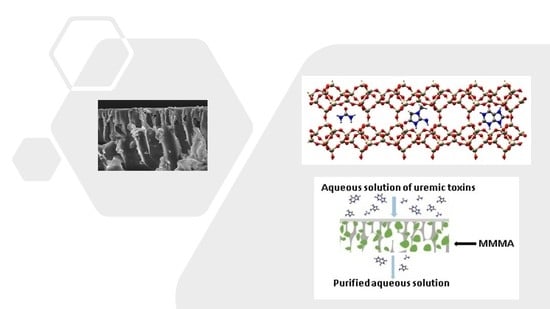
Mixed Matrix Membranes Adsorbers (MMMAs) for the Removal of Uremic Toxins from Dialysate
Abstract
:1. Introduction
1.1. Motivation
1.2. Uremic Toxins Removal
1.2.1. Sorbents
1.2.2. Polymeric Membranes
1.2.3. Mixed Matrix Membranes (MMMs)
1.2.4. Mixed Matrix Membrane Adsorbers
1.3. MMMAs Developed in This Work
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Fillers
2.1.2. Uremic Toxins
2.1.3. Adsorbents
2.1.4. Zeolites Activation
2.2. Membranes
2.2.1. Pure Polymeric Membranes Preparation
2.2.2. MMMAs Preparation
2.3. Membrane Characterization
2.3.1. Morphological Analysis
2.3.2. Water Uptake and Density
2.3.3. Dynamic Contact Angle
2.3.4. Hydraulic Permeability
2.4. Adsorption Properties Characterization
2.4.1. Batch Characterization of the Adsorbents
2.4.2. Batch Characterization of the Membranes
2.4.3. Continuous Removal Capacity for Single-Toxin Solutions and for a Toxin Mixture
3. Results and Discussion
3.1. Materials and Membranes Characterization
3.1.1. Adsorbents Particle Size Analysis
3.1.2. Membrane Morphology
3.1.3. Density and Water Uptake of MMMAs
3.1.4. Hydraulic Permeability of MMMAs
3.2. Batch Adsorption Tests
3.2.1. Adsorbents
3.2.2. MMMAs
3.3. Dynamic Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020, 16, 251. [Google Scholar] [CrossRef] [Green Version]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Agar, J.W. Green dialysis: The environmental challenges ahead. Semin. Dial. 2015, 28, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Agar, J.W.M. Reusing Dialysis Wastewater: The Elephant in the Room. Am. J. Kidney Dis. 2008, 52, 10–12. [Google Scholar] [CrossRef] [PubMed]
- González, J.F. Assessing the Macroeconomic Impact of Water Supply Restrictions Through an Input-Output Analysis. Water Resour. Manag. 2011, 25, 2335–2347. [Google Scholar] [CrossRef]
- Craun, G.F.; Calderon, R.L. Workshop summary: Estimating waterborne disease risks in the United States. J. Water Health 2006, 4, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Agar, J.W.M. Reusing and recycling dialysis reverse osmosis system reject water. Kidney Int. 2015, 88, 653–657. [Google Scholar] [CrossRef] [Green Version]
- Ponson, L.; Arkouche, W.; Laville, M. Toward green dialysis: Focus on water savings. Hemodial Int. 2014, 18, 7–14. [Google Scholar] [CrossRef]
- Van Gelder, M.K.; Jong, J.A.W.; Folkertsma, L.; Guo, Y.; Blüchel, C.; Verhaar, M.C.; Odijk, M.; Van Nostrum, C.F.; Hennink, W.E.; Gerritsen, K.G.F. Urea removal strategies for dialysate regeneration in a wearable artificial kidney. Biomaterials 2020, 234, 119735. [Google Scholar] [CrossRef]
- Blumenkrantz, M.J.; Gordon, A.; Roberts, M.; Lewin, A.J.; Pecker, E.A.; Moran, J.K.; Coburn, J.W.; Maxwell, M.H. Applications of the Redy sorbent system to hemodialysis and peritoneal dialysis. Artif. Organs 1979, 3, 230–236. [Google Scholar] [CrossRef]
- Gura, V.; Rivara, M.B.; Bieber, S.; Munshi, R.; Smith, N.C.; Linke, L.; Kundzins, J.; Beizai, M.; Ezon, C.; Kessler, L.; et al. A wearable artificial kidney for patients with end-stage renal disease. JCI Insight 2016, 1, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, A.; Gura, V.; Ronco, C.; Beizai, M.; Ezon, C.; Rambod, E. A wearable haemodialysis device for patients with end-stage renal failure: A pilot study. Lancet 2007, 370, 2005–2010. [Google Scholar] [CrossRef]
- Lornoy, W.; Becaus, I.; Billiouw, J.M.; Sierens, L.; Van Malderen, P.; D’Haenens, P. Online haemodiafiltration. Remarkable removal of beta2-microglobulin. Long-term clinical observations. Nephrol. Dial. Transplant. 2000, 15 (Suppl. 1), 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avramescu, M.E.; Borneman, Z.; Wessling, M. Mixed-matrix membrane adsorbers for protein separation. J. Chromatogr. A 2003, 1006, 171–183. [Google Scholar] [CrossRef]
