Iminodiacetic Acid (IDA) Cation-Exchange Nonwoven Membranes for Efficient Capture of Antibodies and Antibody Fragments
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Cation-Exchange Membranes
2.3. Membrane Characterization
2.4. Static Protein-Binding Experiments
2.5. Dynamic Protein-Binding Experiments
2.6. DBC100% Measurement by Capturing Spiked IgG and scFv from CHO Supernatants
2.7. Selectivity Evaluation by Separation of IgG from IgG/BSA Mixtures
2.8. Capturing Monoclonal Antibody from Cell Culture Supernatant with Five Reuse Cycles
3. Results and Discussion
3.1. Preparation and Characterization of CEX-IDA Membranes
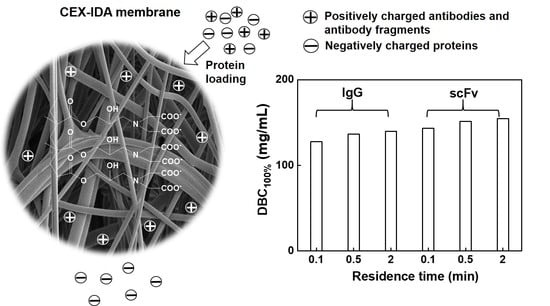
3.2. DBC Evaluation and Comparison
3.3. Evaluation of CEX-IDA Membranes in Competitive Conditions
3.4. Membrane Selectivity Evaluation
3.5. Capture of a Monoclonal Antibody from Cell Culture Supernatant
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grilo, A.L.; Mantalaris, A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. 2019, 37, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, J.X.; Gowtham, Y.K.; Harcum, S.W.; Husson, S.M. Antibody purification from CHO cell supernatant using new multimodal membranes. Biotechnol. Prog. 2017, 33, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Hardick, O.; Dods, S.; Stevens, B.; Bracewell, D.G. Nanofiber adsorbents for high productivity downstream processing. Biotechnol. Bioeng. 2013, 110, 1119–1128. [Google Scholar] [CrossRef]
- Fan, J.X.; Luo, J.Q.; Song, W.J.; Chen, X.R.; Wan, Y.H. Directing membrane chromatography to manufacture alpha(1)-antitrypsin from human plasma fraction IV. J. Chromatogr. A 2015, 1423, 63–70. [Google Scholar] [CrossRef]
- Orr, V.; Zhong, L.Y.; Moo-Young, M.; Chou, C.P. Recent advances in bioprocessing application of membrane chromatography. Biotechnol. Adv. 2013, 31, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Yao, D.X.; Yang, H.; Yin, J.; Wang, H.; Zhang, Y.F.; Meng, J.Q. Surface modification of cellulose membranes to prepare a high-capacity membrane adsorber for monoclonal antibody purification via hydrophobic charge-induction chromatography. Ind. Eng. Chem. Res. 2018, 57, 13235–13246. [Google Scholar] [CrossRef]
- Hou, Y.; Brower, M.; Pollard, D.; Kanani, D.; Jacquemart, R.; Kachuik, B.; Stout, J. Advective hydrogel membrane chromatography for monoclonal antibody purification in bioprocessing. Biotechnol. Prog. 2015, 31, 974–982. [Google Scholar] [CrossRef]
- Li, Z.; Gu, Q.; Coffman, J.L.; Przybycien, T.; Zydney, A.L. Continuous precipitation for monoclonal antibody capture using countercurrent washing by microfiltration. Biotechnol. Prog. 2019, 35, 8. [Google Scholar] [CrossRef]
- Fields, C.; Li, P.; O’Mahony, J.J.; Lee, G.U. Advances in affinity ligand-functionalized nanomaterials for biomagnetic separation. Biotechnol. Bioeng. 2016, 113, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.J.; Bennett, A.L.; Ning, W.J.; Tan, H.Y.; Berwanger, J.D.; Zeng, X.Q.; Bruening, M.L. Monoclonal antibody capture and analysis using porous membranes containing immobilized peptide mimotopes. Anal. Chem. 2018, 90, 12161–12167. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.X.; Si, Y.; Duan, C.; Yan, Z.S.; Liu, L.F.; Yu, J.Y.; Ding, B. Highly carboxylated, cellular structured, and underwater superelastic nanofibrous aerogels for efficient protein separation. Adv. Funct. Mater. 2019, 29, 1808234. [Google Scholar] [CrossRef]
