Charged Lipids Influence Phase Separation in Cell-Sized Liposomes Containing Cholesterol or Ergosterol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liposome Preparation Protocol
2.3. Microscopic Observation
2.4. Statistical Analysis
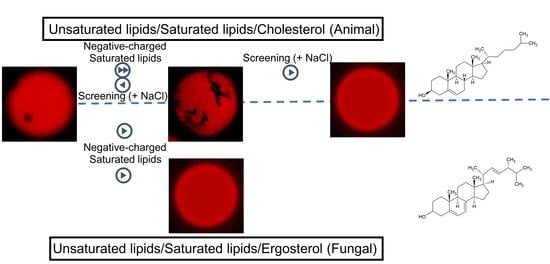
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoda, T.; Ichinohe, S.; Yokosawa, Y. Rapid analysis of minerals in oysters using microwave decomposition and inductively coupled plasma atomic emission spectrometry. Aquacult. Rep. 2021, 19, 100585. [Google Scholar] [CrossRef]
- Yoda, Y.; Ichinohe, S.; Yokosawa, Y. Effect of microwave decomposition on inductively coupled plasma spectrometry analysis of minerals in oysters. Fish. Sci. 2019, 85, 1089–1098. [Google Scholar] [CrossRef]
- Terahara, N.; Sano, M.; Ito, M. A Bacillus Flagellar Motor That Can Use Both Na+ and K+ as a Coupling Ion Is Converted by a Single Mutation to Use Only Na+. PLoS ONE 2012, 7, e46248. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.J.; Nicolson, G.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Vestergaard, M.; Hamada, T.; Takagi, M. Using model membranes for the study of amyloid beta: Lipid interactions and neurotoxicity. Biotechnol. Bioeng. 2008, 99, 753–763. [Google Scholar] [CrossRef]
- Yabuuchi, S.; Endo, S.; Baek, K.; Hoshino, K.; Tsujino, Y.; Vestergaard, M.C.; Takagi, M. Raft-dependent endocytic movement and intracellular cluster formation during T cell activation triggered by concanavalin A. J. Biosci. Bioeng. 2017, 124, 685–693. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P.; Bray, D.; Watson, J. Molecular Biology of the Cell, Classic Textbook, 5th ed.Garland Science: New York, NY, USA, 2012. [Google Scholar]
- Himeno, H.; Shimokawa, N.; Komura, S.; Andelman, D.; Hamada, T.; Takagi, M. Charge-induced phase separation in lipid membranes. Soft Matter 2014, 10, 7959–7967. [Google Scholar] [CrossRef] [Green Version]
- Shimokawa, N.; Hishida, M.; Seto, H.; Yoshikawa, K. Phase separation of a mixture of charged and neutral lipids on a giant vesicle induced by small cations. Chem. Phys. Lett. 2010, 496, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Klose, C.; Ejsing, C.S.; García-Sáez, A.J.; Kaiser, H.-J.; Sampaio, J.L.; Surma, M.A.; Shevchenko, A.; Schwille, P.; Simons, K.S. Yeast Lipids can phase-separate into micrometer-scale membrane domains. J. Biol. Chem. 2010, 285, 30224–30232. [Google Scholar] [CrossRef]
- Wachtler, V.; Balasubramanian, M.K. Yeast lipid rafts?–An emerging view. Trends Cell Biol. 2006, 16, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Grudzinski, W.; Sagan, J.; Welc, R.; Luchowski, R.; Gruszecki, W.I. Molecular organization, localization and orientation of antifungal antibiotic amphotericin B in a single lipid bilayer. Sci. Rep. 2016, 6, 32780. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Suga, K.; Umakoshi, H. Roles of sterol derivatives in regulating the properties of phospholipid bilayer systems. Langmuir 2016, 32, 6176–6184. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Suga, K.; Umakoshi, H. Ergosterol-induced ordered phase in ternary lipid mixture systems of unsaturated and saturated phospholipid membranes. J. Phys. Chem. B 2019, 123, 6161–6168. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Suga, K.; Kuhl, T.L.; Umakoshi, H. Melting-temperature-dependent interactions of ergosterol with unsaturated and saturated lipids in model membranes. Langmuir 2019, 35, 10640–10647. [Google Scholar] [CrossRef]
- Stevens, M.M.; Honerkamp-Smith, A.R.; Keller, S.L. Solubility limits of cholesterol, lanosterol, ergosterol, stigmasterol, and β-sitosterol in electroformed lipid vesicles. Soft Matter 2010, 6, 9061–9068. [Google Scholar] [CrossRef]
- Beattie, M.E.; Veatch, S.L.; Stottrup, B.L.; Keller, S.L. Sterol structure determines miscibility versus melting transitions in lipid vesicles. Biophys. J. 2005, 89, 1760–1768. [Google Scholar] [CrossRef] [Green Version]
