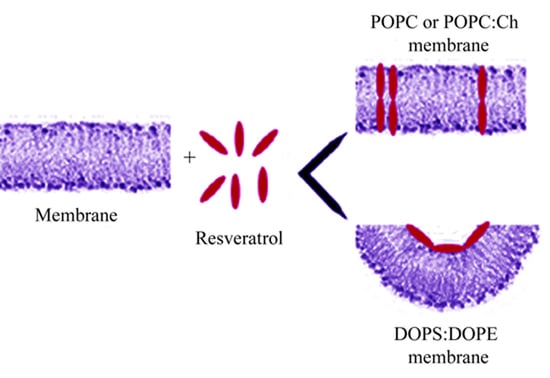
Study of Resveratrol’s Interaction with Planar Lipid Models: Insights into Its Location in Lipid Bilayers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Experimental
2.2.1. Preparation of Resveratrol Solution
2.2.2. Preparation of PLMs
2.2.3. Determination of Conductance of Channel-Like Events
2.2.4. Determination of PLM Capacitance
3. Results
3.1. Membrane Stability
3.2. Resveratrol Interaction with POPC PLMs
3.3. Resveratrol Interaction with POPC:Ch PLM
3.4. Resveratrol Interaction with DOPS:DOPE PLMs
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Brittes, J.; Lúcio, M.; Nunes, C.; Lima, J.L.; Reis, S. Effects of Resveratrol on Membrane Biophysical Properties: Relevance for Its Pharmacological Effects. Chem. Phys. Lipids 2010, 163, 747–754. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and Antifungal Properties of Resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Perin, C.P.; Cassader, M. Anti-inflammatory and Antioxidant Effects of Resveratrol in Healthy Smokers a Randomized, Double-Blind, Placebo-Controlled, Cross-over Trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Lançon, A.; Frazzi, R.; Latruffe, N. Anti-Oxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Resveratrol in Ocular Diseases. Molecules 2016, 21, 304. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Multiple Molecular Targets of Resveratrol: Anti-Carcinogenic Mechanisms. Arch. Biochem. Biophys. 2009, 486, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [Green Version]
- Baxter, R.A. Anti-aging Properties of Resveratrol: Review and Report of a Potent New Antioxidant Skin Care Formulation. J. Cosmet. Dermatol. 2008, 7, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.Q. Role of Resveratrol in Regulating Cutaneous Functions. Evid. Based Complement. Alternat. Med. 2020, 2020, 2416837. [Google Scholar] [CrossRef] [Green Version]
- Braidy, N.; Jugder, B.E.; Poljak, A.; Jayasena, T.; Mansour, H.; Nabavi, S.M.; Sachdev, P.; Grant, R. Resveratrol as a Potential Therapeutic Candidate for the Treatment and Management of Alzheimer’s Disease. Curr. Top. Med. Chem. 2016, 16, 1951–1960. [Google Scholar] [CrossRef]
- Granzotto, A.; Zatta, P. Resveratrol and Alzheimer’s Disease: Message in a Bottle on Red Wine and Cognition. Front. Aging Neurosci. 2014, 6, 95. [Google Scholar] [CrossRef]
- Hartman, R.E.; Shah, A.; Fagan, A.M.; Schwetye, K.E.; Parsadanian, M.; Schulman, R.N.; Finn, M.B.; Holtzman, D.M. Pomegranate Juice Decreases Amyloid Load and Improves Behavior in a Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2006, 24, 506–515. [Google Scholar] [CrossRef]
- Koukoulitsa, C.; Villalonga-Barber, C.; Csonka, R.; Alexi, X.; Leonis, G.; Dellis, D.; Hamelink, E.; Belda, O.; Steele, B.R.; Micha-Screttas, M.; et al. Biological and Computational Evaluation of Resveratrol Inhibitors against Alzheimer’s Disease. J. Enzyme Inhib. Med. Chem. 2016, 31, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Potent Anti-Amyloidogenic and Fibril-Destabilizing Effects of Polyphenols in Vitro: Implications for the Prevention and Thera-Peutics of Alzheimer’s Disease. J. Neurochem. 