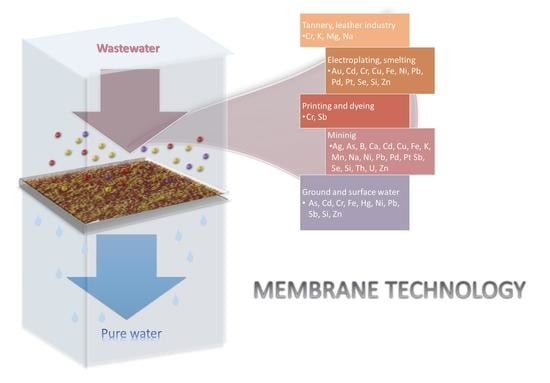
Recovery of Metals from Wastewater—State-of-the-Art Solutions with the Support of Membrane Technology
Abstract
:1. Introduction
2. Removal of Metalloids
2.1. Arsenic (As)
2.2. Boron (B)
2.3. Silica (Si)
| Element | Technology | Basic Process Parameters | Results | Ref. |
|---|---|---|---|---|
| As | NF | Pilot-scale, membrane Dow/FilmTec NF90 with MWCO 100–200 Da, transmembrane pressure 5–20 bar, flow rate 1.2–3.2 L/min, As concentration 100–200 μg, other ions 10–2000 mg/L | Rejection: As(V) 98%, SO4 2− 95%, F− 87%, and NO3− 76% | [15] |
| NF | pilot-scale; membrane NF-300 (Osmonics Inc), TFC polyamide membrane with MWCO 180 Da; operating pressure 7 bar; aqueous feed composition: 180 μg As(V)/L, 5 mg F/L and 84 mg HCO3/L, and pH 8 | Rejection: As(V) 93%, HCO3− 89% and F− 85% | [16] | |
| NF | NF90-4040 (Polyamide Thin-Film Composite (TFC)); operating temperature 28 °C, operating pressure 7 bar; aqueous feed composition: 500 μg/L As | Arsenate removal in 94% | [17] | |
| As, Se | NF | PA-CSBF4 (C-SBF content 40 mg), permeate flux 444 L/m2 h, transmembrane pressure 0.5 bar; aqueous feed composition: pH = 7.0, arsenite and selenite concentration 100 μg/L, NaCl 0.01 mol/L; regenerating agent: NaOH (pH = 9) | Rejection: As(lll) 99%, Se (selenite and selenite) 98% | [22] |
| NF | TFC membrane containing 50 wt% of P[MPC-co-AEMA], aqueous feed composition: 1 mg/L of As and Se ions, pH—7.5, 8.0 and 8.6, transmembrane pressure 10 bar PWP of 8.5 LMH/bar | Rejection: SeO32− 98.2%, SeO42− 99.1% and HAsO42− 99.8% | [25] | |
| B | Multistage RO | Seawater desalination using CDBR process equipped with commercial RO or BF membranes, seawater composition: 35,000 ppm (mainly Na+, Cl−, Ca2+, Mg2+) OPD 56.6 bar energy consumption—2.70 kWh/m3 | Reduction: boron—0.5 ppm, salts -100 ppm, water recovery 65–75% | [33] |
| Si | Adsorption/UF | Brackish water; continuous stirred tank reactor; UFP-30-C-4A hollow fiber (MWCO 30,000 Da); residence time—15 min; agglomerates: iron oxy/hydroxide, adsorbent dosage up to 2 g/L | Rejection Si 93% for 20 mg/L and 67% for 60 mg/L | [37] |
| Ultrafiltration/UF | HFS 60 Silica (Pentair X-Flow, MWCO 10,000 Da); Two streams totalling 6000 m3/day | Rejection Si > 90% | [38] | |
| Tight UF |
Inside-out tubular TiO2/Al2O3 composite membranes (MWCO 95 Da); total solid content in oxide-CMP wastewater: 1333 mg/L (SiO2 1316 mg/L) and pH 9.18, NTU 110; ORP 50.2 mV | Membrane cleaning Removal Si > 90% | [39] |
3. Removal of Heavy Metals
3.1. Chromium (Cr)
3.2. Cobalt (Co)
3.3. Nickel (Ni)
3.4. Copper (Cu)
3.5. Zinc (Zn)
3.6. Cadmium (Cd)
3.7. Mercury (Hg)
3.8. Lead (Pb)
| Element | Technology | Basic Process Parameters | Results | Ref. |
|---|---|---|---|---|
| Cr | RO diafiltration | Tannery industry: Real sludge from TAMEG-Rouiba-SPA—a Leather Industry, Rouiba, Algeria, conc. in mg/L: Cr 50, Fe 4.64, Ni 0.27, Cu 1.54, B 0.12, Ca 81, K 79.8, Mg 67.2, Na 259, P 0.36, S 58.3, Si 9.7, Sr 0.97. RO membrane: SW30 (polyamide thin film composite), DOW Chemical Company Diafiltration membrane: polyethersulfone (PES) MF membrane top-coated with a chitosan layer | RO: More than 95% rejections for all inorganic salts (99.2% for Cr). Diafiltration: Recovery of Cr (III) in RO retentate with the addition of acidified water to pH 3.6. Retain 97% Cr(III), with selectivity for NH4+ (4.2), Cl− (5), K+ (12.9), Na+ (14.6) and Mg2+, Ca2+, S2− (>45), due to Cr (III) adsorption on the chitosan membrane and high permeability of other ions. Desorption of Cr(III) at pH 2: recovery of 92.5% Cr(III) from RO concentrate. The solution can be reused in the tannery process. | [85] |
| NF, RO | Tannery industry: Real sludge from TAMEG-Rouiba-SPA—a Leather Industry, Rouiba, Algeria NF: NF270 and NF90 membranes, RO (BW30 and SW30) and polyethersulfone (PES) MF membrane coated by chitosan | Best option: RO in the first step with SW30, second step selective recovery of Cr(III) in the second step from the retentate using a modified chitosan membrane (permeate with <0.01% Cr). New chitosan membrane: Cr removal >99%, more than 8 and 6 times higher compared to monovalent cations (Na+ and K+) and divalent cations (Mg2+ and Ca2+), respectively. | [86] | |
| UF | Tannery industry: Sludge from the tannery industries, Site-2, Unnao, UP. UF: polyvinylidene fluoride/titanium dioxide solar active photocatalytic membrane | The UF membrane has an excellent rejection and reduction ability from Cr(VI) to Cr(III): 97.59% and 91.73% for the model solution and 90% and 85% for real wastewater. | [87] | |
| RO, electro-cogulation | Leather industry wastewater from Al-Nahrawan, Iraq, conc. in g/L: Cr(III) 1.6. Hybrid process: electrocoagulation (EC) and RO (feed solution electrolyte after EC, 0.12 g/L of Cr) | Rejection of Cr 88.8% after EC and 99.89% after EC/RO; recovery percentage ranged between 8.03 and 25.31%. | [88] | |
| UF, NF, RO, ED | Tannery industry: Sludge from the leather company in Fujian, concentration in mg/L: Ca 250–280, Mg 100–200, Na 1500–1600, chroma 600–1000 UF: PVC, PES membranes, cut off 65, 100, 150 kDa | Process flow chart: flocculation, sedimentation, UF, NF, RO, and ED. Flocculation-UF process with 150 kDa PVC membrane to remove the suspended solids and macromolecular NF process to improve recovery rate, ED for the desalting stage. | [89] | |
