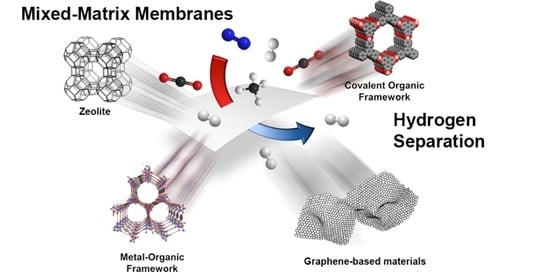
Recent Progress in Mixed-Matrix Membranes for Hydrogen Separation
Abstract
:1. Introduction
2. Current Hydrogen Generation Market and Challenges
3. Hydrogen Purification: Membrane vs. Other Technologies
4. Mixed-Matrix Membranes for Hydrogen Separation
4.1. Zeolite-Based Membranes
4.2. MOF-Based Membranes
4.3. COF-Based Membranes
4.4. Graphene-Based Membranes
4.5. Binary Fillers
5. Critical Evaluation of Hydrogen Separation Performances
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, T.; Zhang, D. A critical review of comparative global historical energy consumption and future demand: The story told so far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Jamil, M.; Ahmad, F.; Jeon, Y.J. Renewable energy technologies adopted by the UAE: Prospects and challenges—A comprehensive overview. Renew. Sustain. Energy Rev. 2016, 55, 1181–1194. [Google Scholar] [CrossRef] [Green Version]
- World Economic Forum. Nature and Net Zero; The World Economic Forum in collarobation with McKinsey & Company, World Economic Forum: Geneva, Switzerland, 2021. [Google Scholar]
- Zerta, M.; Schmidt, P.R.; Stiller, C.; Landinger, H. Alternative World Energy Outlook (AWEO) and the role of hydrogen in a changing energy landscape. Int. J. Hydrogen Energy 2008, 33, 3021–3025. [Google Scholar] [CrossRef]
- Friedlander, B. Touted as Clean, ‘Blue’ Hydrogen May Be Worse Than Gas or Coal. Available online: https://news.cornell.edu/stories/2021/08/touted-clean-blue-hydrogen-may-be-worse-gas-or-coal (accessed on 15 July 2021).
- Atilhan, S.; Park, S.; El-Halwagi, M.M.; Atilhan, M.; Moore, M.; Nielsen, R.B. Green hydrogen as an alternative fuel for the shipping industry. Curr. Opin. Chem. Eng. 2021, 31, 100668. [Google Scholar] [CrossRef]
- van Renssen, S. The hydrogen solution? Nat. Clim. Chang. 2020, 10, 799–801. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The role of green and blue hydrogen in the energy transition—A technological and geopolitical perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- DiChristopher, T. Experts Explain Why Green Hydrogen Costs Have Fallen and Will Keep Falling. Available online: https://www.spglobal.com/marketintelligence/en/news-insights/latest-news-headlines/experts-explain-why-green-hydrogen-costs-have-fallen-and-will-keep-falling-63037203 (accessed on 12 July 2020).
- Collins, L. A Wake-Up Call on Green Hydrogen: The Amount of Wind and Solar Needed Is Immense. Available online: https://www.rechargenews.com/transition/a-wake-up-call-on-green-hydrogen-the-amount-of-wind-and-solar-needed-is-immense/2-1-776481 (accessed on 12 July 2021).
- Hulst, N.v. The Clean Hydrogen Future Has Already Begun. Available online: https://www.iea.org/commentaries/the-clean-hydrogen-future-has-already-begun (accessed on 12 July 2020).
- Shao, L.; Low, B.T.; Chung, T.-S.; Greenberg, A.R. Polymeric membranes for the hydrogen economy: Contemporary approaches and prospects for the future. J. Membr. Sci. 2009, 327, 18–31. [Google Scholar] [CrossRef]
- Shu, L.; Xie, L.-H.; Meng, Y.; Liu, T.; Zhao, C.; Li, J.-R. A thin and high loading two-dimensional MOF nanosheet based mixed-matrix membrane for high permeance nanofiltration. J. Membr. Sci. 2020, 603, 118049. [Google Scholar] [CrossRef]
- Park, S.; Jeong, H.-K. Transforming polymer hollow fiber membrane modules to mixed-matrix hollow fiber membrane modules for propylene/propane separation. J. Membr. Sci. 2020, 612, 118429. [Google Scholar] [CrossRef]
- Barooah, M.; Mandal, B. Synthesis, characterization and CO2 separation performance of novel PVA/PG/ZIF-8 mixed matrix membrane. J. Membr. Sci. 2019, 572, 198–209. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Li, W.; Goh, K.; Chuah, C.Y.; Bae, T.-H. Mixed-matrix carbon molecular sieve membranes using hierarchical zeolite: A simple approach towards high CO2 permeability enhancements. J. Membr. Sci. 2019, 588, 117220. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Lee, J.; Bae, T.-H. Graphene-based membranes for H2 separation: Recent progress and future perspective. Membranes 2020, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Giancarlo, T. Hydrogen Production & Distribution; International Energy Agency-Energy Technology System Analysis Programme: USA, 2014; Available online: https://iea-etsap.org/E-TechDS/PDF/P12_H2_Feb2014_FINAL%203_CRES-2a-GS%20Mz%20GSOK.pdf (accessed on 12 July 2020).
- Liu, K.; Song, C.; Subramani, V. Hydrogen and Syngas Production and Purification Technologies; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Robert, R. Life Cycle Emissions of Hydrogen. Available online: https://4thgeneration.energy/life-cycles-emissions-of-hydrogen/ (accessed on 14 January 2021).
- IEA. The Future of Hydrogen. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 14 January 2021).
- Angeli, S.D.; Monteleone, G.; Giaconia, A.; Lemonidou, A.A. State-of-the-art catalysts for CH4 steam reforming at low temperature. Int. J. Hydrogen Energy 2014, 39, 1979–1997. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A short review on Ni based catalysts and related engineering issues for methane steam reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Salameh, Z. Renewable Energy System Design; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Energy Efficiency & Renewable Energy. Hydrogen Production: Biomass Gasification. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-biomass-gasification#:~:text=Biomass%20gasification%20is%20a%20mature,and%20other%20products%2C%20without%20combustion (accessed on 28 June 2021).