2003, 87, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Pasinetti, G.M.; Wang, J.; Ho, L.; Zhao, W.; Dubner, L. Roles of Resveratrol and Other Grape-Derived Polyphenols in Alzheimer’s Disease Prevention and Treatment. Biochim. Biophys. Acta 2015, 1852, 1202–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rege, S.D.; Geetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective Effects of Resveratrol in Alzheimer Disease Pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef] [Green Version]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective Action of Resveratrol. Biochim. Biophys. Acta 2015, 1852, 1195–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yashiro, T.; Nanmoku, M.; Shimizu, M.; Inoue, J.; Sato, R. Resveratrol Increases the Expression and Activity of the Low Density Lipoprotein Receptor in Hepatocytes by the Proteolytic Activation of the Sterol Regulatory Element-Binding Proteins. Atherosclerosis 2012, 220, 369–374. [Google Scholar] [CrossRef]
- Voloshyna, I.; Hussaini, S.M.; Reiss, A.B. Resveratrol in Cholesterol Metabolism and Atherosclerosis. J. Med. Food 2012, 15, 763–773. [Google Scholar] [CrossRef]
- Galfi, P.; Jakus, J.; Molnar, T.; Neogrady, S.; Csordas, A. Divergent Effects of Resveratrol, a Polyphenolic Phytostilbene, on Free Radical Levels and Type of Cell Death Induced by the Histone Deacetylase Inhibitors Butyrate and Trichostatin A. J. Steroid Biochem. Mol. Biol. 2005, 94, 39–47. [Google Scholar] [CrossRef]
- Frankel, E.N.; Waterhouse, A.L.; Kinsella, J.E. Inhibition of Human LDL Oxidation by Resveratrol. Lancet 1993, 341, 1103–1104. [Google Scholar] [CrossRef]
- Jensen, M.D.; Sheng, W.; Simonyi, A.; Johnson, G.S.; Sun, A.Y.; Sun, G.Y. Involvement of Oxidative Pathways in Cytokine-Induced Secretory Phospholipase A2-IIA in Astrocytes. Neurochem. Int. 2009, 55, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.Y.; Shelat, P.B.; Jensen, M.B.; He, Y.; Sun, A.Y.; Simonyi, A. Phospholipases a2 and Inflammatory Responses in the Central Nervous System. Neuromol. Med. 2010, 12, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.; Chintala, R.; Duddukuri, G.R. Targeting Arachidonic Acid Pathway by Natural Products for Cancer Prevention and Therapy. Semin. Cancer Biol. 2016, 40, 48–81. [Google Scholar] [CrossRef]
- Fei, Q.; Kent, D.; Botello-Smith, W.M.; Nur, F.; Nur, S.; Alsamarah, A.; Chatterjee, P.; Lambros, M.; Luo, Y. Molecular Mechanism of Resveratrol’s Lipid Membrane Protection. Sci. Rep. 2018, 8, 1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabris, S.; Momo, F.; Ravagnan, G.; Stevanato, R. Antioxidant Properties of Resveratrol and Piceid on Lipid Peroxidation in Micelles and Monolamellar Liposomes. Biophys. Chem. 2008, 135, 76–83. [Google Scholar] [CrossRef]
- Ghellinck, d.A.; Shen, C.; Fragneto, G.; Klösgen, B. Probing the Position of Resveratrol in Lipid Bilayers: A Neutron Reflectivity Study. Colloids Surf. B Biointerfaces 2015, 134, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Suga, K.; Hayashi, K.; Okamoto, Y.; Umakoshi, H. Multi-Level Characterization of the Membrane Properties of Resveratrol-Incorporated Liposomes. J. Phys. Chem. B 2017, 121, 4091–4098. [Google Scholar] [CrossRef]
- Cardia, M.C.; Caddeo, C.; Lai, F.; Fadda, A.M.; Sinico, C.; Luhmer, M. H NMR Study of the Interaction of Trans-Resveratrol with Soybean Phosphatidylcholine Liposomes. Sci. Rep. 2019, 9, 17736. [Google Scholar] [CrossRef]