| FO | Wastewater from the processing of Acrylonitrile Butadiene Styrene/Polycarbonate plastics, conc. in g/L: Cr(VI) 50.9 FO: Aquaporin Inside membrane hollow fibre FO (AIM™ HFFO) modules, DS: 1 M NaCl | Rejection of Cr(VI) up to 99.74%, due to electrostatic repulsion between the negative charged membrane surface and the anions (HCrO4− and Cr2O72−). The membrane material is damaged due to the oxidizing character of Cr(VI) and should be modified. | [90] | |
| RO | Electroplating wastewater: from BIA Kunststoff- und Galvanotechnik GmbH & Co. KG, conc. in g/L: Cr(III) 0.77, B(OH)3 7.18, SO42− 7.12. RO: polyamide thin-film composite Flmtec SW30-2540, DuPont | Rejections of boric acid 93.8%, Cr(III) 99.9%, sulfate 99.6% for sulfate with 8.4 g/L Cr(III) in RO retentate. Hull cell electroplating tests showed that the deposition of cold-hued chromium layers is possible directly from the retentate solution. | [91] | |
| FO | Sewage sludge: model based on real effluents, conc, in mg/L Cr(VI) 10, COD (C6H12O6·H2O) 500, TP (KH2PO4) 20, NH4Cl 20 FO: with TFC membrane, DS: temperature-sensitive hydrogels based on sodium alginate | High removal in the process is obtained: Cr(VI): 96.9%–97.4%, COD: 97.1%–97.4%, TP: 97.7%–99.6%, and NH4+Cl: 76.8%–77.9% with high water flux. | [92] | |
| Cr, Sb | FO | Printing and dyeing factory: conc. in wastewater, in ppb total Cr 23.93, Sb 0.43, aniline 46.03 FO: with a flat thin–film composite (TFC) membrane, draw solution (DS): 1.5 wt.% LiCl. | Rejection of Cr, Sb, and aniline, after 10 h of FO operation, 99, 98, 99.5%, respectively. Cr was classified mainly as Cr(VI). | [93] |
| Ni, Cu, Zn, Cd, U, Pb, Th, K | RO | Mining industry: leaching solution of phosphogypsum from the Al-Qaim fertilizers complex at the Al-Anbar government | RO removal of Ni, Cu, Zn, Cd, U, Pb, Th, K,) with maximum rejection: 76.6, 77.5, 80.2, 81, 90.9, 92.9, 93.9%, respectively. | [94] |
| Sb, As, Ni, Zn, Fe | RO | Mining industry: wastewater treatment plant: Costerfield, Mandalay Resources Ltd., Victoria, Australia. Sludge from underground gold-antimony mining, processing plant, water treatment plant, evaporation, and tailing storage facilities, max. conc. in the feed in mg/L: Sb 50.2, As 0.047, Ni 0.03, Zn 0.104, Fe 1.19, Cd 0.0001, Cr 0.001, Cu 0.004, Pb 0.002 RO: 96 polyamide membranes DOW™ FILMTEC™ BW30-440i | RO efficiency, reduction in the concentration of Sb, As, Ni, Zn, Fe by 95, 66, 82, 48 and 10%, respectively, in the RO permeate compared to the feed water. Membranes, due to their tendency to fouling and damage in harsh conditions, require pre-cleaning of the feed solution. | [95] |
| Cr, Pb, Cd, As, Ni, Sb | RO, NF | Municipal sewage treatment: surface water in the Democratic Republic of Congo; conc. in ppm Cr 0.06, Pb, Cd, As, Sb < 0.05, Ni 0.03 RO: polyamide urea X-20 membrane, NF: NF90 and NF 270 membranes from Lenntech Water Treatment Solutions | RO removal of Cr(III), Pb(II), Cd(II), As(III), Ni(II), Sb(III) with a rejection of 99.2, 98.8, 98.6, 99.2, 98.4, 98.8%, respectively. NF removal lower than RO, with a rejection of 98.2, 76.9, 92.3, 52.5, 97.8, 64.1%, respectively. NF is the best option for the removal of heavy metals from low-concentration wastewater, while RO is for a very high concentration. | [96] |
| Cu, Zn, Ag, Pb | RO | San Pedro Porphyry Deposit in the San Rafael Massif, RO commercial membrane | Rejection above 90% for Cu, Zn, Ag, and Pb. Metal osmotic differentiation at low temperatures favored atypical Ag-bearing ore paragenesis. | [97] |
| Cu | RO, FO | Acid mine drainage (AMD) formed by the natural oxidation of sulfide minerals, such as pyrite, NF: TFNC membrane FO: 1 M ammonium dihydrogen phosphate and ammonium sulfate as draw solutions. | The NF process showed a high copper concentration capacity (0.6 to 2.4 g/L) and a good total rejection of species (~82%) and a high water recovery of 80% in FO. The combined NF-solvent extraction with LIX 84-IC resulted in a high recovery of water and Cu from AMD. | [98] |
| Cr, Fe, Ni, Cu, Zn, Pb, Au | Electrochemical-osmotic (EOS) system with NF membranes | Electroplating wastewater was collected from UniMetal Surface Finishing Company, Waterfield, CT, USA, conc. in mg/L: Cr 11.31, Fe 9.53, Ni 63.42, Cu 312.54, Zn 24.62, Pb 2.81, Au < 1 EOS: polyelectrolyte multilayer NF membranes | Water/salt selectivity of the PMNF membrane up to 25.1 L/mol, water production rate of 6.06 L/m2h and the power density of 1.18 mW/cm2 by treating synthetic electroplating wastewater, 2.63 and 1.21. | [99] |
| Fe, Zn, Na, As, Ca, Cu, Ni, Mn | NF | Hydrometallurgical copper industry, conc. in mg/L: Fe(II) 6390, Fe(III) 4566, Zn 722, Na 649, As 508, Ca 500, Cu 230, Ni 98, Mn 60 NF: extreme acid-resistant Duracid membrane. | Metal rejections of more than 90%, H+, could permeate across the membrane. | [100] |
| Hg | UF, adsorption | Industrial wastewater from industrial site in California, conc. in ppm Hg 0.05, Na 357, Mg 26, Ca 52, K17 Three-step process: primary filtration using a PVDF membrane to remove particulates; UF membrane to remove mercury sulfide NPs, and adsorption with thiol-functionalized membranes to remove dissolved mercury | The UF membrane was able to effectively remove mercury sulfide nanoparticles from wastewater, thiol membranes were also found to be effective at removing dissolved mercury, with adsorption efficiencies of up to 97% observed over a 20 h period. | [101] |