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef] [Green Version]
- Brauns, J.; Turek, T. Alkaline water electrolysis powered by renewable energy: A review. Processes 2020, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combus. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Maric, R.; Yu, H. Proton exchange membrane water electrolysis as a promising technology for hydrogen production and energy storage. In Nanostructures in Energy Generation, Transmission and Storage; IntechOpen: London, UK, 2019; p. 13. [Google Scholar]
- Ockwig, N.W.; Nenoff, T.M. Membranes for hydrogen separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Lee, Y.; Bae, T.-H. Potential of adsorbents and membranes for SF6 capture and recovery: A review. Chem. Eng. J. 2020, 404, 126577. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T. Pressure Swing Adsorption Technology for Hydrogen Production; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Linde. Hydrogen Recovery by Pressure Swing Adsorption; Linde AG: Pullach, Germany, 2021. [Google Scholar]
- Li, W.; Chuah, C.Y.; Yang, Y.; Bae, T.-H. Nanocomposites formed by in situ growth of NiDOBDC nanoparticles on graphene oxide sheets for enhanced CO2 and H2 storage. Micropor. Mesopor. Mater. 2018, 265, 35–42. [Google Scholar] [CrossRef]
- Al-Mufachi, N.; Rees, N.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Aasadnia, M.; Mehrpooya, M.; Ghorbani, B. A novel integrated structure for hydrogen purification using the cryogenic method. J. Clean. Prod. 2020, 278, 123872. [Google Scholar] [CrossRef]
- Terrien, P.; Lockwood, F.; Granados, L.; Morel, T. CO2 capture from H2 plants: Implementation for EOR. Energy Procedia 2014, 63, 7861–7866. [Google Scholar] [CrossRef] [Green Version]
- Airliquide. CRYOCAPTM: Cryogenic Solution for CO2 Capture, a World Premiere; Airliquide: Paris, France, 2015; p. 12. [Google Scholar]
- Schorer, L.; Schmitz, S.; Weber, A. Membrane based purification of hydrogen system (MEMPHYS). Int. J. Hydrogen Energy 2019, 44, 12708–12714. [Google Scholar] [CrossRef]
- Bernardo, G.; Araújo, T.; da Silva Lopes, T.; Sousa, J.; Mendes, A. Recent advances in membrane technologies for hydrogen purification. Int. J. Hydrogen Energy 2020, 45, 7313–7338. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Yang, Y.; Gong, H.; Li, W.; Karahan, H.E.; Guiver, M.D.; Wang, R.; Bae, T.-H. Harnessing filler mMaterials for enhancing biogas separation membranes. Chem. Rev. 2018, 118, 8655–8769. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Kim, K.; Lee, J.; Koh, D.-Y.; Bae, T.-H. CO2 absorption using membrane contactors: Recent progress and future perspective. Ind. Eng. Chem. Res. 2019, 59, 6773–6794. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Li, W.; Samarasinghe, S.; Sethunga, G.; Bae, T.-H. Enhancing the CO2 separation performance of polymer membranes via the incorporation of amine-functionalized HKUST-1 nanocrystals. Micropor. Mesopor. Mater. 2019, 290, 109680. [Google Scholar] [CrossRef]
- Peramanu, S.; Cox, B.; Pruden, B. Economics of hydrogen recovery processes for the purification of hydroprocessor purge and off-gases. Int. J. Hydrogen Energy 1999, 24, 405–424. [Google Scholar] [CrossRef]
- Goh, K.; Jiang, W.; Karahan, H.E.; Zhai, S.; Wei, L.; Yu, D.; Fane, A.G.; Wang, R.; Chen, Y. All-carbon nanoarchitectures as gigh-performance separation membranes with superior stability. Adv. Funct. Mater. 2015, 25, 7348–7359. [Google Scholar] [CrossRef]
- Yang, E.; Alayande, A.B.; Goh, K.; Kim, C.-M.; Chu, K.-H.; Hwang, M.-H.; Ahn, J.-H.; Chae, K.-J. 2D materials-based membranes for hydrogen purification: Current status and future prospects. Int. J. Hydrogen Energy 2021, 46, 11389–11410. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Ismail, A.F. Enhancing hydrogen gas separation performance of thin film composite membrane through facilely blended polyvinyl alcohol and PEBAX. Int. J. Hydrogen Energy 2021, 46, 19737–19748. [Google Scholar] [CrossRef]
- Noble, R.D. Perspectives on mixed matrix membranes. J. Membr. Sci. 2011, 378, 393–397. [Google Scholar] [CrossRef]
- Qian, S.; Xia, L.; Yang, L.; Wang, X.; Suo, X.; Cui, X.; Xing, H. Defect-free mixed-matrix membranes consisting of anion-pillared metal-organic frameworks and poly(ionic liquid)s for separation of acetylene from ethylene. J. Membr. Sci. 2020, 611, 118329. [Google Scholar] [CrossRef]
- Zhao, Y.; Jung, B.T.; Ansaloni, L.; Ho, W.W. Multiwalled carbon nanotube mixed matrix membranes containing amines for high pressure CO2/H2 separation. J. Membr. Sci. 2014, 459, 233–243. [Google Scholar] [CrossRef]