- Dhondt, A.; Vanholder, R.; Van Biesen, W.; Lameire, N. The removal of uremic toxins. Kidney Int. 2000, 58 (Suppl. 76), S47–S59. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, H.D.; Marten, R.; Gullberg, C.A. How to catch urea? Considerations on urea removal from hemofiltrate. Artif. Organs 1981, 5, 278–285. [Google Scholar] [CrossRef]
- Lesaffer, G.; De Smet, R.; Lameire, N.; Dhondt, A.; Duym, P.; Vanholder, R. Intradialytic removal of protein-bound uraemic toxins: Role of solute characteristics and of dialyser membrane. Nephrol. Dial. Transplant. 2000, 15, 50–57. [Google Scholar] [CrossRef]
- Blaney, T.L.; Lindan, O.; Sparks, R.E. Adsorption: A step forward toward a wearable artificial kidney. Trans. Am. Soc. Artif. Intern. Organs 1966, 12, 7. [Google Scholar]
- Ma, Y.; Li, S.; Tonelli, M.; Unsworth, L.D. Adsorption-based strategies for removing uremic toxins from blood. Microporous Mesoporous Mater. 2021, 319, 111035. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Sowa, M.; Simka, W. Urea removal from aqueous solutions—A review. J. Appl. Electrochem. 2016, 46, 1011–1029. [Google Scholar] [CrossRef] [Green Version]
- Wernert, V.; Schäf, O.; Ghobarkar, H.; Denoyel, R. Adsorption properties of zeolites for artificial kidney applications. Microporous Mesoporous Mater. 2005, 83, 101–113. [Google Scholar] [CrossRef]
- Bergé-Lefranc, D.; Pizzala, H.; Paillaud, J.L.; Schäf, O.; Vagner, C.; Boulet, P.; Kuchta, B.; Denoyel, R. Adsorption of small uremic toxin molecules on MFI type zeolites from aqueous solution. Adsorption 2008, 14, 377–387. [Google Scholar] [CrossRef]
- Bergé-Lefranc, D.; Vagner, C.; Calaf, R.; Pizzala, H.; Denoyel, R.; Brunet, P.; Ghobarkar, H.; Schäf, O. In vitro elimination of protein bound uremic toxin p-cresol by MFI-type zeolites. Microporous Mesoporous Mater. 2012, 153, 288–293. [Google Scholar] [CrossRef]
- Cheah, W.K.; Sim, Y.L.; Yeoh, F.Y. Amine-functionalized mesoporous silica for urea adsorption. Mater. Chem. Phys. 2016, 175, 151–157. [Google Scholar] [CrossRef]
- Harm, S.; Falkenhagen, D.; Hartmann, J. Pore size—A key property for selective toxin removal in blood purification. Int. J. Artif. Organs 2014, 37, 668–678. [Google Scholar] [CrossRef]
- Pavlenko, D.; Giasafaki, D.; Charalambopoulou, G.; Van Geffen, E.; Gerritsen, K.G.F.; Steriotis, T.; Stamatialis, D. Carbon Adsorbents with Dual Porosity for Efficient Removal of Uremic Toxins and Cytokines from Human Plasma. Sci. Rep. 2017, 7, 1–7. [Google Scholar]
- Cheng, Y.-C.; Fu, C.-C.; Hsiao, Y.-S.; Chien, C.-C.; Juang, R.-S. Clearance of low molecular-weight uremic toxins p-cresol, creatinine, and urea from simulated serum by adsorption. J. Mol. Liq. 2018, 252, 203–210. [Google Scholar] [CrossRef]
- Yang, C.-X.; Liu, C.; Cao, Y.-M.; Yan, X.-P. Metal–organic framework MIL-100(Fe) for artificial kidney application. RSC Adv. 2014, 4, 40824–40827. [Google Scholar] [CrossRef]
- Wernert, V.; Schäf, O.; Faure, V.; Brunet, P.; Dou, L.; Berland, Y.; Boulet, P.; Kuchta, B.; Denoyel, R. Adsorption of the uremic toxin p-cresol onto hemodialysis membranes and microporous adsorbent zeolite silicalite. J. Biotechnol. 2006, 123, 164–173. [Google Scholar]
- Kraus, M.A.; Frommer, M.A.; Nemas, M.; Gutman, R. Urea-rejecting membranes and their application in the development of a miniature artificial kidney. J. Membr. Sci. 1976, 1, 115–127. [Google Scholar] [CrossRef]
- Ofsthun, N.J.; Stennett, A.K. An Integrated Membrane/Sorbent PD Approach to a Wearable Artificial Kidney. In World Congress on Medical Physics and Biomedical Engineering; Dössel, O., Schlegel, W.C., Eds.; IFMBE Proceedings: Munich, Germany; Springer: Berlin/Heidelberg, Germany, 2009; Volume 25/7. [Google Scholar]