| Cr, Pb, Fe, Zn, Si | MF/RO | Wastewater treatment plant located in an industrial area known as an “Organized Industrial Zone” (OIZ), conc. in mg/L Cr 1.5, Ob 1.5, Cd 0.1, Fe 10, Cu 3, Zn 5, Hg 0.05 RO membranes (BW30, HP and LE) for chemical treatment and ceramic microfiltration (MF) as pretreatment steps. | The removal efficiencies for various contaminants in the wastewater ranged from 40 to86.3% for chemical oxygen demand (COD), 97.6 to 99% for S ions, 69.2 to 94.9% for Cr ions, 89.3 to 100% for Pb ions, 66.3 to 98.2% for Fe ions, 97.5 to 99.7% for Zn ions, 95.1 to 99.5% for Si ions, and 79.1 to 100% for total phosphorus. | [102] |
| Pb, Zn, Cd | UF, RO | Pb-Zn smelter wastewater from the smelter in Zhuzhou, China, conc. in mg/L Ca 600–900, Zn 1.5–5, Fe 0.4–0.7, Cu 0.1–0.5, Pb 0.2–1.2, Hg 0.01–0.1, Cd 0.3–0.9, As 0.3–0.5, Ba 0.025–0.035, Sr 0.2–0.4 UF: PVDF membrane RO: polyamide thin film composite membrane | The removal of Cd(II) is nearly 100% at pH 5.5, while the rejection of Pb(II) is less than 60% and the rejection of Zn(II) is also less than 60%. When the pH is increased to 7.0, the removal rate of Pb(II) approaches 100%, while the removal rates of Cd(II) and Zn(II) are lower. | [103] |
| Pb, Zn | Wastewater for a smelting plant located in the central-south of China, conc. in mg/L Ca 600–900, Zn 1.5–5, Fe 0.4–0.7, Cu 0.1–0.5, Pb 0.2–1.2, Hg 0.01–0.1, Cd 0.3–0.9, As 0.3–0.5, Ba 0.025–0.035, Sr 0.2–0.4 Several steps process: 1st coagulation-flocculation-sedimentation (CFS), 2nd multi-media filtration (MMF) as a pretreatment for UF, 3rd UF as a pretreatment for RO | The process had a wastewater recovery rate of 87.4% or higher, with salt, heavy metal ions, and conductivity rejection rates of 97% or higher. The resulting reclaimed water had a conductivity of 220 µS/cm. | [104] | |
| Zn | Adsorption, RO | Wastewater from the Esfarayen Steel Industrial Complex, Malysia, conc. in mg/L Cu 0.83, Mn 1.56, Zn 4.02, Fe 23.30, Al 1.46 adsorption with activated carbon as pretreatment for RO. | Removal efficiencies of 98.1% for dissolved solids, 97.4% for electrocoagulation, 100% for Zn and 95.3% for turbidity. Additionally, the system was found to be resistant to high concentrations of contaminants, with removal efficiencies of more than 90%. | [105] |
4. Platinum Group Metals
5. Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Papaioannou, E.H.; Mazzei, R.; Bazzarelli, F.; Piacentini, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Agri-Food Industry Waste as Resource of Chemicals: The Role of Membrane Technology in Their Sustainable Recycling. Sustainability 2022, 14, 1483. [Google Scholar] [CrossRef]
- Staszak, K.; Wieszczycka, K. Membrane applications in the food industry. Phys. Sci. Rev. 2022. [Google Scholar] [CrossRef]
- Brinkmann, T.; Santonja, G.G.; Yükseler, H.; Roudier, S.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for Common Waste Water and Waste Gas Treatment/Management Systems in the Chemical Sector; Publications Office of the European Union: Luxembourg, 2016; ISBN 9789279619960. [Google Scholar]
- Ahmed, S.F.; Mehejabin, F.; Momtahin, A.; Tasannum, N.; Faria, N.T.; Mofijur, M.; Hoang, A.T.; Vo, D.-V.N.; Mahlia, T.M.I. Strategies to improve membrane performance in wastewater treatment. Chemosphere 2022, 306, 135527. [Google Scholar] [CrossRef] [PubMed]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Kabay, N.; Shirazi, M.M.A.; Güler, E.; Bryjak, M. Grand Challenges in Membrane Modules and Processes. Front. Membr. Sci. Technol. 2022, 1, 4. [Google Scholar] [CrossRef]
- Guiver, M.D. Field Grand Challenge for Membrane Science and Technology. Front. Membr. Sci. Technol. 2022, 1, 2. [Google Scholar] [CrossRef]
- Fatoki, J.O.; Badmus, J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022, 5, 100052. [Google Scholar] [CrossRef]
- Issa, N.B.; Rajaković-Ognjanović, V.N.; Marinković, A.D.; Rajaković, L.V. Separation and determination of arsenic species in water by selective exchange and hybrid resins. Anal. Chim. Acta 2011, 706, 191–198. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Siddique, T.A.; Dutta, N.K.; Choudhury, N.R. Nanomaterials Nanofiltration for Arsenic Removal: Challenges, Recent Developments, and Perspectives. Nanomaterials 2020, 10, 1323. [Google Scholar] [CrossRef]
- Worou, C.N.; Chen, Z.-L.; Bacharou, T. Arsenic removal from water by nanofiltration membrane: Potentials and limitations. Water Pract. Technol. 2021, 16, 291–319. [Google Scholar] [CrossRef]
- Vrijenhoek, E.M.; Waypa, J.J. Arsenic removal from drinking water by a “loose” nanofiltration membrane. Desalination 2000, 130, 265–277. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Marathe, K.V.; Rathod, V.K. A pilot scale concurrent removal of fluoride, arsenic, sulfate and nitrate by using nanofiltration: Competing ion interaction and modelling approach. J. Water Process Eng. 2016, 13, 153–167. [Google Scholar] [CrossRef]
- Padilla, A.P.; Saitua, H. Performance of simultaneous arsenic, fluoride and alkalinity (bicarbonate) rejection by pilot-scale nanofiltration. Desalination 2010, 257, 16–21. [Google Scholar] [CrossRef]