- Ismail, A.; Goh, P.; Sanip, S.; Aziz, M. Transport and separation properties of carbon nanotube-mixed matrix membrane. Sep. Purif. Technol. 2009, 70, 12–26. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-tuned intrinsically ultramicroporous polymers redefine the permeability/selectivity upper bounds of membrane-based air and hydrogen separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Wei, Y.; Li, L.; Zhang, T.; Wang, H.; Xue, J.; Ding, L.-X.; Wang, S.; Caro, J.; Gogotsi, Y. MXene molecular sieving membranes for highly efficient gas separation. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.X.; Drayton, J.A.; Smith, Z.P. The perfluoropolymer upper bound. AIChE J. 2019, 65, e16700. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Kosinov, N.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. Recent developments in zeolite membranes for gas separation. J. Membr. Sci. 2016, 499, 65–79. [Google Scholar] [CrossRef]
- Morris, R.E.; Wheatley, P.S. Gas storage in nanoporous materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.; Kim, J. Predicting performance limits of methane gas storage in zeolites with an artificial neural network. J. Mater. Chem. A 2019, 7, 2709–2716. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Bae, T.-H. Enhanced performance of carbon molecular sieve membranes incorporating zeolite nanocrystals for air separation. Membranes 2021, 11, 489. [Google Scholar] [CrossRef]
- Mei, W.; Du, Y.; Wu, T.; Gao, F.; Wang, B.; Duan, J.; Zhou, J.; Zhou, R. High-flux CHA zeolite membranes for H2 separations. J. Membr. Sci. 2018, 565, 358–369. [Google Scholar] [CrossRef]
- Zhou, M.; Korelskiy, D.; Ye, P.; Grahn, M.; Hedlund, J. A uniformly oriented MFI membrane for improved CO2 separation. Angew. Chem. Int. Ed. 2014, 126, 3560–3563. [Google Scholar] [CrossRef]
- Yu, L.; Nobandegani, M.; Hedlund, J. High performance fluoride MFI membranes for efficient CO2/H2 separation. J. Membr. Sci. 2020, 616, 118623. [Google Scholar] [CrossRef]
- Hu, L.; Cheng, J.; Li, Y.; Liu, J.; Zhang, L.; Zhou, J.; Cen, K. Composites of ionic liquid and amine-modified SAPO 34 improve CO2 separation of CO2-selective polymer membranes. Appl. Surf. Sci. 2017, 410, 249–258. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mohammadi, T.; Bakhtiari, O. Effective hydrogen purification from methane via polyimide Matrimid® 5218- deca-dodecasil 3R type zeolite mixed matrix membrane. Energy 2017, 141, 2100–2107. [Google Scholar] [CrossRef]
- Ahmad, J.; Hägg, M.-B. Preparation and characterization of polyvinyl acetate/zeolite 4A mixed matrix membrane for gas separation. J. Membr. Sci. 2013, 427, 73–84. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Shahidi, K.; Mohammadi, T. Sorption properties of hydrogen-selective PDMS/zeolite 4A mixed matrix membrane. Int. J. Hydrogen Energy 2012, 37, 17275–17284. [Google Scholar] [CrossRef]
- Esmaeili, N.; Boyd, S.E.; Brown, C.L.; Mac, A. Gray, E.; Webb, C.J. Improving the gas-separation properties of PVAc-zeolite 4A mixed-matrix membranes through nano-sizing and silanation of the zeolite. ChemPhysChem 2019, 20, 1590–1606. [Google Scholar] [CrossRef]
- Eden, C.L.; Daramola, M.O. Evaluation of silica sodalite infused polysulfone mixed matrix membranes during H2/CO2 separation. Mater. Today Proc. 2021, 38, 522–527. [Google Scholar] [CrossRef]
- Hu, C.-C.; Cheng, P.-H.; Chou, S.-C.; Lai, C.-L.; Huang, S.-H.; Tsai, H.-A.; Hung, W.-S.; Lee, K.-R. Separation behavior of amorphous amino-modified silica nanoparticle/polyimide mixed matrix membranes for gas separation. J. Membr. Sci. 2020, 595, 117542. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Iacob, C.; Nechifor, G.; Niculescu, V.-C. High Selective Mixed Membranes Based on Mesoporous MCM-41 and MCM-41-NH2 Particles in a Polysulfone Matrix. Front. Chem. 2019, 7, 332. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ding, X.; Zhao, H.; Fu, J.; Xin, Q.; Zhang, Y. Pebax-based mixed matrix membranes containing hollow polypyrrole nanospheres with mesoporous shells for enhanced gas permeation performance. J. Membr. Sci. 2020, 602, 117968. [Google Scholar] [CrossRef]
- Tseng, H.-H.; Chuang, H.-W.; Zhuang, G.-L.; Lai, W.-H.; Wey, M.-Y. Structure-controlled mesoporous SBA-15-derived mixed matrix membranes for H2 purification and CO2 capture. Int. J. Hydrogen Energy 2017, 42, 11379–11391. [Google Scholar] [CrossRef]
- Zornoza, B.; Esekhile, O.; Koros, W.J.; Téllez, C.; Coronas, J. Hollow silicalite-1 sphere-polymer mixed matrix membranes for gas separation. Sep. Purif. Technol. 2011, 77, 137–145. [Google Scholar] [CrossRef]
- Zornoza, B.; Téllez, C.; Coronas, J.; Esekhile, O.; Koros, W.J. Mixed matrix membranes based on 6FDA polyimide with silica and zeolite microsphere dispersed phases. AIChE J. 2015, 61, 4481–4490. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Seoane, B.; Coronas, J.; Gascon, I.; Etxeberria Benavides, M.; Karvan, O.; Caro, J.; Kapteijn, F.; Gascon, J. Metal-organic framework based mixed matrix membranes: A solution for highly efficient CO2 capture? Chem. Soc. Rev. 2015, 44, 2421–2454. [Google Scholar] [CrossRef] [Green Version]
- Denny, M.S.; Moreton, J.C.; Benz, L.; Cohen, S.M. Metal–organic frameworks for membrane-based separations. Nat. Rev. Mater. 2016, 1, 16078. [Google Scholar] [CrossRef]
- Hu, Z.; Kang, Z.; Qian, Y.; Peng, Y.; Wang, X.; Chi, C.; Zhao, D. Mixed matrix membranes containing UiO-66(Hf)-(OH)2 metal–organic framework nanoparticles for efficient H2/CO2 separation. Ind. Eng. Chem. Res. 2016, 55, 7933–7940. [Google Scholar] [CrossRef]