- Abidin, M.N.Z.; Goh, P.S.; Said, N.; Ismail, A.F.; Othman, M.H.D.; Abdullah, M.S.; Ng, B.C.; Hasbullah, H.; Kadir, S.H.S.A.; Kamal, F.; et al. Polysulfone/aminosilanized poly(methyl methacrylate) dual layer hollow fiber membrane for uremic toxin separation. Separ. Purif. Technol. 2020, 236, 116216. [Google Scholar]
- Tijink, M.S.L.; Wester, M.; Sun, J.; Saris, A.; Bolhuis-Versteeg, L.A.; Saiful, S.; Joles, J.A.; Borneman, Z.; Wessling, M.; Stamatialis, D.F. A novel approach for blood purification: Mixed-matrix membranes combining diffusion and adsorption in one step. Acta Biomater. 2013, 8, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Tijink, M.; Kooman, J.; Wester, M.; Sun, J.; Saiful, S.; Joles, J.; Borneman, Z.; Wessling, M.; Stamatialis, D. Mixed matrix membranes: A new asset for blood purification therapies. Blood Purif. 2014, 37, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Tijink, M.S.; Wester, M.; Glorieux, G.; Gerritsen, K.G.; Sun, J.; Swart, P.C.; Borneman, Z.; Wessling, M.; Vanholder, R.; Joles, J.A.; et al. Mixed matrix hollow fiber membranes for removal of protein-bound toxins from human plasma. Biomaterials 2013, 34, 7819–7828. [Google Scholar] [CrossRef] [PubMed]
- Geremia, I.; Bansal, R.; Stamatialis, D. In vitro assessment of mixed matrix hemodialysis membrane for achieving endotoxin-free dialysate combined with high removal of uremic toxins from human plasma. Acta Biomater. 2019, 90, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Ter Beek, O.E.M.; van Gelder, M.K.; Lokhorst, C.; Hazenbrink, D.H.M.; Lentferink, B.H.; Gerritsen, K.G.F.; Stamatialis, D. In vitro study of dual layer mixed matrix hollow fiber membranes for outside-in filtration of human blood plasma. Acta Biomater. 2021, 123, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, M.Z.; Wathoniyyah, M.; Khasanah, M.; Rahardjo, Y.; Wafiroh, S. Incorporation of graphene oxide in polyethersulfone mixed matrix membranes to enhance hemodialysis membrane performance. RSC Adv. 2018, 8, 931. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.C.; Hsiao, Y.S.; Ke, J.W.; Syu, W.L.; Liu, T.Y.; Liu, S.H.; Juang, R.S. Adsorptive removal of p-cresol and creatinine from simulated serum using porous polyethersulfone mixed-matrix membranes. Sep. Purif. Technol. 2020, 245, 116884. [Google Scholar] [CrossRef]
- Lu, L.; Samarasekera, C.; Yeow, J.T. Creatinine adsorption capacity of electrospun polyacrylonitrile (PAN)-zeolite nanofiber membranes for potential artificial kidney applications. J. Appl. Polym. Sci. 2015, 132, 42418. [Google Scholar] [CrossRef]
- Lu, L.; Chen, C.; Samarasekera, C.; Yeow, J.T.W. Influence of zeolite shape and particle size on their capacity to adsorb uremic toxin as powders and as fillers in membranes. J. Biomedical. Mater. Res. B: Appl. Biomater. 2017, 105B, 1594–1601. [Google Scholar] [CrossRef]
- Namekawa, K.; Schreiber, M.T.; Aoyagia, T.; Ebara, M. Fabrication of zeolite–polymer composite nanofibers for removal of uremic toxins from kidney failure patients. Biomater. Sci. 2014, 2, 674. [Google Scholar] [CrossRef] [PubMed]
- Haghdoost, F.; Bahrami, S.H.; Barzin, J.; Ghaee, A. Preparation and characterization of electrospun polyethersulfone/polyvinylpyrrolidone-zeolite core–shell composite nanofibers for creatinine adsorption. Sep. Purif. Technol. 2021, 257, 117881. [Google Scholar] [CrossRef]
- Saiful, S. Mixed Matrix Membrane Adsorbers for Protein and Blood Purification. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2007. [Google Scholar]
- Available online: https://www.zeolith-bentonit-versand.eu/en/zeolite/fine-powder.html (accessed on 29 December 2021).
