol. 1994, 65, 1029–1036. [Google Scholar] [CrossRef]
- Crigger, M.; Bogle, G.C.; Garrett, S.; Gantes, B.G. Repair following treatment of circumferential periodontal defects in dogs with collagen and expanded polytetrafluoroethylene barrier membranes. J. Periodontol. 1996, 67, 403–413. [Google Scholar] [CrossRef]
- Yukna, C.N.; Yukna, R.A. Multi-center evaluation of bioabsorbable collagen membrane for guided tissue regeneration in human Class II furcations. J. Periodontol. 1996, 67, 650–657. [Google Scholar] [CrossRef]
- De Olyveira, G.M.; dos Santos, M.L.; dos Santos, R.C.; Costa, L.M.M.; Daltro, P.B.; Basmaii, P.; de Cerquerira, D.G.; Guastaldi, A.C. Physically Modified Bacterial Cellulose Biocomposites for Guided Tissue Regeneration. Sci. Adv. Mater. 2015, 7, 1657–1664. [Google Scholar] [CrossRef]
- Yoshino, A.; Tabuchi, M.; Uo, M.; Tatsumi, H.; Hideshima, K.; Kondo, S.; Sekine, J. Applicability of bacterial cellulose as an alternative to paper points in endodontic treatment. Acta Biomater. 2013, 9, 6116–6122. [Google Scholar] [CrossRef]
- Koike, T.; Sha, J.; Bai, Y.; Matsuda, Y.; Hideshima, K.; Yamada, T.; Kanno, T. Efficacy of Bacterial Cellulose as a Carrier of BMP-2 for Bone Regeneration in a Rabbit Frontal Sinus Model. Materials 2019, 12, 2489. [Google Scholar] [CrossRef] [Green Version]
- Enemark, H.; Sindet-Pedersen, S.; Bundgaard, M. Long-term results after secondary bone grafting of alveolar clefts. J. Oral. Maxillofac. Surg. 1987, 45, 913–919. [Google Scholar] [CrossRef]
- De Olyveira, G.M.; dos Santos, M.L.; Daltro, P.B.; Basmaji, P.; de Cerqueira Daltro, G.; Guastaldi, A.C. Bacterial cellulose/chondroitin sulfate for dental materials scaffolds. J. Biomater. Tissue Eng. 2014, 4, 150–154. [Google Scholar] [CrossRef]
- Codreanu, A.; Balta, C.; Herman, H.; Cotoraci, C.; Mihali, C.V.; Zurbau, N.; Zaharia, C.; Rapa, M.; Stanescu, P.; Radu, I.-C.; et al. Bacterial Cellulose-Modified Polyhydroxyalkanoates Scaffolds Promotes Bone Formation in Critical Size Calvarial Defects in Mice. Materials 2020, 13, 1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Olyveira, G.G.; Dos Santos Riccardi, C.; Dos Santos, M.L.; Costa, L.M.M.; Daltro, P.B.; Basmaji, P.; De Cerqueia Daltro, G.; Guastaldi, A.C. Bacterial celulose nanobiocomposites for periodontal disease. J. Bionanosci. 2014, 8, 319–324. [Google Scholar] [CrossRef]
- De Olyveira, G.M.; Filho, L.X.; Basmaji, P.; Costa, L.M.M. Bacterial nanocellulose for medicine regenerative. J. Nanotechnol. Eng. Med. 2011, 2, 034001. [Google Scholar] [CrossRef]
- User, G.; Manzine Costa, L.M.; Molina De Olyveira, G.; Basmaji, P.; Filho, L.X. Delivered by ingenta to: Bacterial Cellulose Towards Functional Green Composites Materials. J. Bionanoscience 2011, 5, 167–172. [Google Scholar]
- Olyveira, G.; Valido, D.P.; Costa, L.M.M.; Gois, P.B.P.; Xavier Filho, L.; Basmaji, P. First Otoliths/Collagen/Bacterial Cellulose Nanocomposites as a Potential Scaffold for Bone Tissue Regeneration. J. Biomater. Nanobiotechnol. 2011, 2, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Zellin, G.; Linde, A. Effects of different osteopromotive membrane porosities on experimental bone neogenesis in rats. Biomaterials 1996, 17, 695–702. [Google Scholar] [CrossRef]