- Lin, R.; Villacorta Hernandez, B.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Cao, L.; Tao, K.; Huang, A.; Kong, C.; Chen, L. A highly permeable mixed matrix membrane containing CAU-1-NH2 for H2 and CO2 separation. Chem. Commun. 2013, 49, 8513–8515. [Google Scholar] [CrossRef]
- Ma, X.; Wu, X.; Caro, J.; Huang, A. Polymer composite membrane with penetrating ZIF-7 sheets displays high hydrogen permselectivity. Angew. Chem. Int. Ed. 2019, 131, 16302–16306. [Google Scholar] [CrossRef]
- Perez, E.V.; Kalaw, G.J.D.; Ferraris, J.P.; Balkus, K.J.; Musselman, I.H. Amine-functionalized (Al) MIL-53/VTEC™ mixed-matrix membranes for H2/CO2 mixture separations at high pressure and high temperature. J. Membr. Sci. 2017, 530, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, D.; Kong, C.; Zhou, F.; Jiang, T.; Chen, L. Design of thin and tubular MOFs-polymer mixed matrix membranes for highly selective separation of H2 and CO2. Sep. Purif. Technol. 2019, 220, 197–205. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Bouša, D.; Šturala, J.; Sofer, Z.; Shaliutina-Kolešová, A.; Gardenö, D.; Friess, K. Surface and interface engineering in CO2-philic based UiO-66-NH2-PEI mixed matrix membranes via covalently bridging PVP for effective hydrogen purification. Int. J. Hydrogen Energy 2021, 46, 5449–5458. [Google Scholar] [CrossRef]
- Ma, C.; Urban, J.J. Hydrogen-bonded polyimide/metal-organic framework hybrid membranes for ultrafast separations of multiple gas pairs. Adv. Funct. Mater. 2019, 29, 1903243. [Google Scholar] [CrossRef]
- Smith, S.J.D.; Ladewig, B.P.; Hill, A.J.; Lau, C.H.; Hill, M.R. Post-synthetic Ti exchanged UiO-66 metal-organic frameworks that deliver exceptional gas permeability in mixed matrix membranes. Sci. Rep. 2015, 5, 7823. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Navarro, M.; Lhotka, M.; Zornoza, B.; Téllez, C.; de Vos, W.M.; Benes, N.E.; Konnertz, N.M.; Visser, T.; Semino, R.; et al. Enhanced gas separation performance of 6FDA-DAM based mixed matrix membranes by incorporating MOF UiO-66 and its derivatives. J. Membr. Sci. 2018, 558, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Satheeshkumar, C.; Yu, H.J.; Kim, S.; Lee, J.S.; Seo, M.; Kim, M. Pore engineering of covalently connected metal–organic framework nanoparticle–mixed-matrix membrane composites for molecular separation. ACS Appl. Nano Mater. 2020, 3, 9356–9362. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Jie, X.; Liu, D.; Cao, Y. Improved interfacial affinity and CO2 separation performance of asymmetric mixed matrix membranes by incorporating [ostmodified MIL-53(Al). ACS Appl. Mater. Interfaces 2016, 8, 22696–22704. [Google Scholar] [CrossRef]
- Smith, S.J.D.; Konstas, K.; Lau, C.H.; Gozukara, Y.M.; Easton, C.D.; Mulder, R.J.; Ladewig, B.P.; Hill, M.R. Post-synthetic annealing: Linker self-exchange in UiO-66 and its effect on polymer–metal organic framework interaction. Cryst. Growth Des. 2017, 17, 4384–4392. [Google Scholar] [CrossRef]
- Al-Maythalony, B.A.; Alloush, A.M.; Faizan, M.; Dafallah, H.; Elgzoly, M.A.A.; Seliman, A.A.A.; Al-Ahmed, A.; Yamani, Z.H.; Habib, M.A.M.; Cordova, K.E.; et al. Tuning the interplay between selectivity and permeability of ZIF-7 mixed matrix membranes. ACS Appl. Mater. Interfaces 2017, 9, 33401–33407. [Google Scholar] [CrossRef]
- Sánchez-Laínez, J.; Zornoza, B.; Orsi, A.F.; Łozińska, M.M.; Dawson, D.M.; Ashbrook, S.E.; Francis, S.M.; Wright, P.A.; Benoit, V.; Llewellyn, P.L.; et al. Synthesis of ZIF-93/11 hybrid nanoparticles via post-synthetic modification of ZIF-93 and their use for H 2/CO2 separation. Chem. A Eur. J. 2018, 24, 11211–11219. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Moon, S.J.; Wang, H.H.; Kim, S.; Lee, Y.M. Mixed matrix membranes with a thermally rearranged polymer and ZIF-8 for hydrogen separation. J. Membr. Sci. 2019, 582, 381–390. [Google Scholar] [CrossRef]
- Xiang, F.; Marti, A.M.; Hopkinson, D.P. Layer-by-layer assembled polymer/MOF membrane for H2/CO2 separation. J. Membr. Sci. 2018, 556, 146–153. [Google Scholar] [CrossRef]
- Park, S.; Cho, K.Y.; Jeong, H.-K. Polyimide/ZIF-7 mixed-matrix membranes: Understanding the in situ confined formation of the ZIF-7 phases inside a polymer and their effects on gas separations. J. Mater. Chem. A 2020, 8, 11210–11217. [Google Scholar] [CrossRef]
- Mei, X.; Yang, S.; Lu, P.; Zhang, Y.; Zhang, J. Improving the Selectivity of ZIF-8/polysulfone-mixed matrix membranes by polydopamine modification for H2/CO2 separation. Front. Chem. 2020, 8, 528. [Google Scholar] [CrossRef]