- Herigstad, M.O.; Dimartino, S.; Boi, C.; Sarti, G.C. Experimental characterization of the transport phenomena, adsorption, and elution in a protein A affinity monolithic medium. J. Chromatogr. A 2015, 1407, 130–138. [Google Scholar] [CrossRef]
- Rajesh, S.; Schneiderman, S.; Crandall, C.; Fong, H.; Menkhaus, T.J. Synthesis of cellulose-graft-polypropionic acid nanofiber cation-exchange membrane adsorbers for high-efficiency separations. ACS Appl. Mater. Interfaces 2017, 9, 41055–41065. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Wickramasinghe, S.R.; Qian, X.H. Membrane chromatography for protein purifications from ligand design to functionalization. Sep. Sci. Technol. 2017, 52, 299–319. [Google Scholar] [CrossRef]
- Muthukumar, S.; Muralikrishnan, T.; Mendhe, R.; Rathore, A.S. Economic benefits of membrane chromatography versus packed bed column purification of therapeutic proteins expressed in microbial and mammalian hosts. J. Chem. Technol. Biotechnol. 2017, 92, 59–68. [Google Scholar] [CrossRef]
- Somasundaram, B.; Pleitt, K.; Shave, E.; Baker, K.; Lua, L.H.L. Progression of continuous downstream processing of monoclonal antibodies: Current trends and challenges. Biotechnol. Bioeng. 2018, 115, 2893–2907. [Google Scholar] [CrossRef]
- Ning, W.J.; Wijeratne, S.; Dong, J.L.; Bruening, M.L. Immobilization of carboxymethylated polyethylenimine-metal-ion complexes in porous membranes to selectively capture his-tagged protein. ACS Appl. Mater. Interfaces 2015, 7, 2575–2584. [Google Scholar] [CrossRef]
- Fu, Q.X.; Wang, X.Q.; Si, Y.; Liu, L.F.; Yu, J.Y.; Ding, B. Scalable fabrication of electrospun nanofibrous membranes functionalized with citric acid for high-performance protein adsorption. ACS Appl. Mater. Interfaces 2016, 8, 11819–11829. [Google Scholar] [CrossRef]
- Dou, X.Y.; Wang, Q.; Li, Z.L.; Ju, J.P.; Wang, S.; Hao, L.Y.; Sui, K.Y.; Xia, Y.Z.; Tan, Y.Q. Seaweed-derived electrospun nanofibrous membranes for ultrahigh protein adsorption. Adv. Funct. Mater. 2019, 29, 1905610. [Google Scholar] [CrossRef]
- Keating, J.J.; Imbrogno, J.; Belfort, G. Polymer brushes for membrane separations: A review. ACS Appl. Mater. Interfaces 2016, 8, 28383–28399. [Google Scholar] [CrossRef] [PubMed]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.A. Surface-initiated controlled radical polymerization: State-of-the-art, opportunities, and challenges in surface and interface engineering with polymer brushes. Chem. Rev. 2017, 117, 4667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajesh, S.; Crandall, C.; Schneiderman, S.; Menkhaus, T.J. Cellulose-graft-polyethyleneamidoamine anion-exchange nanofiber membranes for simultaneous protein adsorption and virus filtration. ACS Appl. Nano Mater. 2018, 1, 3321–3330. [Google Scholar] [CrossRef]