- Biguetti, C.C.; De Olive, A.H.; Healy, K.; Mahmoud, R.H.; Custódio, I.D.C.; Constantino, D.H.; Ervolino, E.; Duarte, M.A.H.; Fakhouri, W.D.; Matsumoto, M.A. Medication-related osteonecrosis of the jaws after tooth extractio in senescent female mice trated with zoledronic acid: Microtomographic, histological and immunohistochemical characterization. PLoS ONE 2019, 14, e0214173. [Google Scholar] [CrossRef] [Green Version]
- Mada, E.Y.; Santos, A.C.C.; Fonseca, A.C.; Biguetti, C.C.; Neves, F.T.A.; Saraiva, P.P.; Matsumoto, M.A. Effects of green tea and bisphosphonate association on dental socket repair of rats. Arch. Oral. Biol. 2017, 75, 1–7. [Google Scholar] [CrossRef]
- Maciel, J.; Momesso, G.A.; Ramalho-Ferreira, G.; Consolaro, R.B.; Perri de Carvalho, P.S.; Faverani, L.P.; Farnezi Bassi, A.P. Bone Healing Evaluation in Critical-Size Defects Treated With Xenogenous Bone Plus Porcine Collagen. Implantdentistry 2017, 26, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Palin, L.P.; Polo, T.O.B.; Batista, F.R.S.; Gomes-Ferreira, P.H.S.; Garcia Junior, I.R.; Rossi, A.C.; Freire, A.; Faverani, L.P.; Sumida, D.H.; Okamoto, R. Daily melatonin administration improves osseointegration in pinealectomized rats. J. Appl. Oral. Sci. 2018, 10, e20170470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassumi, J.S.; Mulinari-Santos, G.; Fabris, A.L.D.S.; Jacob, R.G.M.; Gonçalves, A.; Rossi, A.C.; Freire, A.R.; Faverani, L.P.; Okamoto, R. Alveolar bone healing in rats: Micro-CT, immunohistochemical and molecular analysis. J. Appl. Oral. Sci. 2018, 18, e20170326. [Google Scholar] [CrossRef] [PubMed]
- Yogui, F.C.; Momesso, G.A.C.; Faverani, L.P.; Polo, T.O.B.; Ramalho-Ferreira, G.; Hassumi, J.S.; Rossi, A.C.; Freire, A.R.; Prado, F.B.; Okamoto, R. A SERM increasing the expression of the osteoblastogenesis and mineralization-related proteins and improving quality of bone tissue in an experimental model of osteoporosis. J. Appl. Oral. Sci. 2018, 26, e20170329. [Google Scholar] [CrossRef] [PubMed]
- Faverani, L.P.; Polo, T.O.B.; Ramalho-Ferreira, G.; Momesso, G.A.C.; Hassumi, J.S.; Rossi, A.C.; Freire, A.R.; Prado, F.B.; Luvizuto, E.R.; Gruber, R.; et al. Raloxifene but not alendronate can compensate the impaired osseointegration in osteoporotic rats. Clin. Oral. Investig. 2018, 22, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Luvizuto, E.R.; de Oliveira, J.C.S.; Gomes-Ferreira, P.H.S.; Pereira, C.C.S.; Faverani, L.P.; Antoniali, C.; Okamoto, R. Immunohistochemical response in rats of beta-tricalcium phosphate (TCP) with or without BMP-2 in the production of collagen matrix critical defects. Acta Histochem. 2017, 119, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.; Hassumi, J.S.; Gomes-Ferreira, P.H.; Polo, T.O.; Ferreira, G.R.; Faverani, L.P.; Okamoto, R. Short term sodium alendronate administration improves the peri- implant bone quality in osteoporotic animals. J. Appl. Oral. Sci. 2017, 25, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Ramalho-Ferreira, G.; Faverani, L.P.; Momesso, G.A.C.; Luvizuto, E.R.; de Oliveira Puttini, I.; Okamoto, R. Effect of antiresorptive drugs in the alveolar bone healing. A histometric and immunohistochemical study in ovariectomized rats. Clin. Oral. Investig. 2017, 21, 1485–1494. [Google Scholar] [CrossRef]