- Bacia, K.; Schwille, P.; Kurzchalia, T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl. Acad. Sci. USA 2005, 102, 3272–3277. [Google Scholar] [CrossRef] [Green Version]
- Yoda, T.; Vestergaard, M.C.; Akazawa-Ogawa, Y.; Yoshida, Y.; Hamada, T.; Takagi, M. Dynamic response of a cholesterol-containing model membrane to oxidative stress. Chem. Lett. 2010, 39, 1273–1274. [Google Scholar] [CrossRef] [Green Version]
- Vestergaard, M.C.; Yoda, T.; Hamada, T.; Akazawa Ogawa, Y.; Yoshida, Y.; Takagi, M. The effect of oxycholesterols on thermo-induced membrane dynamics. Biochim. Biophys. Acta 2011, 1808, 2245–2251. [Google Scholar] [CrossRef]
- Vestergaard, M.C.; Yoda, T.; Hamada, T.; Akazawa, Y.; Yoshida, Y.; Takagi, M. Thermo-responsiveness of auto-oxidized cholesterol-containing lipid membranes, observed in real-time. In Proceedings of the 2011 International Symposium on Micro-NanoMechatronics and Human Science, Nagoya, Japan, 6–9 November 2011; pp. 451–455. [Google Scholar]
- Dhingra, S.; Morita, M.; Yoda, T.; Vestergaard, M.C.; Hamada, T.; Takagi, M. Dynamic transformation of a cell-sized liposome containing ganglioside. In Proceedings of the 2011 International Symposium on Micro-NanoMechatronics and Human Science, Nagoya, Japan, 6–9 November 2011; pp. 461–465. [Google Scholar]
- Yoda, T.; Phan, H.T.T.; Vestergaard, M.C.; Hamada, T.; Takagi, M. Thermo-induced dynamics of membranes and liquid crystals containing cholesterol derivatives. In Proceedings of the 2012 International Symposium on Micro-NanoMechatronics and Human Science (MHS), Nagoya, Japan, 4–7 November 2012; pp. 87–92. [Google Scholar]
- Chahal, B.; Vestergaard, M.C.; Yoda, T.; Morita, M.; Takagi, M. Structure-Dependent Membrane Interaction and bioactivity of Flavonoids with Lipid bilayers. In Proceedings of the 2012 International Symposium on Micro-NanoMechatronics and Human Science (MHS), Nagoya, Japan, 4–7 November 2012; pp. 106–110. [Google Scholar]
- Yoda, T.; Vestergaard, M.C.; Hamada, T.; Le, P.T.M.; Takagi, M. Thermo-induced vesicular dynamics of membranes containing cholesterol derivatives. Lipids 2012, 47, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.T.; Hata, T.; Morita, M.; Yoda, T.; Hamada, T.; Vestergaard, M.C.; Takagi, M. The effect of oxysterols on the interaction of Alzheimer’s amyloid beta with model membranes. Biochim. Biophys. Acta 2013, 1828, 2487–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhingra, S.; Morita, M.; Yoda, T.; Vestergaard, M.C.; Hamada, T.; Takagi, M. Dynamic morphological changes induced by GM1 and protein interactions on the surface of cell-sized liposomes. Materials 2013, 6, 2522–2533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, H.T.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Vestergaard, M.C. Structure-dependent interactions of polyphenols with a biomimetic membrane system. Biochim. Biophys. Acta 2014, 1838, 2670–2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Phan, H.T.T.; Yoda, T.; Shimokawa, N.; Vestergaard, M.C.; Takagi, M. Effects of capsaicin on biomimetic membranes. Biomimetics 2019, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Yoda, T.; Saito, T. Size of cells and physicochemical properties of membranes are related to flavor production during sake brewing in the yeast Saccharomyces cerevisiae. Membranes 2020, 10, 440. [Google Scholar] [CrossRef]
- Yoda, T.; Ogura, A.; Saito, T. Influence of ethyl caproate on the size of lipid vesicles and yeast cells. Biomimetics 2020, 5, 16. [Google Scholar] [CrossRef]
- Yoda, T.; Yamada, Y.; Chounan, Y. Effects of isovaleraldehyde on cell-sized lipid bilayer vesicles. Biophys. Chem. 2021, 279, 106698. [Google Scholar] [CrossRef]
- Yoda, T. Quality evaluation of drinks based on liposome shape changes induced by flavor molecules. ACS Omega 2022, 7, 5679–5686. [Google Scholar] [CrossRef]
- Avanti Polar Lipids. Phase Transition Temperatures for Glycerophospholipids. Available online: https://avantilipids.com/tech-support/physical-properties/phase-transition-temps (accessed on 19 September 2022).