- Bhut, B.V.; Wickramasinghe, S.R.; Husson, S.M. Preparation of high-capacity, weak anion-exchange membranes for protein separations using surface-initiated atom transfer radical polymerization. J. Membr. Sci. 2008, 325, 176–183. [Google Scholar] [CrossRef]
- Chenette, H.C.S.; Robinson, J.R.; Hobley, E.; Husson, S.M. Development of high-productivity, strong cation-exchange adsorbers for protein capture by graft polymerization from membranes with different pore sizes. J. Membr. Sci. 2012, 423, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, J.K.; Bothof, C.A.; Atan, S.C.; Fitzsimons, R.T.; Griesgraber, G.W.; Hembre, J.I. Ion exchange ligand design: Improving membrane adsorber efficiencies by spacer arm manipulation. React. Funct. Polym. 2019, 136, 181–188. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zheng, Y.; Gurgel, P.V.; Carbonell, R.G. Affinity membrane development from PBT nonwoven by photo-induced graft polymerization, hydrophilization and ligand attachment. J. Membr. Sci. 2013, 428, 562–575. [Google Scholar] [CrossRef]
- Miyoshi, K.; Saito, K.; Shiraishi, T.; Sugo, T. Introduction of taurine into polymer brush grafted onto porous hollow-fiber membrane. J. Membr. Sci. 2005, 264, 97–103. [Google Scholar] [CrossRef]
- Wang, J.; Faber, R.; Ulbricht, M. Influence of pore structure and architecture of photo-grafted functional layers on separation performance of cellulose-based macroporous membrane adsorbers. J. Chromatogr. A 2009, 1216, 6490–6501. [Google Scholar] [CrossRef] [PubMed]
- He, D.M.; Ulbricht, M. Preparation and characterization of porous anion-exchange membrane adsorbers with high protein-binding capacity. J. Membr. Sci. 2008, 315, 155–163. [Google Scholar] [CrossRef]
- Knudsen, H.L.; Fahrner, R.L.; Xu, Y.; Norling, L.A.; Blank, G.S. Membrane ion-exchange chromatography for process-scale antibody purification. J. Chromatogr. A 2001, 907, 145–154. [Google Scholar] [CrossRef]
- Liu, H.Y.; Gurgel, P.V.; Carbonell, R.G. Preparation and characterization of anion exchange adsorptive nonwoven membranes with high protein binding capacity. J. Membr. Sci. 2015, 493, 349–359. [Google Scholar] [CrossRef]
- Lemma, I.S.M.; Boi, C.; Carbonell, R.G. Nonwoven ion-exchange membranes with high protein binding capacity for bioseparations. Membranes 2021, 11, 18. [Google Scholar] [CrossRef]
- Hassel, K.J.; Moresoli, C. Role of pH and Ionic strength on weak cation exchange macroporous hydrogel membranes and IgG capture. J. Membr. Sci. 2016, 498, 158–166. [Google Scholar] [CrossRef]
- Staby, A.; Jacobsen, J.H.; Hansen, R.G.; Bruus, U.K.; Jensen, I.H. Comparison of chromatographic ion-exchange resins—V Strong and weak cation-exchange resins. J. Chromatogr. A 2006, 1118, 168–179. [Google Scholar] [CrossRef]
- Yuan, Y.Q.; Gao, X.L.; Wei, Y.; Wang, X.Y.; Wang, J.; Zhang, Y.S.; Gao, C.J. Enhanced desalination performance of carboxyl functionalized graphene oxide nanofiltration membranes. Desalination 2017, 405, 29–39. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, G.D.; Yu, H.J.; Jin, Y.; Cao, Y.M. Fabrication of a highly permeable composite nanofiltration membrane via interfacial polymerization by adding a novel acyl chloride monomer with an anhydride group. J. Membr. Sci. 2019, 570, 403–409. [Google Scholar] [CrossRef]