- Bonechi, C.; Martini, S.; Ciani, L.; Lamponi, S.; Rebmann, H.; Rossi, C.; Ristori, S. Using Liposomes as Carriers for Polyphenolic Compounds: The Case of Trans-Resveratrol. PLoS ONE 2012, 7, e41438. [Google Scholar] [CrossRef] [PubMed]
- Balanc, B.; Ota, A.; Djordjevic, V.; Sentjurc, M.; Nedovic, V.; Bugarski, B.; Poklar, U.N. Resveratrol-Loaded Liposomes: Interaction of Resveratrol with Phospholipids. Eur. J. Lipid Sci. Technol. 2015, 117, 1615–1626. [Google Scholar] [CrossRef]
- Neves, A.R.; Nunes, C.; Reis, S. Resveratrol Induces Ordered Domains Formation in Biomembranes: Implication for Its Pleiotropic Action. Biochim. Biophys. Acta 2016, 1858, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Neves, A.R.; Nunes, C.; Reis, S. New Insights on the Biophysical Interaction of Resveratrol with Biomembrane Models: Relevance for Its Biological Effects. J. Phys. Chem. B 2015, 119, 11664–11672. [Google Scholar] [CrossRef]
- Martinović, N.; Abramovič, H.; Poklar, U.N. Inhibition of Copper-Induced Lipid Peroxidation by Sinapic Acid and Its Derivatives in Correlation to Their Effect on the Membrane Structural Properties. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.; Loureiro, A.; Pereira, M. The Biophysical Interaction of Ferulic Acid with Liposomes as Biological Membrane Model: The Effect of the Lipid Bilayer Composition. J. Mol. Liq. 2020. [Google Scholar] [CrossRef]
- Gutiérrez, M.E.; García, A.F.; Madariaga, A.M.; Sagrista, M.L.; Casadó, F.J.; Mora, M. Interaction of Tocopherols and Phenolic Compounds with Membrane Lipid Components: Evaluation of Their Antioxidant Activity in a Liposomal Model System. Life Sci. 2003, 72, 2337–2360. [Google Scholar] [CrossRef]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of Liposomal Membrane Fluidity by Flavonoids and Isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Nagayama, M.; Tanaka, T.; Furusawa, M.; Kashimata, M.; Takeuchi, H. Membrane-Rigidifying Effects of Anti-cancer Dietary Factors. Biofactors 2002, 16, 45–56. [Google Scholar] [CrossRef]
- Selvaraj, S.; Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Dose-Dependent Interaction of Trans-Resveratrol with Biomembranes: Effects on Antioxidant Property. J. Med. Chem. 2013, 56, 970–981. [Google Scholar] [CrossRef]
- Shahane, G.; Ding, W.; Palaiokostas, M.; Orsi, M. Physical Properties of Model Biological Lipid Bilayers: Insights from All-Atom Molecular Dynamics Simulations. J. Mol. Model. 2019, 25, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugova, T.N.; Balgavý, P. Molecular Volumes of DOPC and DOPS in Mixed Bilayers of Multilamellar Vesicles. Phys. Chem. Chem. Phys. 2014, 16, 18211–18216. [Google Scholar] [CrossRef] [PubMed]
- Rand, R.P.; Fuller, N.L. Structural Dimensions and Their Changes in a Reentrant Hexagonal-Lamellar Transition of Phospholipids. Biophys. J. 1994, 66, 2127–2138. [Google Scholar] [CrossRef] [Green Version]
- Suetsugu, S.; Kurisu, S.; Takenawa, T. Dynamic Shaping of Cellular Membranes by Phospholipids and Membrane-Deforming Proteins. Physiol. Rev. 2014, 94, 1219–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tien, H.; Ottova, A.-L. Membrane Biophysics: As Viewed from Experimental Bilayer Lipid Membranes; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Tien, H.; Ottova, A.-L. The Lipid Bilayer Concept and Its Experimental Realization: From Soap Bubbles, Kitchen Sink, to Bilayer Lipid Membranes. J. Membr. Sci. 2001, 189, 83–117. [Google Scholar] [CrossRef]