- Dimova, R.; Stano, P.; Marques, C.M.; Walde, P. Chapter 1, Preparation methods for giant unilamellar vesicles. In The Giant Vesicle Book; Dimova, R., Marques, C.M., Eds.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Hamada, T.; Kishimoto, Y.; Nagasaki, T.; Takagi, M. Lateral phase separation in tense membranes. Soft Matter 2011, 7, 9061–9068. [Google Scholar] [CrossRef]
- Ishii, K.; Hamada, T.; Hatakeyama, M.; Sugimoto, R.; Nagasaki, T.; Takagi, M. Reversible control of exo-and endo-budding transitions in a photosensitive lipid membrane. ChemBioChem 2009, 10, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Veatch, S.L.; Keller, S.L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 2003, 85, 3074–3083. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.H.; Clair, J.J.; Juhasz, J. Phase equilibria in DOPC/DPPC-d62/cholesterol mixtures. Biophys. J. 2009, 96, 521–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, R.F.M.; Borst, J.; Fedorov, A.; Prieto, M.; Visser, A.J.W.G. Complexity of lipid domains and rafts in giant unilamellar vesicles revealed by combining imaging and microscopic and macroscopic time-resolved fluorescence. Biophys. J. 2007, 93, 539–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Ito, H.; Higuchi, Y.; Bohinc, K.; Shimokawa, N.; Takagi, M. Three-phase coexistence in binary charged lipid membranes in a hypotonic solution. Langmuir 2021, 37, 9683–9693. [Google Scholar] [CrossRef]
- Yoda, T. Phase Separation in Liposomes Determined by Ergosterol and Classified Using Machine Learning. Microsc. Microanal. 2022; 28, 2130–2137. [Google Scholar] [CrossRef]
- Kubsch, B.; Robinson, T.; Lipowsky, R.; Dimova, R. Solution Asymmetry and Salt Expand Fluid-Fluid Coexistence Regions of Charged Membranes. Biophys. J. 2016, 110, 2581–2584. [Google Scholar] [CrossRef] [Green Version]
- Pataraia, S.; Liu, Y.; Lipowsky, R.; Dimova, R. Effect of cytochrome c on the phase behavior of charged multicomponent lipid membranes. Biochim. Biophys. Acta 2014, 1838, 2036–2045. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Baek, K.; Shimokawa, N.; Takagi, M. Effect of temperature on raft-dependent endocytic cluster formation during activation of Jurkat T cells by concanavalin A. J. Biosci. Bioeng. 2019, 127, 479–485. [Google Scholar] [CrossRef]
- Uchida, K.; Obayashi, K.; Minamihata, K.; Wakabayashi, R.; Goto, M.; Shimokawa, N.; Takagi, M.; Kamiya, N. Artificial Palmitoylation of Proteins Controls the Lipid Domain-Selective Anchoring on Biomembranes and the Raft-Dependent Cellular Internalization. Langmuir 2022, 38, 9640–9648. [Google Scholar] [CrossRef]
- Qian, T.; Li, C.; He, R.; Wan, C.; Liu, Y.; Yu, H. Calcium-dependent and -independent lipid transfer mediated by tricalbins in yeast. J. Biol. Chem. 2021, 296, 100729. [Google Scholar] [CrossRef]
- Rothman, J.E.; Lenard, J. Membrane Asymmetry: The nature of membrane asymmetry provides clues to the puzzle of how membranes are assembled. Science 1977, 195, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkleij, A.J.; Post, J.A. Membrane Phospholipid Asymmetry and Signal Transduction. J. Membr. Biol. 2000, 178, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Yoshikawa, K. Cell-Sized Liposomes and Droplets: Real-World Modeling of Living Cells. Materials 2012, 5, 2292–2305. [Google Scholar] [CrossRef]
- Hamada, T.; Miura, Y.; Komatsu, Y.; Kishimoto, Y.; Vestergaard, M.; Takagi, M. Construction of Asymmetric Cell-Sized Lipid Vesicles from Lipid-Coated Water-in-Oil Microdroplets. J. Phys. Chem. B 2008, 112, 14678–14681. [Google Scholar] [CrossRef]
- Laradji, M.; Kumar, P.S. Anomalously slow domain growth in fluid membranes with asymmetric transbilayer lipid distribution. Phys. Rev. E 2006, 73, 040901. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoda, T. Charged Lipids Influence Phase Separation in Cell-Sized Liposomes Containing Cholesterol or Ergosterol. Membranes 2022, 12, 1121. https://doi.org/10.3390/membranes12111121
Yoda T. Charged Lipids Influence Phase Separation in Cell-Sized Liposomes Containing Cholesterol or Ergosterol. Membranes. 2022; 12(11):1121. https://doi.org/10.3390/membranes12111121
Chicago/Turabian StyleYoda, Tsuyoshi. 2022. "Charged Lipids Influence Phase Separation in Cell-Sized Liposomes Containing Cholesterol or Ergosterol" Membranes 12, no. 11: 1121. https://doi.org/10.3390/membranes12111121
APA StyleYoda, T. (2022). Charged Lipids Influence Phase Separation in Cell-Sized Liposomes Containing Cholesterol or Ergosterol. Membranes, 12(11), 1121. https://doi.org/10.3390/membranes12111121