- Zhang, W.; Ying, Y.; Ma, J.; Guo, X.; Huang, H.; Liu, D.; Zhong, C. Mixed matrix membranes incorporated with polydopamine-coated metal-organic framework for dehydration of ethylene glycol by pervaporation. J. Membr. Sci. 2017, 527, 8–17. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhang, S.; Hu, L.; Jin, J. Interfacial Design of Mixed Matrix Membranes for Improved Gas Separation Performance. Adv. Mater. 2016, 28, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; He, S.; Han, G.; Long, J.; Li, S.; Lau, C.H.; Zhang, S.; Shao, L. Aqueous One-Step Modulation for Synthesizing Monodispersed ZIF-8 Nanocrystals for Mixed-Matrix Membrane. ACS Appl. Mater. Interfaces 2021, 13, 11296–11305. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Peng, Y.; Hu, Z.; Qian, Y.; Chi, C.; Yeo, L.Y.; Tee, L.; Zhao, D. Mixed matrix membranes composed of two-dimensional metal–organic framework nanosheets for pre-combustion CO2 capture: A relationship study of filler morphology versus membrane performance. J. Mater. Chem. A 2015, 3, 20801–20810. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Jia, C.; Wang, Y.; Zhai, L.; Wang, Q.; Zhao, D. Ultrathin mixed matrix membranes containing two-dimensional metal-organic framework nanosheets for efficient CO2/CH4 separation. J. Membr. Sci. 2017, 539, 213–223. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Liu, X.; Gottmer, S.; Chu, L.; Gascon, J.; Kapteijn, F. Thin mixed matrix and dual layer membranes containing metal-organic framework nanosheets and Polyactive™ for CO2 capture. J. Membr. Sci. 2019, 570–571, 226–235. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.; Ban, Y.; Jin, H.; Jiao, W.; Liu, X.; Yang, W. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 2014, 346, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabrés i Xamena, F.X.; Gascon, J. Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2014, 14, 48. [Google Scholar] [CrossRef]
- Şahin, F.; Topuz, B.; Kalıpçılar, H. Synthesis of ZIF-7, ZIF-8, ZIF-67 and ZIF-L from recycled mother liquors. Micropor. Mesopor. Mater. 2018, 261, 259–267. [Google Scholar] [CrossRef]
- Chen, R.; Yao, J.; Gu, Q.; Smeets, S.; Baerlocher, C.; Gu, H.; Zhu, D.; Morris, W.; Yaghi, O.M.; Wang, H. A two-dimensional zeolitic imidazolate framework with a cushion-shaped cavity for CO2 adsorption. Chem. Commun. 2013, 49, 9500–9502. [Google Scholar] [CrossRef]
- Kim, S.; Shamsaei, E.; Lin, X.; Hu, Y.; Simon, G.P.; Seong, J.G.; Kim, J.S.; Lee, W.H.; Lee, Y.M.; Wang, H. The enhanced hydrogen separation performance of mixed matrix membranes by incorporation of two-dimensional ZIF-L into polyimide containing hydroxyl group. J. Membr. Sci. 2018, 549, 260–266. [Google Scholar] [CrossRef]
- Deng, J.; Dai, Z.; Deng, L. H2-selective Troger’s base polymer based mixed matrix membranes enhanced by 2D MOFs. J. Membr. Sci. 2020, 610, 118262. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, Y.; Zhang, F.; Zhang, S.; Wang, Z.; Jin, J. MOF nanosheet-based mixed matrix membranes with metal-organic coordination interfacial interaction for gas separation. ACS Appl. Mater. Interfaces 2020, 12, 49101–49110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Huang, Y.; Peng, Y.; Huang, Z.; Ma, Q.; Zhang, H. Two-dimensional metal-organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef]
- Duan, K.; Wang, J.; Zhang, Y.; Liu, J. Covalent organic frameworks (COFs) functionalized mixed matrix membrane for effective CO2/N2 separation. J. Membr. Sci. 2019, 572, 588–595. [Google Scholar] [CrossRef]
- Yang, Y.; Chuah, C.Y.; Nie, L.; Bae, T.-H. Enhancing the mechanical strength and CO2/CH4 separation performance of polymeric membranes by incorporating amine-appended porous polymers. J. Membr. Sci. 2019, 569, 149–156. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, G. Microporous Organic Materials for Membrane-Based Gas Separation. Adv. Mater. 2018, 30, 1700750. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ben, T.; Qiu, S.; Valtchev, V. Two-dimensional COF–three-dimensional MOF dual-layer membranes with unprecedentedly high H2/CO2 selectivity and ultrahigh gas permeabilities. ACS Appl. Mater. Interfaces 2020, 12, 52899–52907. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Das, S.; Xing, G.; Ben, T.; Valtchev, V.; Qiu, S. Fabrication of COF-MOF composite membranes and their highly selective separation of H2/CO2. J. Am. Chem. Soc. 2016, 138, 7673–7680. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Peng, M.; Strauss, I.; Mundstock, A.; Meng, H.; Caro, J. MOF-in-COF molecular sieving membrane for selective hydrogen separation. Nat. Commun. 2021, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Feng, S.; Fan, L.; Pang, J.; Fan, W.; Kong, G.; Kang, Z.; Sun, D. Covalent organic frameworks combined with graphene oxide to fabricate membranes for H2/CO2 separation. Sep. Purif. Technol. 2019, 223, 10–16. [Google Scholar] [CrossRef]
- Fan, H.; Peng, M.; Strauss, I.; Mundstock, A.; Meng, H.; Caro, J. High-flux vertically aligned 2D covalent organic framework membrane with enhanced hydrogen separation. J. Am. Chem. Soc. 2020, 142, 6872–6877. [Google Scholar] [CrossRef]
- Yang, Y.; Goh, K.; Weerachanchai, P.; Bae, T.-H. 3D covalent organic framework for morphologically induced high-performance membranes with strong resistance toward physical aging. J. Membr. Sci. 2019, 574, 235–242. [Google Scholar] [CrossRef]