- Oortgiesen, D.P.; Plachokova, A.S.; Geenen, C.; Meijer, G.J.; Walboomers, X.F.; van den Beucken, J.J.; Jansen, J.A. Alkaline phosphatase immobilization onto Bio-Gide and Bio-Oss for periodontal and bone regeneration. J. Clin. Periodontol. 2012, 39, 546–555. [Google Scholar] [CrossRef]
- Vajgel, A.; Mardas, N.; Farias, B.C.; Petrie, A.; Cimões, R.; Donos, N. A systematic review on the critical size defect model. Clin. Oral. Implant. Res. 2014, 25, 879–893. [Google Scholar] [CrossRef]
- Kozloysky, A.; Aboodi, G.; Moses, O.; Tal, H.; Artzi, Z.; Weinreb, M.; Nemcovsky, C.E. Bio-degradation of a resorbable collagen membrane (Bio-Gide) applied in a double-layer technique in rats. Clin. Oral. Implant. Res. 2009, 20, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; An, S.J.; Bae, E.B.; Gwon, H.J.; Park, J.S.; Jeong, S.I.; Jeon, Y.C.; Lee, S.H.; Lim, Y.M.; Huh, J.B. The effect of bacterial celulose membrane compared with collagen membrane on guided bone regeneration. J. Adv. Prosthodont. 2015, 7, 484–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, Z.; Qureshi, J.; Alshahrani, M.A.; Nassar, H.; Ikeda, Y.; Glogauer, M.; Ganss, B. Collagen based barrier Membr. for periodontal guided bone regeneration applications. Odontology 2016, 105, 1–12. [Google Scholar] [CrossRef] [PubMed]









| Membranes | 7 | 15 | ||
|---|---|---|---|---|
| Cells | Vessels | Cells | Vessels | |
| Collagen | 10.22 ± 2.08 * | 7.14 ± 1.05 | 5.20 ± 2.40 | 9.98 ± 2.22 |
| Bacterial | 14.06 ± 3.11 | 5.46 ± 2.33 | 8.02 ± 1.25 | 5.04 ± 1.29 |
| p = 0.019 | p = 0.163 | p = 0.072 | p < 0.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farnezi Bassi, A.P.; Bizelli, V.F.; Brasil, L.F.d.M.; Pereira, J.C.; Al-Sharani, H.M.; Momesso, G.A.C.; Faverani, L.P.; Lucas, F.d.A. Is the Bacterial Cellulose Membrane Feasible for Osteopromotive Property? Membranes 2020, 10, 230. https://doi.org/10.3390/membranes10090230
Farnezi Bassi AP, Bizelli VF, Brasil LFdM, Pereira JC, Al-Sharani HM, Momesso GAC, Faverani LP, Lucas FdA. Is the Bacterial Cellulose Membrane Feasible for Osteopromotive Property? Membranes. 2020; 10(9):230. https://doi.org/10.3390/membranes10090230
Chicago/Turabian StyleFarnezi Bassi, Ana Paula, Vinícius Ferreira Bizelli, Leticia Freitas de Mendes Brasil, Járede Carvalho Pereira, Hesham Mohammed Al-Sharani, Gustavo Antonio Correa Momesso, Leonardo P. Faverani, and Flavia de Almeida Lucas. 2020. "Is the Bacterial Cellulose Membrane Feasible for Osteopromotive Property?" Membranes 10, no. 9: 230. https://doi.org/10.3390/membranes10090230
APA StyleFarnezi Bassi, A. P., Bizelli, V. F., Brasil, L. F. d. M., Pereira, J. C., Al-Sharani, H. M., Momesso, G. A. C., Faverani, L. P., & Lucas, F. d. A. (2020). Is the Bacterial Cellulose Membrane Feasible for Osteopromotive Property? Membranes, 10(9), 230. https://doi.org/10.3390/membranes10090230