- Mockel, D.; Staude, E.; Guiver, M.D. Static protein adsorption, ultrafiltration behavior and cleanability of hydrophilized polysulfone membranes. J. Membr. Sci. 1999, 158, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Sano, S.; Kato, K.; Ikada, Y. Introduction of functional-groups onto the surface of polyethylene for protein immobilization. Biomaterials 1993, 14, 817–822. [Google Scholar] [CrossRef]
- Wrzosek, K.; Gramblicka, M.; Polakovic, M. Influence of ligand density on antibody binding capacity of cation-exchange adsorbents. J. Chromatogr. A 2009, 1216, 5039–5044. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.; Wimbish, R.; Gurgel, P.V.; Pourdeyhimi, B.; Carbonell, R.G. Reducing diffusion limitations in Ion exchange grafted membranes using high surface area nonwovens. J. Membrane Sci. 2016, 514, 53–64. [Google Scholar] [CrossRef]
- Farkas, T.; Zhong, G.M.; Guiochon, G. Validity of Darcy's law at low flow-rates in liquid chromatography. J. Chromatogr. A 1999, 849, 35–43. [Google Scholar] [CrossRef]
- Herigstad, M.O.; Gurgel, P.V.; Carbonell, R.G. Transport and binding characterization of a novel hybrid particle impregnated membrane material for bioseparations. Biotechnol. Prog. 2011, 27, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Boi, C.; Malavasi, A.; Carbonell, R.G.; Gilleskie, G. A direct comparison between membrane adsorber and packed column chromatography performance. J. Chromatogr. A 2020, 1612, 460629. [Google Scholar] [CrossRef]
- Schwellenbach, J.; Taft, F.; Villain, L.; Strube, J. Preparation and characterization of high capacity, strong cation-exchange fiber based adsorbents. J. Chromatogr. A 2016, 1447, 92–106. [Google Scholar] [CrossRef]
- Screening and Optimization of the Loading Conditions on Capto S. Available online: https://cdn.cytivalifesciences.com/dmm3bwsv3/AssetStream.aspx?mediaformatid=10061&destinationid=10016&assetid=14818 (accessed on 14 June 2021).
- Staby, A.; Sand, M.B.; Hansen, R.G.; Jacobsen, J.H.; Andersen, L.A.; Gerstenberg, M.; Bruus, U.K.; Jensen, I.H. Comparison of chromatographic ion-exchange resins III. Strong cation-exchange resins. J. Chromatogr. A 2004, 1034, 85–97. [Google Scholar] [CrossRef]
- Ramos-de-la-Pena, A.M.; Gonzalez-Valdez, J.; Aguilar, O. Protein A chromatography: Challenges and progress in the purification of monoclonal antibodies. J. Sep. Sci. 2019, 42, 1816–1827. [Google Scholar] [CrossRef]
- Tao, Y.Y.; Ibraheem, A.; Conley, L.; Cecchini, D.; Ghose, S. Evaluation of high-capacity cation exchange chromatography for direct capture of monoclonal antibodies from high-titer cell culture processes. Biotechnol. Bioeng. 2014, 111, 1354–1364. [Google Scholar] [CrossRef]
- Vogel, J.H.; Nguyen, H.; Giovannini, R.; Ignowski, J.; Garger, S.; Salgotra, A.; Tom, J. A new large-scale manufacturing platform for complex biopharmaceuticals. Biotechnol. Bioeng. 2012, 109, 3049–3058. [Google Scholar] [CrossRef]
- Miesegaes, G.R.; Lute, S.; Strauss, D.M.; Read, E.K.; Venkiteshwaran, A.; Kreuzman, A.; Shah, R.; Shamlou, P.; Chen, D.; Brorson, K. Monoclonal antibody capture and viral clearance by cation exchange chromatography. Biotechnol. Bioeng. 2012, 109, 2048–2058. [Google Scholar] [CrossRef]