- Hou, R.; O’Loughlin, R.; Ackroyd, J.; Liu, Q.; Doherty, C.M.; Wang, H.; Hill, M.R.; Smith, S.J.D. Greatly enhanced gas selectivity in mixed-matrix membranes through size-controlled hyper-cross-linked polymer additives. Ind. Eng. Chem. Res. 2020, 59, 13773–13782. [Google Scholar] [CrossRef]
- Hou, R.; Ghanem, B.S.; Smith, S.J.D.; Doherty, C.M.; Setter, C.; Wang, H.; Pinnau, I.; Hill, M.R. Highly permeable and selective mixed-matrix membranes for hydrogen separation containing PAF-1. J. Mater. Chem. A 2020, 8, 14713–14720. [Google Scholar] [CrossRef]
- Lau, C.H.; Konstas, K.; Thornton, A.W.; Liu, A.C.; Mudie, S.; Kennedy, D.F.; Howard, S.C.; Hill, A.J.; Hill, M.R. Gas-separation membranes loaded with porous aromatic frameworks that improve with age. Angew. Chem. Int. Ed. 2015, 54, 2669–2673. [Google Scholar] [CrossRef]
- Berlanga, I.; Ruiz-González, M.L.; González-Calbet, J.M.; Fierro, J.L.G.; Mas-Ballesté, R.; Zamora, F. Delamination of Layered Covalent Organic Frameworks. Small 2011, 7, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Bunck, D.N.; Dichtel, W.R. Bulk Synthesis of exfoliated two-dimensional polymers using hydrazone-linked covalent organic frameworks. J. Am. Chem. Soc. 2013, 135, 14952–14955. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Peng, Y.; Qian, Y.; Yuan, D.; Addicoat, M.A.; Heine, T.; Hu, Z.; Tee, L.; Guo, Z.; Zhao, D. Mixed matrix membranes (MMMs) comprising exfoliated 2D covalent organic frameworks (COFs) for efficient CO2 separation. Chem. Mater. 2016, 28, 1277–1285. [Google Scholar] [CrossRef]
- Cao, X.; Xu, H.; Dong, S.; Xu, J.; Qiao, Z.; Zhao, S.; Wang, J.; Wang, Z. Preparation of high-performance and pressure-resistant mixed matrix membranes for CO2/H2 separation by modifying COF surfaces with the groups or segments of the polymer matrix. J. Membr. Sci. 2020, 601, 117882. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Z.; Lu, K.; Fang, W.; Bi, X.; Zhang, Y.; Jin, J. In-situ generation of polymer molecular sieves in polymer membranes for highly selective gas separation. J. Membr. Sci. 2021, 630, 119302. [Google Scholar] [CrossRef]
- Goh, K.; Karahan, H.E.; Wei, L.; Bae, T.-H.; Fane, A.G.; Wang, R.; Chen, Y. Carbon nanomaterials for advancing separation membranes: A strategic perspective. Carbon 2016, 109, 694–710. [Google Scholar] [CrossRef]
- Yang, E.; Goh, K.; Chuah, C.Y.; Wang, R.; Bae, T.-H. Asymmetric mixed-matrix membranes incorporated with nitrogen-doped graphene nanosheets for highly selective gas separation. J. Membr. Sci. 2020, 615, 118293. [Google Scholar] [CrossRef]
- Castarlenas, S.; Téllez, C.; Coronas, J. Gas separation with mixed matrix membranes obtained from MOF UiO-66-graphite oxide hybrids. J. Membr. Sci. 2017, 526, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Samarasinghe, S.A.S.C.; Bae, T.-H. Enhancing CO2/CH4 separation performance and mechanical strength of mixed-matrix membrane via combined use of graphene oxide and ZIF-8. J. Ind. Eng. Chem. 2018, 67, 156–163. [Google Scholar] [CrossRef]
- Goh, K.; Karahan, H.E.; Yang, E.; Bae, T.-H. Graphene-based membranes for CO2/CH4 separation: Key challenges and perspectives. Appl. Sci. 2019, 9, 2784. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chuah, C.Y.; Nie, L.; Bae, T.-H. Enhanced CO2/CH4 selectivity and mechanical strength of mixed-matrix membrane incorporated with NiDOBDC/GO composite. J. Ind. Eng. Chem. 2019, 74, 118–125. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Chu, Z.; Jin, W.; Xu, N. Subnanometer two-simensional graphene oxide channels for ultrafast gas Sieving. ACS Nano 2016, 10, 3398–3409. [Google Scholar] [CrossRef]
- Lin, H.; Liu, R.; Dangwal, S.; Kim, S.-J.; Mehra, N.; Li, Y.; Zhu, J. Permselective H2/CO2 separation and desalination of hybrid GO/rGO membranes with controlled pre-cross-linking. ACS Appl. Mater. Interfaces 2018, 10, 28166–28175. [Google Scholar] [CrossRef]
- Ibrahim, A.F.; Banihashemi, F.; Lin, Y. Graphene oxide membranes with narrow inter-sheet galleries for enhanced hydrogen separation. Chem. Commun. 2019, 55, 3077–3080. [Google Scholar] [CrossRef]
- Xin, Q.; Ma, F.; Zhang, L.; Wang, S.; Li, Y.; Ye, H.; Ding, X.; Lin, L.; Zhang, Y.; Cao, X. Interface engineering of mixed matrix membrane via CO2-philic polymer brush functionalized graphene oxide nanosheets for efficient gas separation. J. Membr. Sci. 2019, 586, 23–33. [Google Scholar] [CrossRef]
- Cheng, L.; Guan, K.; Liu, G.; Jin, W. Cysteamine-crosslinked graphene oxide membrane with enhanced hydrogen separation property. J. Membr. Sci. 2020, 595, 117568. [Google Scholar] [CrossRef]
- Nie, L.; Chuah, C.Y.; Bae, T.H.; Lee, J.M. Graphene-based advanced membrane applications in organic solvent nanofiltration. Adv. Funct. Mater. 2020, 31, 2006949. [Google Scholar] [CrossRef]
- Brodie, B.C., XIII. On the atomic weight of graphite. Phil. Trans. R. Soc. 1859, 149, 249–259. [Google Scholar]
- Samarasinghe, S.; Chuah, C.Y.; Yang, Y.; Bae, T.-H. Tailoring CO2/CH4 separation properties of mixed-matrix membranes via combined use of two-and three-dimensional metal-organic frameworks. J. Membr. Sci. 2018, 557, 30–37. [Google Scholar] [CrossRef]
