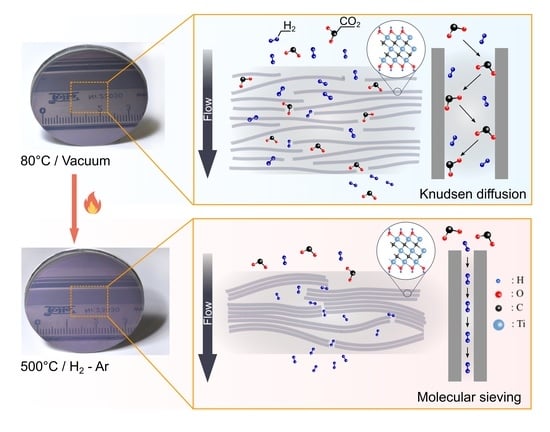
Ti3C2 MXene Membranes for Gas Separation: Influence of Heat Treatment Conditions on D-Spacing and Surface Functionalization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ti3C2 MXene Synthesis
2.2. Membrane Processing
2.3. Permeation Tests
2.4. Characterization
3. Results
3.1. MXenes Synthesis and Membrane Processing
3.2. Gas Permeation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van Der Bruggen, B. Covalent Organic Frameworks for Membrane Separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Van der Bruggen, B. An MXene-Based Membrane for Molecular Separation. Environ. Sci. Nano 2020, 7, 1289–1304. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the Right Stuff: The Trade-off between Membrane Permeability and Selectivity. Science 2017, 356, 1138–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Jin, W.; Xu, N. Two-Dimensional-Material Membranes: A New Family of High-Performance Separation Membranes. Angew. Chem.-Int. Ed. 2016, 55, 13384–13397. [Google Scholar] [CrossRef] [PubMed]
- Mi, B. Graphene Oxide Membranes for Ionic and Molecular Sieving. Science 2014, 343, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Zornoza, B.; Seoane, B.; Zamaro, J.M.; Téllez, C.; Coronas, J. Combination of MOFs and Zeolites for Mixed-Matrix Membranes. ChemPhysChem 2011, 12, 2781–2785. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Rehman, F.; Ali, U.; Ali, A.; Iqbal, M.; Thebo, K.H. Recent Advances in MXene-Based Separation Membranes. ChemBioEng Rev. 2021, 8, 110–120. [Google Scholar] [CrossRef]
- Varoon, K.; Zhang, X.; Elyassi, B.; Brewer, D.D.; Gettel, M.; Kumar, S.; Lee, J.A.; Maheshwari, S.; Mittal, A.; Sung, C.Y.; et al. Dispersible Exfoliated Zeolite Nanosheets and Their Application as a Selective Membrane. Science 2011, 334, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakhe, P.; Prehn, E.M.; Habib, T.; Lutkenhaus, J.L.; Radovic, M.; Mannan, M.S.; Green, M.J. Process Safety Analysis for Ti3C2Tx MXene Synthesis and Processing. Ind. Eng. Chem. Res. 2019, 58, 1570–1579. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, D.; Ma, J.; Tong, M.; Zhang, W.; Huang, H.; Yang, Q.; Zhong, C. A GO-Assisted Method for the Preparation of Ultrathin Covalent Organic Framework Membranes for Gas Separation. J. Mater. Chem. A 2016, 4, 13444–13449. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and Optical Properties of 2D Transition Metal Carbides and Nitrides (MXenes). Adv. Mater. 2018, 30, 1804779P. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; Kim, S.J.; Cho, S.Y.; Choi, J.; Maleski, K.; Lee, B.J.; Jung, H.T.; Gogotsi, Y.; Lee, Y.; Ahn, C.W. An Investigation into the Factors Governing the Oxidation of Two-Dimensional Ti3C2 MXene. Nanoscale 2019, 11, 8387–8393. [Google Scholar] [CrossRef]
- Ding, L.X.; Wei, Y.; Li, L.; Zhang, T.; Wang, H.; Xue, J.; Ding, L.X.; Wang, S.; Caro, J.; Gogotsi, Y. MXene Molecular Sieving Membranes for Highly Efficient Gas Separation. Nat. Commun. 2018, 9, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Liu, G.; Ji, Y.; Liu, Q.; Cheng, L.; Guan, K.; Zhang, M.; Liu, G.; Xiong, J.; Yang, J.; et al. 2D MXene Nanofilms with Tunable Gas Transport Channels. Adv. Funct. Mater. 2018, 28, 1801511. [Google Scholar] [CrossRef]