- Urmann, M.; Graalfs, H.; Joehnck, M.; Jacob, L.R.; Frech, C. Cation-exchange chromatography of monoclonal antibodies Characterization of a novel stationary phase designed for production-scale purification. MAbs 2010, 2, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Deldari, S.; Guo, H.; Narahari, C.R.; Bates, R.C.; Swanson, R.; Ghose, S.; Li, Z.J.; Frey, D.D. Evaluation of chromatofocusing as a capture method for monoclonal antibody products. J. Chromatogr. A 2018, 1568, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Naik, A.D.; Hashimoto, Y.; Menegatti, S.; Carbonell, R.G. Optimization of sequence, display, and mode of operation of IgG-binding peptide ligands to develop robust, high-capacity affinity adsorbents that afford high IgG product quality. Int. J. Mol. Sci. 2019, 20, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattoli, F.; Boi, C.; Sorci, M.; Sarti, G. Adsorption of pure recombinant MBP-fusion proteins on amylose affinity membranes. J. Membr. Sci. 2006, 273, 2–11. [Google Scholar] [CrossRef]








| Nonwoven Membrane | Ligand Density (µmol/mg) | DBC at 0.5 min RT (mg/mL) | SBC (mg/g) | Pressure Drop (kPa/cm) at 146.7 cm/h |
|---|---|---|---|---|
| CEX-IDA | 5.5 | 96.0 | 605.2 (Figure S4) | 82.5 |
| CEX-SO3 | 5.8 | 99.2 | 712.9 | 87.2 |
| Protein Used | RT (min) | DBC100% (mg/mL) | DBC100% (µmol/mL) | HCP (LRV) | DNA (LRV) |
|---|---|---|---|---|---|
| IgG | 0.1 | 127.5 | 0.85 | 0.8 | 0.7 |
| IgG | 0.5 | 136.5 | 0.91 | 1.0 | 0.9 |
| IgG | 2.0 | 139.6 | 0.93 | 0.9 | 0.7 |
| scFv | 0.1 | 143.2 | 5.7 | 1.2 | 1.2 |
| scFv | 0.5 | 151.4 | 6.1 | 1.0 | 1.0 |
| scFv | 2.0 | 154.5 | 6.2 | 1.2 | 1.0 |
| Purification Cycle | DBC 1 (mg/mL) | Recovery (%) | Purity (%) | HCP (LRV) | DNA (LRV) | Aggregates 2 (%) |
|---|---|---|---|---|---|---|
| 1 | 81.8 | 94.2 | 87.9 | 0.4 | 1.0 | 1.4 |
| 2 | 86.4 | 99.5 | 87.2 | 0.3 | 1.1 | 1.5 |
| 3 | 82.1 | 94.6 | 87.6 | 0.3 | 1.1 | 1.4 |
| 4 | 83.7 | 96.5 | 87.9 | 0.4 | 1.2 | 1.4 |
| 5 | 85.0 | 97.9 | 87.7 | 0.4 | 1.1 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Boi, C.; Lemma, S.M.; Lavoie, J.; Carbonell, R.G. Iminodiacetic Acid (IDA) Cation-Exchange Nonwoven Membranes for Efficient Capture of Antibodies and Antibody Fragments. Membranes 2021, 11, 530. https://doi.org/10.3390/membranes11070530
Fan J, Boi C, Lemma SM, Lavoie J, Carbonell RG. Iminodiacetic Acid (IDA) Cation-Exchange Nonwoven Membranes for Efficient Capture of Antibodies and Antibody Fragments. Membranes. 2021; 11(7):530. https://doi.org/10.3390/membranes11070530
Chicago/Turabian StyleFan, Jinxin, Cristiana Boi, Solomon Mengistu Lemma, Joseph Lavoie, and Ruben G. Carbonell. 2021. "Iminodiacetic Acid (IDA) Cation-Exchange Nonwoven Membranes for Efficient Capture of Antibodies and Antibody Fragments" Membranes 11, no. 7: 530. https://doi.org/10.3390/membranes11070530
APA StyleFan, J., Boi, C., Lemma, S. M., Lavoie, J., & Carbonell, R. G. (2021). Iminodiacetic Acid (IDA) Cation-Exchange Nonwoven Membranes for Efficient Capture of Antibodies and Antibody Fragments. Membranes, 11(7), 530. https://doi.org/10.3390/membranes11070530