- Valero, M.; Zornoza, B.; Téllez, C.; Coronas, J. Mixed matrix membranes for gas separation by combination of silica MCM-41 and MOF NH2-MIL-53(Al) in glassy polymers. Micropor. Mesopor. Mater. 2014, 192, 23–28. [Google Scholar] [CrossRef]
- Galve, A.; Sieffert, D.; Staudt, C.; Ferrando, M.; Güell, C.; Téllez, C.; Coronas, J. Combination of ordered mesoporous silica MCM-41 and layered titanosilicate JDF-L1 fillers for 6FDA-based copolyimide mixed matrix membranes. J. Membr. Sci. 2013, 431, 163–170. [Google Scholar] [CrossRef]
- Zornoza, B.; Seoane, B.; Zamaro, J.M.; Téllez, C.; Coronas, J. Combination of MOFs and zeolites for mixed-matrix membranes. ChemPhysChem 2011, 12, 2781–2785. [Google Scholar] [CrossRef]
- Lin, H.; He, Z.; Sun, Z.; Vu, J.; Ng, A.; Mohammed, M.; Kniep, J.; Merkel, T.C.; Wu, T.; Lambrecht, R.C. CO2-selective membranes for hydrogen production and CO2 capture—Part I: Membrane development. J. Membr. Sci. 2014, 457, 149–161. [Google Scholar] [CrossRef]
- Thür, R.; Van Havere, D.; Van Velthoven, N.; Smolders, S.; Lamaire, A.; Wieme, J.; Van Speybroeck, V.; De Vos, D.; Vankelecom, I.F.J. Correlating MOF-808 parameters with mixed-matrix membrane (MMM) CO2 permeation for a more rational MMM development. J. Mater. Chem. A 2021, 9, 12782–12796. [Google Scholar] [CrossRef]









| Hydrogen Generation Technology | Common Impurity |
|---|---|
| Steam methane reforming | CO, CO2 and CH4 |
| Partial oxidation of hydrocarbons | CO, CO2 and CH4 |
| Gasification (coal/oil/biomass) | Light hydrocarbons, CO, CO2, CH4, O2, and N2 |
| Electrolysis of water | CH4, O2, N2, CO2, and CO |
| Membrane | Separation Performance | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|
| Filler | Polymer/Support | Test Condition | Permeation Area (cm2) | P(H2) (GPU) | Selectivity | |||
| H2/CO2 | H2/CH4 | H2/N2 | ||||||
| IL/SAPO-34 (1:4) | Pebax® MH1657/PEGDME with ceramic | 1 bar, 20 °C | - | 4.9 (+188%) | 0.11 (+31%) | 1.2 (+9%) | - | [67] |
| IL/SAPO-34-NH2 (1:4) | Pebax® MH1657/PEGDME with ceramic | 1 bar, 20 °C | - | 2.2 (+29%) | 0.05 (−17%) | 1.9 (+73%) | - | [67] |
| IL/SAPO-34-NH2 (1:2) | Pebax® MH1657/PEGDME with ceramic | 1 bar, 20 °C | - | 2.3 (+35%) | 0.05 (−17%) | 2.6 (+136%) | - | [67] |
| SAPO-34 | Pebax® MH1657/PEGDME with ceramic | 1 bar, 20 °C | - | 1.5 (−12%) | 0.06 (0%) | 1.4 (+27%) | - | [67] |
| DD3R (20 wt%) | Matrimid® 5218 | 1 bar, 25 °C | 11.95 | 34.9 a (+105%) | - | 375 (+188%) | - | [68] |
| Zeolite 4A (25 wt%) | PVAc | - | 2.3–2.5 | 3.8 a (−36%) | 1.6 (−20%) | - | 156 (+42%) | [69] |
| Zeolite 4A (15 wt%) | PVAc | 0.75 bar, 30 °C | - | 5.8 a (0%) | 5.3 (+13%) | - | 117 (+22%) | [71] |
| Modified zeolite 4A (15 wt%) | PVAc | 0.75 bar, 30 °C | - | 5.6 a (−3%) | 6.1 (+30%) | - | 143 (+49%) | [71] |
| Hydroxyl sodalite (5 wt%) | PSF | - | - | 21.8 (+169%) | 1.1 (−78%) | - | 1.1 (−78%) | [72] |
| Silica sodalite (15 wt%) | PSF | - | - | 22.8 (+182%) | 1.1 (−78%) | - | 1.0 (−80%) | [72] |
| HZS (8 wt%) | 6FDA-DAM | 2 bar, 35 °C | 28 | 541 a (+13%) | 0.77 (−3%) | 25 (+47%) | 21 (+62%) | [78] |
| Membrane | Separation Performance | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|
| Filler | Polymer/Support | Test Condition | Permeation Area (cm2) | P(H2) (GPU) | Selectivity | |||
| H2/CO2 | H2/CH4 | H2/N2 | ||||||
| CAU-1-NH2 (15 wt%) | PMMA | 3 bar, 25 °C | 0.02 | 11,000 a (+122%) | 13 (+333%) | - | - | [84] |
| CBMN (2 wt%) | 6FDA-Durene-DABA | 3 bar, 25 °C | - | 410 a (−24%) | 1.2 (−14%) | 29 (+45%) | 42 (+83%) | [113] |
| [Cu2(ndc)2(dabco)]n ns (30 wt%) | PBI | 2 bar, 35 °C | - | 12.1 a (+236%) | 11 (+21%) | - | - | [104] |
| MIL-53(Al)-NH2 (20 wt%) | PI-1388 (VTECTM) | 5 bar, 35 °C | 16 | 5.4 a (+8%) | 5.4 (+8%) | - | - | [86] |
| NH2-CAU-1 (20 wt%) | PMMA with ceramic | 2 bar | - | 92 (+475%) | 33 (+400%) | - | - | [87] |
| NH2-MIL-53 (20 wt%) | PMMA with ceramic | 2 bar | - | 72 (+350%) | 45 (+582%) | - | - | [87] |
| nZIF-7 | PEI | 2 bar, 35 °C | - | 1.1 (−45%) | 3.2 (−68%) | 42 (+324%) | 54 (+38%) | [95] |
| PSM-nZIF-7 | PEI | 2 bar, 35 °C | - | 8.6 (+330%) | 9.9 (−1%) | 23 (+132%) | 13 (−67%) | [95] |
| UiO-66 (3 wt%) | PAA/PVP | - | - | 1.2 (−56%) | 20.3 (+62%) | - | - | [98] |
| UiO-66-NH2 (55 wt%) | 6FDA-DAM:DABA (3:2) | 3 bar, 35 °C | - | 2932 a (+1529%) | 1.2 (+20%) | 34 (+3%) | 24 (+4%) | [89] |
| UiO-66-NH2 (40 wt%) | 6FDA-DAM | 3 bar, 35 °C | - | 1810 a (+165%) | 0.7 (−13%) | 10 (−29%) | 10 (−23%) | [89] |
| UiO-66-NH2 (40 wt%) | 6FDA-BPDA-DAM (1:1) | 3 bar, 35 °C | - | 1086 a (+198%) | 0.8 (+14%) | 12 (+14%) | 13 (+13%) | [89] |
| UiO-66-NH2 (18 wt%) | PVP/PEI | 1 bar, 25 °C | - | 31 a (+244%) | 0.08 (−81%) | - | - | [88] |
| UiO-66(Hf)-(OH)2 (10 wt%) | PBI | 2 bar, 35 °C | - | 8.2 a (+128%) | 12 (+33%) | - | - | [82] |
| Oriented & Penetrating ZIF-7 | PI | 2 bar, 100 °C | - | 889 (+1357%) b | 92 (+889%) b | - | 128 (+1424%) b | [85] |
| ZIF-7-I | 6FDA-DAM with α-alumina | - | - | 921 a (+56%) | 2 (+100%) | 67 (+68%) | 36 (+16%) | [99] |
| ZIF-7-III | 6FDA-DAM with α-alumina | - | - | 322 a (−45%) | 4 (+300%) | 172 (+330%) | 59 (+90%) | [99] |