- Müller, P.; Rudin, D.; Tien, T.; Weacott, W. Reconstitution of Cell Membrane Structure in Vitro and Its Trasformation into an Excitable System. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Tien, T.; Mountz, J.; Martinosi, A. Protein-Lipid Interaction in Bilayer Lipid Membranes (BLM). In Enzyme of Biological Membranes; NY Plenum: New York, NY, USA, 1977; pp. 139–170. [Google Scholar]
- Micelli, S.; Gallucci, E.; Meleleo, D.; Stipani, V.; Picciarelli, V. Mitochondrial Porin Incorporation into Black Lipid Membranes: Ionic and Gating Contribution to the Total Current. Bioelectrochemistry 2002, 57, 97–106. [Google Scholar] [CrossRef]
- Faroux, J.M.; Ureta, M.M.; Tymczyszyn, E.E.; Gómez-Zavaglia, A. An Overview of Peroxidation Reactions Using Liposomes as Model Systems and Analytical Methods as Monitoring Tools. Colloids Surf. B Biointerfaces 2020, 195, 111254. [Google Scholar] [CrossRef]
- Koller, D.; Lohner, K. The Role of Spontaneous Lipid Curvature in the Interaction of Interfacially Active Peptides with Membranes. Biochim. Biophys. Acta 2014, 1838, 2250–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullis, P.R.; de Kruijff, B. Lipid Polymorphism and the Functional Roles of Lipids in Biological Membranes. Biochim. Biophys. Acta 1979, 559, 399–420. [Google Scholar] [CrossRef]
- Koukoulitsa, C.; Durdagi, S.; Siapi, E.; Villalonga-Barber, C.; Alexi, X.; Steele, B.R.; Micha, M.-S.; Alexis, M.N.; Tsantili-Kakoulidou, A.; Mavromoustakos, T. Comparison of Thermal Effects of Stilbenoid Analogs in Lipid Bilayers Using Differential Scanning Calorimetry and Molecular Dynamics: Correlation of Thermal Effects and Topographical Position with Antioxidant Activity. Eur. Biophys. J. 2011, 40, 865–875. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fu, Y.; Yang, P.; Liu, X.; Li, Y.; Gu, Z. ROS Scavenging Biopolymers for Anti-Inflammatory Diseases: Classification and Formulation. Adv. Mater. Interfaces 2020, 7. [Google Scholar] [CrossRef]
- Rand, R.P.; Fuller, N.L.; Gruner, S.M.; Parsegian, V.A. Membrane Curvature, Lipid Segregation, and Structural Transitions for Phospholipids under Dual-Solvent Stress. Biochemistry 1990, 29, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Sodt, A.J.; Pastor, R.W. Molecular Modeling of Lipid Membrane Curvature Induction by a Peptide: More Than Simply Shape. Biophys. J. 2014, 106, 1958–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haney, E.F.; Nathoo, S.; Vogel, H.J.; Prenner, E.J. Induction of Non-lamellar Lipid Phases by Antimicrobial Peptides: A Potential Link to Mode of Action. Chem. Phys. Lipids 2010, 163, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, E.; Meleleo, D.; Micelli, S.; Picciarelli, V. Magainin 2 Channel Formation in Planar Lipid Membranes: The Role of Lipid Polar Groups and Ergosterol. Eur. Biophys. J. 2003, 32, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Mitani, Y.; Akada, K.Y.; Murase, O.; Yoneyama, S.; Zasloff, M.; Miyajima, K. Mechanism of Synergism between Antimicrobial Peptides Magainin 2 and PGLa. Biochemistry 1998, 37, 15144–15153. [Google Scholar] [CrossRef]