- Arshadi, F.; Mohammad, M.; Hosseini, E.; Ahmadi, H.; Asadnia, M.; Orooji, Y.; Korayem, A.H.; Noorbakhsh, A.; Razmjou, A. The Effect of D-Spacing on the Ion Selectivity Performance of MXene Membrane. J. Memb. Sci. 2021, 639, 119752. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and Characterization of Graphene Oxide Paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Hadler-Jacobsen, J.; Fagerli, F.H.; Kaland, H.; Schnell, S.K. Stacking Sequence, Interlayer Bonding, Termination Group Stability and Li/Na/Mg Diffusion in MXenes. ACS Mater. Lett. 2021, 3, 1369–1376. [Google Scholar] [CrossRef]
- Fan, Y.; Wei, L.; Meng, X.; Zhang, W.; Yang, N.; Jin, Y.; Wang, X.; Zhao, M.; Liu, S. An Unprecedented High-Temperature-Tolerance 2D Laminar MXene Membrane for Ultrafast Hydrogen Sieving. J. Memb. Sci. 2019, 569, 117–123. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Zhuang, Y.; Liu, G.; Xing, W.; Jing, W. Adjustable Interlayer Spacing of Ultrathin MXene-Derived Membranes for Ion Rejection. J. Memb. Sci. 2019, 591, 117350. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and Delamination of Layered Carbides and Carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, F.; Zimmermann, K.; Alamoudi, M.; Abkar, L.; Barbeau, B.; Mohseni, M.; Kandasubramanian, B.; Smith, K. Application of MXenes for Air Purification, Gas Separation and Storage: A Review. Renew. Sustain. Energy Rev. 2022, 164, 112527. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Kan, A.S.; Chumakov, A.P.; Konovalov, O.V.; Valeev, R.G.; Eliseev, A.A. MXene-Based Gas Separation Membranes with Sorption Type Selectivity. J. Memb. Sci. 2021, 621, 118994. [Google Scholar] [CrossRef]
- Dash, A.; Vaßen, R.; Guillon, O.; Gonzalez-Julian, J. Molten Salt Shielded Synthesis of Oxidation Prone Materials in Air. Nat. Mater. 2019, 18, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Naguib, M.; Unocic, R.R.; Armstrong, B.L.; Nanda, J. Large-Scale Delamination of Multi-Layers Transition Metal Carbides and Carbonitrides “MXenes”. Dalt. Trans. 2015, 44, 9353–9358. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive Two-Dimensional Titanium Carbide “clay” with High Volumetric Capacitance. Nature 2015, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at Every Step, from Their Precursors to Single Flakes and Assembled Films. Prog. Mater. Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic Interference Shielding with 2D Transition Metal Carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Zhang, Y.; Gao, X.; Yuan, Y.; Su, B.; Gao, C. Declining Flux and Narrowing Nanochannels under Wrinkles of Compacted Graphene Oxide Nanofiltration Membranes. Carbon 2016, 108, 568–575. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Hu, T.; Hu, M.; Gao, B.; Li, W.; Wang, X. Screening Surface Structure of MXenes by High-Throughput Computation and Vibrational Spectroscopic Confirmation. J. Phys. Chem. C 2018, 122, 18501–18509. [Google Scholar] [CrossRef]
- Yazdanparast, S.; Soltanmohammad, S.; Fash-White, A.; Tucker, G.J.; Brennecka, G.L. Synthesis and Surface Chemistry of 2D TiVC Solid-Solution MXenes. ACS Appl. Mater. Interfaces 2020, 12, 20129–20137. [Google Scholar] [CrossRef] [PubMed]