- Byler, M.; Gerasimowicz, W.V.; Stockette, V.M.; Eberl, D.D. Infrared spectroscopic examination of the interaction of urea with the naturally occurring zeolite clinoptilolite. Microchem. J. 1991, 44, 130–139. [Google Scholar] [CrossRef]
- Saiapina, Y.; Pyeshkova, V.M.; Soldatkin, O.O.; Melnik, V.G.; Akata Kurç, B.; Walcarius, A.; Dzyadevych, S.V.; Jaffrezic-Renault, N. Conductometric enzyme biosensors based on natural zeolite clinoptilolite for urea determination. Mater. Sci. Eng. C 2011, 31, 1490–1497. [Google Scholar] [CrossRef]
- Maghsoodi, M.R.; Najafi, N.; Reyhanitabar, A.; Oustan, S. Hydroxyapatite nanorods, hydrochar, biochar, and zeolite for controlled-release urea fertilizers. Geoderma 2020, 379, 114644. [Google Scholar] [CrossRef]
- Acevedo, B.; Barriocanal, C. Simultaneous adsorption of Cd2+ and reactive dye on mesoporous nanocarbons. RSC Adv. 2015, 5, 95247–95255. [Google Scholar] [CrossRef] [Green Version]
- Kraus, M.; Trommler, U.; Holzer, F.; Kopinke, F.-D.; Roland, U. Competing adsorption of toluene and water on various zeolites. Chem. Eng. J. 2018, 351, 356–363. [Google Scholar] [CrossRef]
- Amdebrhan, B.T. Evaluating the Performance of Activated Carbon, Polymeric, and Zeolite Adsorbents for Volatile Organic Compounds Control. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2018. [Google Scholar]
- Mansouri, N.; Rikhtegari, N.; Panahi, H.A.; Atabi, F.; Shahraki, B.K. Porosity, Characterization and Structural properties of natural zeolite- clinoptilolite-as a sorbent. Environ. Prot. Eng. 2013, 39, 139–152. [Google Scholar]
- Jamieson, H.L.; Yin, H.; Waller, A.; Khosravi, A.; Lind, M.L. Impact of acids on the structure and composition of Linde Type A zeolites for use in reverse osmosis membranes for recovery of urine-containing wastewaters. Microporous Mesoporous Mater. 2015, 201, 50–60. [Google Scholar] [CrossRef]
- Valdiviés-Cruz, K.; Lam, A.; Zicovich-Wilson, C.M. Full Mechanism of Zeolite Dealumination in Aqueous Strong Acid Medium: Ab Initio Periodic Study on H.-Clinoptilolite. J. Phys. Chem. C 2017, 121, 2652–2660. [Google Scholar] [CrossRef]
- Saljoughi, E.; Sadrzadeh, M.; Mohammadi, T. Effect of preparation variables on morphology and pure water permeation flux through asymmetric cellulose acetate membranes. J. Memb. Sci. 2009, 326, 627–634. [Google Scholar] [CrossRef]
- Kameda, T.; Ito, S.; Yoshioka, T. Kinetic and equilibrium studies of urea adsorption onto activated carbon: Adsorption mechanism. J. Disper. Sci. Technol. 2017, 38, 1063–1066. [Google Scholar] [CrossRef]









| Toxin | MW (g/mol) | cN (mg/mL) | cMAX (mg/mL) | λUV-VIS (nm) |
|---|---|---|---|---|
| Urea | 60.06 | 0.4 | 4.6 | 200 |
| Creatinine | 113.12 | 0.012 | 0.24 | 235 |
| Uric acid | 168.11 | 0.067 | 0.15 | 290 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pascale, M.; De Angelis, M.G.; Boi, C. Mixed Matrix Membranes Adsorbers (MMMAs) for the Removal of Uremic Toxins from Dialysate. Membranes 2022, 12, 203. https://doi.org/10.3390/membranes12020203
De Pascale M, De Angelis MG, Boi C. Mixed Matrix Membranes Adsorbers (MMMAs) for the Removal of Uremic Toxins from Dialysate. Membranes. 2022; 12(2):203. https://doi.org/10.3390/membranes12020203
Chicago/Turabian StyleDe Pascale, Matilde, Maria Grazia De Angelis, and Cristiana Boi. 2022. "Mixed Matrix Membranes Adsorbers (MMMAs) for the Removal of Uremic Toxins from Dialysate" Membranes 12, no. 2: 203. https://doi.org/10.3390/membranes12020203
APA StyleDe Pascale, M., De Angelis, M. G., & Boi, C. (2022). Mixed Matrix Membranes Adsorbers (MMMAs) for the Removal of Uremic Toxins from Dialysate. Membranes, 12(2), 203. https://doi.org/10.3390/membranes12020203