- Yesylevskyy, S.; Rivel, T.; Ramseyer, C. Curvature Increases Permeability of the Plasma Membrane for Ions, Water and the Anti-cancer Drugs Cisplatin and Gemcitabine. Sci. Rep. 2019, 9, 17214. [Google Scholar] [CrossRef] [Green Version]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.E.; Lee, D.K.; Ramamoorthy, A. Determining the Effects of Lipophilic Drugs on Membrane Structure by Solid-State NMR Spectroscopy: The Case of the Antioxidant Curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef] [Green Version]
- Basso, L.G.; Rodrigues, R.Z.; Naal, R.M.; Costa-Filho, A.J. Effects of the Antimalarial Drug Primaquine on the Dynamic Structure of Lipid Model Membranes. Biochim. Biophys. Acta 2011, 1808, 55–64. [Google Scholar] [CrossRef] [Green Version]






| [Res] = 10 µM | [Res] = 20 µM | |||
|---|---|---|---|---|
| Vs mV | Λc ± SE nS | F ± SD | Λc ± SE nS | F ± SD |
| 120 | 0.012 ± 0.003 | 12.28 ± 1.08 | ||
| 100 | 0.022 ± 0.002 | 13.90 ± 1.48 | ||
| 80 | 0.019 ± 0.006 | 14.47 ± 0.74 | ||
| 60 | 0.029 ± 0.004 | 9.88 ± 0.52 | 0.034 ± 0.001 | 12.20 ± 0.55 |
| 40 | 0.046 ± 0.003 | 13.03 ± 0.52 | ||
| −40 | 0.054 ± 0.003 | 12.50 ± 1.01 | ||
| −60 | 0.030 ± 0.003 | 16.97 ± 0.98 | 0.034 ± 0.001 | 9.71 ± 0.51 |
| −80 | 0.016 ± 0.008 | 30.28 ± 4.16 | ||
| −100 | 0.020 ± 0.001 | 21.93 ± 1.35 | ||
| −120 | 0.016 ± 0.0006 | 18.78 ± 2.21 | ||
| Time | C ± SE µF/cm2 [Res] = 10 µM | C ± SE µF/cm2 [Res] = 20 µM |
|---|---|---|
| T0 | 0.27 ± 0.01 | 0.28 ± 0.01 |
| T1 | 0.11 ± 0.002 | 0.10 ± 0.003 |
| T2 | 0.28 ± 0.02 | 0.29 ± 0.01 |
| T3 | 0.30 ± 0.02 | 0.31 ± 0.02 |
| [Res] = 10 µM | [Res] = 20 µM | |||
|---|---|---|---|---|
| Vs mV | Λc ± SE nS | F ± SD | Λc ± SE nS | F ± SD |
| 120 | 0.020 ± 0.001 | 11.10 ± 0.49 | ||
| 100 | 0.020 ± 0.001 | 9.63 ± 0.62 | ||
| 80 | 0.019 ± 0.002 | 6.80 ± 0.47 | ||
| 40 | 0.065 ± 0.003 | 9.87 ± 0.86 | ||
| −40 | 0.050 ± 0.003 | 6.95 ± 0.70 | ||
| −80 | 0.020 ± 0.002 | 10.78 ± 0.79 | ||
| −100 | 0.021 ± 0.0006 | 12.74 ± 0.60 | ||
| −120 | 0.016 ± 0.0004 | 7.20 ± 1.07 | ||
| Time | C ± SE µF/cm2 [Res] = 10 µM | C ± SE µF/cm2 [Res] = 20 µM |
|---|---|---|
| T0 | 0.30 ± 0.02 | 0.30 ± 0.02 |
| T1 | 0.11 ± 0.002 | 0.12 ± 0.002 |
| T2 | 0.29 ± 0.01 | 0.28 ± 0.01 |
| T3 | 0.30 ± 0.02 | 0.31 ± 0.01 |
| Time | C ± SE µF/cm2 [Res] = 10 µM | C ± SE µF/cm2 [Res] = 20 µM |
|---|---|---|
| T0 | 0.26 ± 0.01 | 0.28 ± 0.01 |
| T1 | 0.10 ± 0.003 | 0.11 ± 0.002 |
| T2 | 0.10 ± 0.002 | 0.11 ± 0.002 |
| T3 | 0.10 ± 0.003 | 0.12 ± 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meleleo, D. Study of Resveratrol’s Interaction with Planar Lipid Models: Insights into Its Location in Lipid Bilayers. Membranes 2021, 11, 132. https://doi.org/10.3390/membranes11020132
Meleleo D. Study of Resveratrol’s Interaction with Planar Lipid Models: Insights into Its Location in Lipid Bilayers. Membranes. 2021; 11(2):132. https://doi.org/10.3390/membranes11020132
Chicago/Turabian StyleMeleleo, Daniela. 2021. "Study of Resveratrol’s Interaction with Planar Lipid Models: Insights into Its Location in Lipid Bilayers" Membranes 11, no. 2: 132. https://doi.org/10.3390/membranes11020132
APA StyleMeleleo, D. (2021). Study of Resveratrol’s Interaction with Planar Lipid Models: Insights into Its Location in Lipid Bilayers. Membranes, 11(2), 132. https://doi.org/10.3390/membranes11020132