- Qian, A.; Hyeon, S.E.; Seo, J.Y.; Chung, C.H. Capacitance Changes Associated with Cation-Transport in Free-Standing Flexible Ti3C2Tx (T[Dbnd]O, F, OH) MXene Film Electrodes. Electrochim. Acta 2018, 266, 86–93. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion Sieving in Graphene Oxide Membranes via Cationic Control of Interlayer Spacing. Nat. Publ. Gr. 2017, 550, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Zhu, J.; Tian, M.; Zheng, S.; Wang, F.; Wang, X.; Wang, L. Ion Sieving by a Two-Dimensional Ti3C2Tx Alginate Lamellar Membrane with Stable Interlayer Spacing. Nat. Commun. 2020, 11, 3540. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Hu, T.; Zhang, H.; Wang, C.; Hu, M.; Yang, J.; Cui, C.; Guang, T.; Li, C.; Shi, C.; et al. Understanding the Lithium Storage Mechanism of Ti3C2Tx MXene. J. Phys. Chem. C 2019, 123, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Sarycheva, A.; Gogotsi, Y. Raman Spectroscopy Analysis of the Structure and Surface Chemistry of Ti3C2T XMXene. Chem. Mater. 2020, 32, 3480–3488. [Google Scholar] [CrossRef]
- Seredych, M.; Shuck, C.E.; Pinto, D.; Alhabeb, M.; Precetti, E.; Deysher, G.; Anasori, B.; Kurra, N.; Gogotsi, Y. High-Temperature Behavior and Surface Chemistry of Carbide MXenes Studied by Thermal Analysis. Chem. Mater. 2019, 31, 3324–3332. [Google Scholar] [CrossRef]
- Sang, X.; Xie, Y.; Yilmaz, D.E.; Lotfi, R.; Alhabeb, M.; Ostadhossein, A.; Anasori, B.; Sun, W.; Li, X.; Xiao, K.; et al. In Situ Atomistic Insight into the Growth Mechanisms of Single Layer 2D Transition Metal Carbides. Nat. Commun. 2018, 9, 2266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Wan, Y.J.; Rajavel, K.; Li, X.M.; Wang, X.Y.; Liao, S.Y.; Lin, Z.Q.; Zhu, P.L.; Sun, R.; Wong, C.P. Electromagnetic Interference Shielding of Ti3C2Tx MXene Modified by Ionic Liquid for High Chemical Stability and Excellent Mechanical Strength. Chem. Eng. J. 2021, 408, 127303. [Google Scholar] [CrossRef]
- Zheng, Z.; Guo, C.; Wang, E.; He, Z.; Liang, T.; Yang, T.; Hou, X. The Oxidation and Thermal Stability of Two-Dimensional Transition Metal Carbides and/or Carbonitrides (MXenes) and the Improvement Based on Their Surface State. Inorg. Chem. Front. 2021, 8, 2164–2182. [Google Scholar] [CrossRef]
- Cao, F.; Zhang, Y.; Wang, H.; Khan, K.; Tareen, A.K.; Qian, W.; Zhang, H.; Ågren, H. Recent Advances in Oxidation Stable Chemistry of 2D MXenes. Adv. Mater. 2022, 34, 2107554. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.; Yu, Y.; Jiang, F.; Wang, Y.; Mi, L.; Yu, Y.; Song, L. Fabrication and Thermal Stability of NH4HF2-Etched Ti3C2 MXene. Ceram. Int. 2017, 43, 6322–6328. [Google Scholar] [CrossRef]
- Chong, J.Y.; Wang, B.; Mattevi, C.; Li, K. Dynamic Microstructure of Graphene Oxide Membranes and the Permeation Flux. J. Memb. Sci. 2018, 549, 385–392. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Springer: Dordrecht, The Netherlands, 1996; ISBN 9780792342489. [Google Scholar]
- Xu, W.L.; Fang, C.; Zhou, F.; Song, Z.; Liu, Q.; Qiao, R.; Yu, M. Self-Assembly: A Facile Way of Forming Ultrathin, High-Performance Graphene Oxide Membranes for Water Purification. Nano Lett. 2017, 17, 2928–2933. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.P.; Wang, L.; Pellegrino, J.; Bunch, J.S. Selective Molecular Sieving through Porous Graphene. Nat. Nanotechnol. 2012, 7, 728–732. [Google Scholar] [CrossRef] [PubMed]