- Harfoush, M.; Mirbagheri, S.A.; Ehteshami, M.; Nejati, S. Arsenic removal from drinking water using low-pressure nanofiltration under various operating conditions. Water Pract. Technol. 2018, 13, 295–302. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Hasib, M.A.; Watanabe, Y. Performance of nanofiltration membrane in a vibrating module (VSEP-NF) for arsenic removal. Desalination 2010, 252, 127–134. [Google Scholar] [CrossRef]
- Wong, W.; Wong, H.Y.; Badruzzaman, A.B.M.; Goh, H.H.; Zaman, M. Recent advances in exploitation of nanomaterial for arsenic removal from water: A review. Nanotechnology 2016, 28, 042001. [Google Scholar] [CrossRef]
- Pérez-Sicairos, S.; Lin, S.W.; Félix-Navarro, R.M.; Espinoza-Gómez, H. Rejection of As(III) and As(V) from arsenic contaminated water via electro-cross-flow negatively charged nanofiltration membrane system. Desalination 2009, 249, 458–465. [Google Scholar] [CrossRef]
- Stefaniak, J.; Dutta, A.; Verbinnen, B.; Shakya, M.; Rene, E.R. Selenium removal from mining and process wastewater: A systematic review of available technologies. J. Water Supply Res. Technol. 2018, 67, 903–918. [Google Scholar] [CrossRef]
- Zeeshan, M.H.; Khan, R.U.; Shafiq, M.; Sabir, A. Polyamide intercalated nanofiltration membrane modified with biofunctionalized core shell composite for efficient removal of Arsenic and Selenium from wastewater. J. Water Process Eng. 2020, 34, 101175. [Google Scholar] [CrossRef]
- He, Y.; Zhao, D.L.; Chung, T.-S. Na+ functionalized carbon quantum dot incorporated thin-film nanocomposite membranes for selenium and arsenic removal. J. Memb. Sci. 2018, 564, 483–491. [Google Scholar] [CrossRef]
- He, Y.; Tang, Y.P.; Ma, D.; Chung, T.-S. UiO-66 incorporated thin-film nanocomposite membranes for efficient selenium and arsenic removal. J. Memb. Sci. 2017, 541, 262–270. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.; Han, G.; Chung, T.-S. Novel thin-film composite nanofiltration membranes consisting of a zwitterionic co-polymer for selenium and arsenic removal. J. Memb. Sci. 2018, 555, 299–306. [Google Scholar] [CrossRef]
- Wan, P.; Yuan, M.; Yu, X.; Zhang, Z.; Deng, B. Arsenate removal by reactive mixed matrix PVDF hollow fiber membranes with UIO-66 metal organic frameworks. Chem. Eng. J. 2020, 382, 122921. [Google Scholar] [CrossRef]
- Kumar, M.; RaoT, S.; Isloor, A.M.; Ibrahim, G.P.S.; Inamuddin; Ismail, N.; Ismail, A.F.; Asiri, A.M. Use of cellulose acetate/polyphenylsulfone derivatives to fabricate ultrafiltration hollow fiber membranes for the removal of arsenic from drinking water. Int. J. Biol. Macromol. 2019, 129, 715–727. [Google Scholar] [CrossRef]
- Ahmad, A.; Rutten, S.; de Waal, L.; Vollaard, P.; van Genuchten, C.; Bruning, H.; Cornelissen, E.; van der Wal, A. Mechanisms of arsenate removal and membrane fouling in ferric based coprecipitation–low pressure membrane filtration systems. Sep. Purif. Technol. 2020, 241, 116644. [Google Scholar] [CrossRef]
- López Zavala, M.Á.; Frías Bouchez, B. Montmorillonite-perlite-iron ceramic membranes for the adsorption/removal of As(III) and other constituents from surface water. Ceram. Int. 2022, 48, 31695–31704. [Google Scholar] [CrossRef]
- Tagliabue, M.; Reverberi, A.P.; Bagatin, R. Boron removal from water: Needs, challenges and perspectives. J. Clean. Prod. 2014, 77, 56–64. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Distillation vs. membrane filtration: Overview of process evolutions in seawater desalination. Desalination 2002, 143, 207–218. [Google Scholar] [CrossRef]
- Kürklü, S.; Velioğlu, S.; Ahunbay, M.G.; Tantekin-Ersolmaz, S.B.; Krantz, W.B. A novel energy-efficient concurrent desalination and boron removal (CDBR) process. Desalination 2017, 423, 79–94. [Google Scholar] [CrossRef]
- Kayaci, S.; Tantekin-Ersolmaz, S.B.; Ahunbay, M.G.; Krantz, W.B. Technical and economic feasibility of the concurrent desalination and boron removal (CDBR) process. Desalination 2020, 486, 114474. [Google Scholar] [CrossRef]
- Neo, J.G.; Japip, S.; Luo, L.; Chung, T.-S.; Weber, M.; Maletzko, C. Hydroxyl-terminated poly(ethyleneimine) polymer enhanced ultrafiltration for boron removal. Sep. Purif. Technol. 2019, 222, 214–220. [Google Scholar] [CrossRef]
- Kumar, R.; Ahmed, M.; Bhadrachari, G.; Al- Muqahwi, S.; Thomas, J.P. Thin-film nanocomposite membrane comprised of a novel phosphonic acid derivative of titanium dioxide for efficient boron removal. J. Environ. Chem. Eng. 2021, 9, 105722. [Google Scholar] [CrossRef]
- Çermikli, E.; Şen, F.; Altıok, E.; Wolska, J.; Cyganowski, P.; Kabay, N.; Bryjak, M.; Arda, M.; Yüksel, M. Performances of novel chelating ion exchange resins for boron and arsenic removal from saline geothermal water using adsorption-membrane filtration hybrid process. Desalination 2020, 491, 114504. [Google Scholar] [CrossRef]
- Shemer, H.; Melki-Dabush, N.; Semiat, R. Removal of silica from brackish water by integrated adsorption/ultrafiltration process. Environ. Sci. Pollut. Res. 2019, 26, 31623–31631. [Google Scholar] [CrossRef]
- Silica removal projects in India use hollow-fibre membranes. Membr. Technol. 2012, 2012, 6. [CrossRef]
- Yang, G.C.C.; Li, C.J. Electrofiltration of silica nanoparticle-containing wastewater using tubular ceramic membranes. Sep. Purif. Technol. 2007, 58, 159–165. [Google Scholar] [CrossRef]