| ZIF-7-mix | 6FDA-DAM with α-alumina | - | - | 478 a (−45%) | 4 (+300%) | 86 (+115%) | 32 (+3%) | [99] |
| ZIF-8 (5 wt%) | PSF | 4 bar, 30 °C | - | 53 a (+43%) | 2.3 (+5%) | 57 (+24%) | 58 (+21%) | [100] |
| ZIF-8 (20 wt%) | 6FDA-Durene | 1 bar, 35 °C | - | 1525 a (+80%) | 1 (+11%) | 16 (+11%) | 14 (+18%) | [102] |
| ZIF-8-PD (20 wt%) | 6FDA-Durene | 1 bar, 35 °C | - | 1320 a (+56%) | 1 (+11%) | 16 (+11%) | 17 (0%) | [102] |
| ZIF-8 (40 wt%) | Matrimid® 5218 | 3.5 bar, 35 °C | - | 400 a (+1329%) | 4 (+33%) | 50 (−52%) | 44 (−50%) | [103] |
| ZIF-8-DA (40 wt%) | Matrimid® 5218 | 3.5 bar, 35 °C | - | 65 a (+132%) | 4 (+33%) | 108 (+4%) | 130 (+48%) | [103] |
| ZIF-8 (20 wt%) | TR polymers (90% conversion) | 1 bar, 35 °C | 3.14 | 1206 a (+189%) | 1.3 (+30%) | 28 (+22%) | 21 (0%) | [97] |
| ZIF-93 (20 wt%) | PBI | 3 bar, 180 °C | 3.14 | 128 a (+184%) | 5 (+25%) | - | - | [96] |
| ZIF-L (20 wt%) | PI | 1 bar | - | 260 a (+18%) | 13 (+550%) | 62 (−11%) | 41 (+3%) | [111] |
| ZIF-L-Co (20 wt%) | TB | 2 bar, 24 °C | - | 1236 a (+312%) | 2 (0%) | 26 (+48%) | 25 (−49%) | [112] |
| ZIF-L-Zn (20 wt%) | TB | 2 bar, 24 °C | - | 898 a (+199%) | 2 (0%) | 25 (+50%) | 23 (−53%) | [112] |
| Membrane | Separation Performance | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|
| Filler | Polymer/Support | Test Condition | Permeation Area (cm2) | P(H2) (GPU) | Selectivity | |||
| H2/CO2 | H2/CH4 | H2/N2 | ||||||
| COFL | PVAm/mPSF | 5 bar | - | 90 (+221%) | 0.1 (0%) | - | - | [131] |
| COFM | PVAm/mPSF | 5 bar | - | 85 (+204%) | 0.085 (−15%) | - | - | [131] |
| COFP | PVAm/mPSF | 5 bar | - | 58 (+107%) | 0.052 (−48%) | - | - | [131] |
| PAOPIM-1 (10 wt%) | PI-COOH | 3 bar | - | 1279 a (+3%) | 1 (+11%) | 16.7 (+14%) | 23.5 (+22%) | [132] |
| NUS-2 (20 wt%) | Ultem | 2 bar, 35 °C | - | 22.7 a (+255%) | 4.6 (+59%) | 103 (+78%) | - | [130] |
| NUS-2 (20 wt%) | PBI | 5 bar, 35 °C | 4.1 a (+14%) | 31.4 (+231%) | - | - | [130] | |
| NUS-3 (20 wt%) | Ultem | 2 bar, 35 °C | - | 33.4 a (+423%) | 2.2 (−24%) | 63 (+9%) | - | [130] |
| NUS-3 (20 wt%) | PBI | 5 bar, 35 °C | 12.2 a (+239%) | 8.9 (−6%) | - | - | [130] | |
| p-DCX (10 wt%) | PTMSP | 2 bar, 25 °C | - | 30,000 a (+53%) | 0.7 (+17%) | 2.8 (0%) | 5.0 (+4%) | [125] |
| V-125 (10 wt%) | PTMSP | 2 bar, 25 °C | - | 11,100 a (−43%) | 0.7 (+17%) | 4.3 (+54%) | 7.6 (+62%) | [125] |
| PAF-1 (10 wt%) | PTMSP | 2 bar, 25 °C | - | 18,400 a (−6%) | 0.7 (+17%) | 4.8 (+72%) | 8.3 (+77%) | [125] |
| PAF-1 (10 wt%) | TPIM-2 | - | - | 4886 a (+196%) | 1 (+25%) | 18.8 (+18%) | 23.4 (+20%) | [126] |
| PAF-1 (10 wt%) | PIM-1 | - | - | 5500 a (+375%) | - | - | 4.5 (+13%) | [127] |
| Membrane | Separation Performance | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|
| Filler | Polymer/Support | Test Condition | Permeation Area (cm2) | P(H2) (GPU) | Selectivity | |||
| H2/CO2 | H2/CH4 | H2/N2 | ||||||
| CGO-76 (C=Cysteamine) | Anodized Al2O3 | 1.5 bar, 25 °C | 3.14 | 52 (−45%) b | 21 (+133%) b | - | - | [143] |
| GO (8 wt%) | PSF | 35 °C | - | 4.7 a (−61%) | - | 29 (−51%) | - | [135] |
| GO (8 wt%) | PI | 35 °C | - | 14 a (−55%) | - | 82 (−39%) | - | [135] |
| GO/PEI | Porous Al2O3 | 25 °C | 3.14 | 1000 a (−83%) | 29 (+625%) | - | - | [139] |
| GO/EDA-2 | Porous Al2O3 | 25 °C | 3.14 | 73 (−42%) b | 23 (+35%) | - | - | [140] |
| Membrane | Separation Performance | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|
| Filler | Polymer/Support | Test Condition | Permeation Area (cm2) | P(H2) (GPU) | Selectivity | |||
| H2/CO2 | H2/CH4 | H2/N2 | ||||||
| UiO-66 + GO (8 wt%) | PSF | 35 °C | - | 12 a (0%) | - | 60 (+2%) | - | [135] |
| UiO-66 + GO (8 wt%) | PI | 35 °C | - | 41 a (+32%) | - | 136 (+1%) | - | [135] |
| MCM-41 + MIL-53(Al)-NH2 (8 wt%) | PSF | 35 °C | 15.2 | 20 a (+67%) | - | 67 (+14%) | - | [147] |
| MCM-41 + MIL-53(Al)-NH2 (8 wt%) | PI | 35 °C | 15.2 | 16 a (−47%) | - | 132 (0%) | - | [147] |
| MCM-41 + JDF-L1 (8 + 4 wt%) | 6FDA-based copolyimide | 35 °C | - | 440 a (+41%) | - | 32 (+68%) | - | [148] |
| HKUST-1 + Silicalite-1 (8 wt%) | PSF | 35 °C | 15.2 | 16 a (+33%) | - | 83 (+38%) | - | [149] |
| ZIF-8 + Silicalite-1 (8 wt%) | PSF | 35 °C | 15.2 | 17 a (+42%) | - | 74 (+23%) | - | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuah, C.Y.; Jiang, X.; Goh, K.; Wang, R. Recent Progress in Mixed-Matrix Membranes for Hydrogen Separation. Membranes 2021, 11, 666. https://doi.org/10.3390/membranes11090666
Chuah CY, Jiang X, Goh K, Wang R. Recent Progress in Mixed-Matrix Membranes for Hydrogen Separation. Membranes. 2021; 11(9):666. https://doi.org/10.3390/membranes11090666
Chicago/Turabian StyleChuah, Chong Yang, Xu Jiang, Kunli Goh, and Rong Wang. 2021. "Recent Progress in Mixed-Matrix Membranes for Hydrogen Separation" Membranes 11, no. 9: 666. https://doi.org/10.3390/membranes11090666
APA StyleChuah, C. Y., Jiang, X., Goh, K., & Wang, R. (2021). Recent Progress in Mixed-Matrix Membranes for Hydrogen Separation. Membranes, 11(9), 666. https://doi.org/10.3390/membranes11090666