| Sample ID | c-Lattice (Å) | d(002) (Å) | Free d-Spacing (nm) | Average Free D-Spacing (nm) |
|---|---|---|---|---|
| Heat treatment temperature 80 °C | ||||
| C1_1 | 26.623 | 13.311 | 0.331 | 0.347 ± 0.003 |
| C1_2 | 27.036 | 13.518 | 0.352 | |
| C1_3 | 26.940 | 13.470 | 0.347 | |
| C1_4 | 26.782 | 13.391 | 0.339 | |
| C1_5 | 26.952 | 13.476 | 0.348 | |
| C2_1 | 27.079 | 13.540 | 0.354 | 0.352 ± 0.003 |
| C2_2 | 27.195 | 13.597 | 0.360 | |
| C2_3 | 26.755 | 13.378 | 0.338 | |
| C2_4 | 26.980 | 13.490 | 0.349 | |
| C2_5 | 27.092 | 13.546 | 0.355 | |
| Heat treatment temperature 500 °C | ||||
| C1_1 | 20.467 | 10.234 | 0.023 | 0.024 ± 0.001 |
| C1_2 | 20.413 | 10.206 | 0.021 | |
| C1_3 | 20.491 | 10.246 | 0.025 | |
| C1_4 | 20.580 | 10.290 | 0.029 | |
| C1_5 | 20.475 | 10.237 | 0.024 | |
| C2_1 | 20.773 | 10.387 | 0.039 | 0.040 ± 0.001 |
| C2_2 | 20.891 | 10.445 | 0.045 | |
| C2_3 | 20.808 | 10.404 | 0.040 | |
| C2_4 | 20.766 | 10.383 | 0.038 | |
| C2_5 | 20.903 | 10.452 | 0.045 | |
| Sample ID | H2 Permeability (Barrer) | H2 Permeance (10³ mol/m² s¹ Pa¹) | CO2 Permeability (Barrer) | CO2 Permeance (10³ mol/m² s¹ Pa¹) | H2/CO2 Selectivity |
|---|---|---|---|---|---|
| Heat treatment temperature 80 °C | |||||
| C1_1 | 6.4 | 33.7 | 2.0 | 10.2 | 3.3 |
| C1_2 | 2.1 | 10.9 | 0.5 | 2.5 | 4.4 |
| C1_3 | 2.0 | 10.5 | 0.7 | 3.6 | 3.0 |
| C2_1 | 16.4 | 254.7 | 4.9 | 71.7 | 3.3 |
| C2_2 | 23.9 | 85.4 | 4.9 | 25.8 | 4.8 |
| C2_3 | 48.8 | 124.9 | 13.7 | 25.8 | 3.6 |
| Heat treatment temperature 500 °C | |||||
| C1_1 | 0.86 | 4.5 | 0.12 | 0.6 | 7.0 |
| C1_2 | 0.54 | 2.8 | 0.09 | 0.5 | 5.8 |
| C1_3 | 0.83 | 4.4 | 0.14 | 0.7 | 6.0 |
| C2_1 | 35.6 | 186.2 | 9.1 | 47.6 | 3.9 |
| C2_2 | 9.7 | 50.8 | 2.5 | 12.9 | 3.9 |
| C2_3 | 28.5 | 149.1 | 7.3 | 37.9 | 3.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emerenciano, A.A.; do Nascimento, R.M.; Barbosa, A.P.C.; Ran, K.; Meulenberg, W.A.; Gonzalez-Julian, J. Ti3C2 MXene Membranes for Gas Separation: Influence of Heat Treatment Conditions on D-Spacing and Surface Functionalization. Membranes 2022, 12, 1025. https://doi.org/10.3390/membranes12101025
Emerenciano AA, do Nascimento RM, Barbosa APC, Ran K, Meulenberg WA, Gonzalez-Julian J. Ti3C2 MXene Membranes for Gas Separation: Influence of Heat Treatment Conditions on D-Spacing and Surface Functionalization. Membranes. 2022; 12(10):1025. https://doi.org/10.3390/membranes12101025
Chicago/Turabian StyleEmerenciano, Aline Alencar, Rubens Maribondo do Nascimento, Ana Paula Cysne Barbosa, Ke Ran, Wilhelm Albert Meulenberg, and Jesus Gonzalez-Julian. 2022. "Ti3C2 MXene Membranes for Gas Separation: Influence of Heat Treatment Conditions on D-Spacing and Surface Functionalization" Membranes 12, no. 10: 1025. https://doi.org/10.3390/membranes12101025
APA StyleEmerenciano, A. A., do Nascimento, R. M., Barbosa, A. P. C., Ran, K., Meulenberg, W. A., & Gonzalez-Julian, J. (2022). Ti3C2 MXene Membranes for Gas Separation: Influence of Heat Treatment Conditions on D-Spacing and Surface Functionalization. Membranes, 12(10), 1025. https://doi.org/10.3390/membranes12101025