- Sheikholeslami, R.; Bright, J. Silica and metals removal by pretreatment to prevent fouling of reverse osmosis membranes. Desalination 2002, 143, 255–267. [Google Scholar] [CrossRef]
- Jo, J.H.; Shin, S.S.; Jeon, S.; Park, S.-J.; Park, H.; Park, Y.-I.; Lee, J.-H. Star polymer-assembled adsorptive membranes for effective Cr(VI) removal. Chem. Eng. J. 2022, 449, 137883. [Google Scholar] [CrossRef]
- Filipowiak, K.; Wieszczycka, K.; Buchwald, T.; Nowicki, M.; Wójcik, G.; Aksamitowski, P.; Staszak, K. Reduction-adsorption of chromium(VI) by using IL-imprinted resin-innovative solution for water purification. J. Mol. Liq. 2021, 343, 116977. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Naghdali, Z.; Kabdaşlı, I.; Sandoval, M.A.; Titchou, F.E.; Malekdar, F.; Nasr, M.; Martínez-Huitle, C.A.; Lichtfouse, E.; Emamjomeh, M.M. Reclamation of forward osmosis reject water containing hexavalent chromium via coupled electrochemical-physical processes. Environ. Technol. 2022. [Google Scholar] [CrossRef]
- Karunakaran, A.; Chaturvedi, A.; Ali, J.; Singh, R.; Agarwal, S.; Garg, M.C. Response surface methodology-based modeling and optimization of chromium removal using spiral-wound reverse-osmosis membrane setup. Int. J. Environ. Sci. Technol. 2022, 19, 5999–6010. [Google Scholar] [CrossRef]
- Pham, M.T.; Nishihama, S.; Yoshizuka, K. Removal of Chromium from Water Environment by Forward Osmosis System. MATEC Web Conf. 2021, 333, 04007. [Google Scholar] [CrossRef]
- Fadhil, S. Sustainable chromium removal by nanofiltration membranes: Application of pore flow model. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Fuoco, I.; Figoli, A.; Criscuoli, A.; Brozzo, G.; De Rosa, R.; Gabriele, B.; Apollaro, C. Geochemical modeling of chromium release in natural waters and treatment by RO/NF membrane processes. Chemosphere 2020, 254, 126696. [Google Scholar] [CrossRef]
- Chen, R.; Qiu, F.; Meng, Q.-W.; Chung, T.-S.; Ge, Q. A cobalt-based forward osmosis draw solute synthesized from lithium-ion battery wastes for cobalt-containing wastewater purification. Desalination 2023, 548, 116279. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; He, M.; Wang, X.; Lv, Y.; Huang, D.; Wang, J.; Miao, R.; Nie, L.; Hao, J. Highly permeable thin film nanocomposite membrane utilizing a MoS2@NH2-UiO-66 interlayer for forward osmosis removal of Co2+, Sr2+ and Cs+ nuclide ions. Appl. Surf. Sci. 2023, 611, 155618. [Google Scholar] [CrossRef]
- Yusaf, A.; Usman, M.; Ahmad, M.; Siddiq, M.; Mansha, A.; Al-Hussain, S.A.; Zaki, M.E.A.; Rehman, H.F. Highly Selective Methodology for Entrapment and Subsequent Removal of Cobalt (II) Ions under Optimized Conditions by Micellar-Enhanced Ultrafiltration. Molecules 2022, 27, 8332. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.; Makhtar, S.N.N.M.; Abdullah, N.; Pauzi, M.Z.M.; Mahpoz, N.M.; Othman, M.H.D.; Jaafar, J.; Abas, K.H.; Fadil, N.A.; Rahman, M.A. Composite zeolite hollow fiber membrane for the removal of nickel using forward osmosis. J. Water Process Eng. 2021, 40, 101806. [Google Scholar] [CrossRef]
- He, M.; Wang, L.; Lv, Y.; Wang, X.; Zhu, J.; Zhang, Y.; Liu, T. Novel polydopamine/metal organic framework thin film nanocomposite forward osmosis membrane for salt rejection and heavy metal removal. Chem. Eng. J. 2020, 389, 124452. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, R.; Chung, T.-S.; Ge, Q. Forward osmosis for heavy metal removal: Multi-charged metallic complexes as draw solutes. Desalination 2022, 539, 115924. [Google Scholar] [CrossRef]
- Kumar, J.; Joshi, H.; Malyan, S.K. Removal of Copper, Nickel, and Zinc Ions from an Aqueous Solution through Electrochemical and Nanofiltration Membrane Processes. Appl. Sci. 2021, 12, 280. [Google Scholar] [CrossRef]
- Moradi, G.; Zinadini, S.; Rajabi, L.; Ashraf Derakhshan, A. Removal of heavy metal ions using a new high performance nanofiltration membrane modified with curcumin boehmite nanoparticles. Chem. Eng. J. 2020, 390, 124546. [Google Scholar] [CrossRef]
- Lin, W.; Jing, L.; Zhang, B. Micellar-Enhanced Ultrafiltration to Remove Nickel Ions: A Response Surface Method and Artificial Neural Network Optimization. Water 2020, 12, 1269. [Google Scholar] [CrossRef]
- Korus, I. Ultrafiltration enhanced with poly(sodium acrylate) as an effective method for separation of heavy metals from multicomponent solutions. Desalin. Water Treat. 2021, 242, 38–46. [Google Scholar] [CrossRef]
- Abdulkarem, E.; Ibrahim, Y.; Naddeo, V.; Banat, F.; Hasan, S.W. Development of Polyethersulfone/α-Zirconium phosphate (PES/α-ZrP) flat-sheet nanocomposite ultrafiltration membranes. Chem. Eng. Res. Des. 2020, 161, 206–217. [Google Scholar] [CrossRef]
- Wołowicz, A.; Staszak, K.; Hubicki, Z. Removal of Copper(II) in the Presence of Sodium Dodecylobenzene Sulfonate from Acidic Effluents Using Adsorption on Ion Exchangers and Micellar-Enhanced Ultrafiltration Methods. Molecules 2022, 27, 2430. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, X.; Li, G.; Fei, G.; Jin, P.; Liu, Y.; Wouters, C.; Meir, G.; Li, Y.; Van der Bruggen, B. Selective removal of heavy metals from saline water by nanofiltration. Desalination 2022, 525, 115380. [Google Scholar] [CrossRef]
- Hamid, M.F.; Abdullah, N.; Yusof, N.; Ismail, N.M.; Ismail, A.F.; Salleh, W.N.W.; Jaafar, J.; Aziz, F.; Lau, W.J. Effects of surface charge of thin-film composite membrane on copper (II) ion removal by using nanofiltration and forward osmosis process. J. Water Process Eng. 2020, 33, 101032. [Google Scholar] [CrossRef]
- Abdullah, W.N.A.S.; Tiandee, S.; Lau, W.; Aziz, F.; Ismail, A.F. Potential use of nanofiltration like-forward osmosis membranes for copper ion removal. Chinese J. Chem. Eng. 2020, 28, 420–428. [Google Scholar] [CrossRef]
- Hamid, M.F.; Abdullah, N.; Yusof, N.; Lau, W.J.; Ismail, A.F.; Wan Salleh, W.N.; Jaafar, J.; Aziz, F. Innovative polymer-complex draw solution for copper(II) removal using forward osmosis. J. Environ. Chem. Eng. 2021, 9, 104854. [Google Scholar] [CrossRef]
- Harharah, R.H.; Abdalla, G.M.T.; Elkhaleefa, A.; Shigidi, I.; Harharah, H.N. A Study of Copper (II) Ions Removal by Reverse Osmosis under Various Operating Conditions. Separations 2022, 9, 155. [Google Scholar] [CrossRef]
- Siddique, J.A. Nanofiltration membrane use for separation of heavy metals from wastewater. In Emerging Techniques for Treatment of Toxic Metals from Wastewater; Elsevier: Amsterdam, The Netherlands, 2023; pp. 523–549. ISBN 9780128228807. [Google Scholar]
- Kahloul, M.; Mahfoudhi, S.; Ounifi, I.; Elabed, B.; Amor, T.B.; Hafiane, A. Green complexation for heavy metals removal from wastewater by Keggin-polyoxometalates enhanced ultrafiltration. Water Sci. Technol. 2022, 86, 1510–1526. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.T.; Pham, T.D.; Verheyen, D.; Nguyen, M.K.; Pham, T.T.; Zhu, J.; Van der Bruggen, B. Fabrication of thin film nanocomposite nanofiltration membrane incorporated with cellulose nanocrystals for removal of Cu(II) and Pb(II). Chem. Eng. Sci. 2020, 228, 115998. [Google Scholar] [CrossRef]
- Bandehali, S.; Parvizian, F.; Moghadassi, A.R.; Hosseini, S.M.; Shen, J.N. Fabrication of thin film-PEI nanofiltration membrane with promoted separation performances: Cr, Pb and Cu ions removal from water. J. Polym. Res. 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Cuhorka, J.; Wallace, E.; Mikulášek, P. Removal of micropollutants from water by commercially available nanofiltration membranes. Sci. Total Environ. 2020, 720, 137474. [Google Scholar] [CrossRef]
- Saeedi-Jurkuyeh, A.; Jafari, A.J.; Kalantary, R.R.; Esrafili, A. A novel synthetic thin-film nanocomposite forward osmosis membrane modified by graphene oxide and polyethylene glycol for heavy metals removal from aqueous solutions. React. Funct. Polym. 2020, 146, 104397. [Google Scholar] [CrossRef]
- Soo, K.W.; Wong, K.C.; Goh, P.S.; Ismail, A.F.; Othman, N. Efficient heavy metal removal by thin film nanocomposite forward osmosis membrane modified with geometrically different bimetallic oxide. J. Water Process Eng. 2020, 38, 101591. [Google Scholar] [CrossRef]
- Ounifi, I.; Ursino, C.; Santoro, S.; Chekir, J.; Hafiane, A.; Figoli, A.; Ferjani, E. Cellulose acetate nanofiltration membranes for cadmium remediation. J. Membr. Sci. Res. 2020, 6, 226–234. [Google Scholar] [CrossRef]
- Bhowmick, K.; Roy, S.; Mukherjee, M.; Sahoo, G.C.; Majumdar, S.; Mondal, P. Removal of cadmium by in-situ Cu nanoparticle enhanced ceramic-supported-polymeric composite NF membrane. Mater. Today Proc. 2021, 47, 1496–1499. [Google Scholar] [CrossRef]
- Meng, Q.; Nan, J.; Mu, Y.; Zu, X.; Guo, M. Study on the treatment of sudden cadmium pollution in surface water by a polymer enhanced ultrafiltration process. RSC Adv. 2021, 11, 7405–7415. [Google Scholar] [CrossRef] [PubMed]
- Can, M.; Arslan, F.; Çelik, M. Modelling of selective retention of Cd-Ni ions from aqueous solutions by polymer enhanced ultrafiltration. Physicochem. Probl. Miner. Process. 2022, 58, 151913. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, S.H. Micellar enhanced ultrafiltration (MEUF) of mercury-contaminated wastewater: Experimental and artificial neural network modeling. J. Water Process Eng. 2020, 33, 101046. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, S.H.; Lee, W. Investigating micellar-enhanced ultrafiltration (MEUF) of mercury and arsenic from aqueous solution using response surface methodology and gene expression programming. Sep. Purif. Technol. 2022, 281, 119880. [Google Scholar] [CrossRef]
- Zhang, H.L.; Cai, H.; Xia, Y.; Zhang, P.; Xiong, S.W.; Gai, J.G. An l-cystine/l-cysteine impregnated nanofiltration membrane with the superior performance of an anchoring heavy metal in wastewater. RSC Adv. 2020, 10, 3438–3449. [Google Scholar] [CrossRef] [Green Version]
- Han, D.S.; Solayman, K.M.D.; Shon, H.K.; Abdel-Wahab, A. Pyrite (FeS2)-supported ultrafiltration system for removal of mercury (II) from water. Emergent Mater. 2021, 4, 1441–1453. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, L.C.; Díaz Barrera, L.E.; Quintanilla-Carvajal, M.X.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Jiménez-Junca, C. Preparation of a Hybrid Membrane from Whey Protein Fibrils and Activated Carbon to Remove Mercury and Chromium from Water. Membranes 2020, 10, 386. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Jye, L.W.; Jaafar, J.; Misdan, N.; Ismail, A.F. Removal of lead(II) by nanofiltration-ranged thin film nanocomposite membrane incorporated UiO-66-NH2: Comparative removal performance between hydraulic-driven and osmotic-driven membrane process. J. Taiwan Inst. Chem. Eng. 2021, 128, 354–369. [Google Scholar] [CrossRef]
- Hanif, A.; Ali, S.; Hanif, M.A.; Rashid, U.; Bhatti, H.N.; Asghar, M.; Alsalme, A.; Giannakoudakis, D.A. A Novel Combined Treatment Process of Hybrid Biosorbent–Nanofiltration for Effective Pb(II) Removal from Wastewater. Water 2021, 13, 3316. [Google Scholar] [CrossRef]
- Babaei, E.; Hashemifard, S.A. Polycarbonate/copper oxide mixed matrix membrane for separation of lead and cadmium from industrial effluents. Sep. Sci. Technol. 2022, 57, 619–636. [Google Scholar] [CrossRef]
- Cao, D.-Q.; Wang, X.; Wang, Q.-H.; Fang, X.-M.; Jin, J.-Y.; Hao, X.-D.; Iritani, E.; Katagiri, N. Removal of heavy metal ions by ultrafiltration with recovery of extracellular polymer substances from excess sludge. J. Memb. Sci. 2020, 606, 118103. [Google Scholar] [CrossRef]
- Zakmout, A.; Sadi, F.; Velizarov, S.; Crespo, J.G.; Portugal, C.A.M. Recovery of Cr(III) from Tannery Effluents by Diafiltration Using Chitosan Modified Membranes. Water 2021, 13, 2598. [Google Scholar] [CrossRef]
- Zakmout, A.; Sadi, F.; Portugal, C.A.M.; Crespo, J.G.; Velizarov, S. Tannery Effluent Treatment by Nanofiltration, Reverse Osmosis and Chitosan Modified Membranes. Membranes 2020, 10, 378. [Google Scholar] [CrossRef]
- Arif, Z.; Sethy, N.K.; Mishra, P.K.; Verma, B. Green approach for the synthesis of ultrafiltration photocatalytic membrane for tannery wastewater: Modeling and optimization. Int. J. Environ. Sci. Technol. 2020, 17, 3397–3410. [Google Scholar] [CrossRef]
- Salman, R.H.; Hassan, H.A.; Abed, K.M.; Al-Alawy, A.F.; Tuama, D.A.; Hussein, K.M.; Jabir, H.A. Removal of chromium ions from a real wastewater of leather industry using electrocoagulation and reverse osmosis processes. AIP Conf. Proc. 2020, 2213, 020186. [Google Scholar]
- Liu, Z.; Lei, M.; Chen, G.; Yuan, J. Treatment of Chromium Removal Wastewater from Tanning by a New Coupling Technology. Processes 2022, 10, 1134. [Google Scholar] [CrossRef]
- Bratovcic, A.; Buksek, H.; Helix-Nielsen, C.; Petrinic, I. Concentrating hexavalent chromium electroplating wastewater for recovery and reuse by forward osmosis using underground brine as draw solution. Chem. Eng. J. 2022, 431, 133918. [Google Scholar] [CrossRef]
- Engstler, R.; Reipert, J.; Karimi, S.; Vukušić, J.L.; Heinzler, F.; Davies, P.; Ulbricht, M.; Barbe, S. A Reverse Osmosis Process to Recover and Recycle Trivalent Chromium from Electroplating Wastewater. Membranes 2022, 12, 853. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, X.; Liang, Y.; Lyu, M.; Huang, Y.; Zhou, H.; Wen, G.; Yu, H.; He, J. Chromium-containing wastewater reclamation via forward osmosis with sewage sludge ash temperature-sensitive hydrogel as draw agent. J. Water Process Eng. 2023, 51, 103422. [Google Scholar] [CrossRef]
- Shao, M.; Li, Y.; Meng, L.; Guo, J.; Gao, Y.; Liu, Y.; Huang, M. Simultaneous removal of antimony, chromium and aniline by forward osmosis membrane: Preparation, performance and mechanism. Desalination 2021, 520, 115363. [Google Scholar] [CrossRef]
- Rashid, W.T.; Alkadira, I.A.; Jalhoom, M.G. Effect of Operating Conditions on Removal Heavy and Radioactive Elements by Reverse Osmosis Membrane. Al-Qadisiyah J. Eng. Sci. 2020, 13, 240–245. [Google Scholar] [CrossRef]
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. Performance evaluation of reverse osmosis process in the post-treatment of mining wastewaters: Case study of Costerfield mining operations, Victoria, Australia. J. Water Process Eng. 2020, 34, 101116. [Google Scholar] [CrossRef]
- Lumami Kapepula, V.; García Alvarez, M.; Sang Sefidi, V.; Buleng Njoyim Tamungang, E.; Ndikumana, T.; Musibono, D.-D.; Van Der Bruggen, B.; Luis, P. Evaluation of Commercial Reverse Osmosis and Nanofiltration Membranes for the Removal of Heavy Metals from Surface Water in the Democratic Republic of Congo. Clean Technol. 2022, 4, 1300–1316. [Google Scholar] [CrossRef]
- Japas, M.S.; Rubinstein, N.A.; Gómez, A.L.R. Revisiting reverse osmosis as a mechanism contributing to metal zoning in porphyry copper deposits. Ore Geol. Rev. 2022, 143, 104746. [Google Scholar] [CrossRef]
- Pino, L.; Beltran, E.; Schwarz, A.; Ruiz, M.C.; Borquez, R. Optimization of nanofiltration for treatment of acid mine drainage and copper recovery by solvent extraction. Hydrometallurgy 2020, 195, 105361. [Google Scholar] [CrossRef]
- Wang, C.; Sun, M.; Wang, X.; Zhang, X.; Zhao, Y.; Huo, M. Enhanced resource recovery from wastewater using electrochemical-osmotic system with nanofiltration membranes. Resour. Conserv. Recycl. 2022, 186, 106555. [Google Scholar] [CrossRef]
- López, J.; Gibert, O.; Cortina, J.L. Evaluation of an extreme acid-resistant sulphonamide based nanofiltration membrane for the valorisation of copper acidic effluents. Chem. Eng. J. 2021, 405, 127015. [Google Scholar] [CrossRef]
- Islam, M.S.; Vogler, R.J.; Abdullah Al Hasnine, S.M.; Hernandez, S.; Malekzadeh, N.; Hoelen, T.P.; Hatakeyama, E.S.; Bhattacharyya, D. Mercury removal from wastewater using cysteamine functionalized membranes. ACS Omega 2020, 5, 22255–22267. [Google Scholar] [CrossRef]
- Ozbey-Unal, B.; Omwene, P.I.; Yagcioglu, M.; Balcik-Canbolat, Ç.; Karagunduz, A.; Keskinler, B.; Dizge, N. Treatment of organized industrial zone wastewater by microfiltration/reverse osmosis membrane process for water recovery: From lab to pilot scale. J. Water Process Eng. 2020, 38, 101646. [Google Scholar] [CrossRef]
- Le, H.-S.; Qiu, Y.-R. Selective separation of Cd(II), Zn(II) and Pb(II) from Pb-Zn smelter wastewater via shear induced dissociation coupling with ultrafiltration. Korean J. Chem. Eng. 2020, 37, 784–791. [Google Scholar] [CrossRef]
- Fu, J. Lead and Zinc Smelting Wastewater Treatment and Reclamation by Coagulation-Flocculation-Sedimentation, Ultrafiltration and Reverse Osmosis Technique. J. Energy Environ. Chem. Eng. 2021, 6, 94. [Google Scholar] [CrossRef]
- Arabi, A.K.; Akram, B.; Mirbagheri, S.A. Industrial wastewater treatment by combining two systems of adsorption column and reverse osmosis. J. Environ. Eng. Sci. 2022, 17, 131–138. [Google Scholar] [CrossRef]
- Hughes, A.E.; Haque, N.; Northey, S.A.; Giddey, S. Platinum Group Metals: A Review of Resources, Production and Usage with a Focus on Catalysts. Resources 2021, 10, 93. [Google Scholar] [CrossRef]
- Crundwell, F.K.; Moats, M.S.; Ramachandran, V.; Robinson, T.G.; Davenport, W.G. Platinum-Group Metals, Production, Use and Extraction Costs. In Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals; Crundwell, F.K., Moats, M.S., Ramachandran, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 395–409. [Google Scholar] [CrossRef]
- Rao, C.R.M.; Reddi, G.S. Platinum group metals (PGM); occurrence, use and recent trends in their determination. TrAC Trends Anal. Chem. 2000, 19, 565–586. [Google Scholar] [CrossRef]
- Mudd, G.M. Sustainability reporting and the platinum group metals: A global mining industry leader? Platin. Met. Rev. 2012, 56, 2–19. [Google Scholar] [CrossRef]
- Ericsson, M.; Tegen, A. Global PGM mining during 40 years—A stable corporate landscape of oligopolistic control. Miner. Econ. 2016, 29, 29–36. [Google Scholar] [CrossRef]
- Taghvaie Nakhjiri, A.; Sanaeepur, H.; Ebadi Amooghin, A.; Shirazi, M.M.A. Recovery of precious metals from industrial wastewater towards resource recovery and environmental sustainability: A critical review. Desalination 2022, 527, 115510. [Google Scholar] [CrossRef]
- Swain, P.; Mallika, C.; Srinivasan, R.; Mudali, U.K.; Natarajan, R. Separation and recovery of ruthenium: A review. J. Radioanal. Nucl. Chem. 2013, 298, 781–796. [Google Scholar] [CrossRef]
- Noah, N.F.M.; Othman, N.; Jusoh, N. Highly selective transport of palladium from electroplating wastewater using emulsion liquid membrane process. J. Taiwan Inst. Chem. Eng. 2016, 64, 134–141. [Google Scholar] [CrossRef]
- Mohdee, V.; Ramakul, P.; Phatanasri, S.; Pancharoen, U. A numerical and experimental investigation on the selective separation of Pd (II) from wastewater using Aliquat 336 via hollow fiber supported liquid membrane. J. Environ. Chem. Eng. 2020, 8, 104234. [Google Scholar] [CrossRef]
- Loreti, M.A.P.; Reis, M.T.A.; Ismael, M.R.C.; Staszak, K.; Wieszczycka, K. Effective Pd(II) carriers for classical extraction and pseudo-emulsion system. Sep. Purif. Technol. 2021, 265, 118509. [Google Scholar] [CrossRef]
- Hanada, T.; Firmansyah, M.L.; Yoshida, W.; Kubota, F.; Kolev, S.D.; Goto, M. Transport of Rhodium(III) from Chloride Media across a Polymer Inclusion Membrane Containing an Ionic Liquid Metal Ion Carrier. ACS Omega 2020, 5, 12989–12995. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zeng, J.; Zeng, Y.; Zhou, H.; Liu, G.; Liu, Y.; Zeng, L.; Jian, J.; Yuan, Z. Preparation and performance of poly(4-vinylpyridine)-b-polysulfone-b-poly(4-vinylpyridine) triblock copolymer/polysulfone blend membrane for separation of palladium (II) from electroplating wastewaters. J. Hazard. Mater. 2020, 384, 121277. [Google Scholar] [CrossRef]
- Jaafar, J.; Nasir, A.M. Grand Challenge in Membrane Fabrication: Membrane Science and Technology. Front. Membr. Sci. Technol. 2022, 1, 3. [Google Scholar] [CrossRef]
- Wang, L.; Rehman, D.; Sun, P.-F.; Deshmukh, A.; Zhang, L.; Han, Q.; Yang, Z.; Wang, Z.; Park, H.-D.; Lienhard, J.H.; et al. Novel Positively Charged Metal-Coordinated Nanofiltration Membrane for Lithium Recovery. ACS Appl. Mater. Interfaces 2021, 13, 16906–16915. [Google Scholar] [CrossRef]
- Purushothaman, M.; Harikrishnan, A.; Senthil Kumar, P.; George, J.; Rangasamy, G.; Vaidyanathan, V.K. Enhancement of antifouling properties, metal ions and protein separation of poly(ether-ether-sulfone) ultrafiltration membranes by incorporation of poly ethylene glycol and n-ZnO. Environ. Res. 2023, 216, 114696. [Google Scholar] [CrossRef]
- Rahighi, R.; Hosseini-Hosseinabad, S.M.; Zeraati, A.S.; Suwaileh, W.; Norouzi, A.; Panahi, M.; Gholipour, S.; Karaman, C.; Akhavan, O.; Khollari, M.A.R.; et al. Two-dimensional materials in enhancement of membrane-based lithium recovery from metallic-ions-rich wastewaters: A review. Desalination 2022, 543, 116096. [Google Scholar] [CrossRef]
- Chadha, U.; Selvaraj, S.K.; Vishak Thanu, S.; Cholapadath, V.; Abraham, A.M.; Zaiyan, M.; Manikandan, M.; Paramasivam, V. A review of the function of using carbon nanomaterials in membrane filtration for contaminant removal from wastewater. Mater. Res. Express 2022, 9, 012003. [Google Scholar] [CrossRef]
- Huang, X.; Tian, F.; Chen, G.; Wang, F.; Weng, R.; Xi, B. Preparation and Characterization of Regenerated Cellulose Membrane Blended with ZrO2 Nanoparticles. Membranes 2021, 12, 42. [Google Scholar] [CrossRef]
- Kwon, O.; Choi, Y.; Kang, J.; Kim, J.H.; Choi, E.; Woo, Y.C.; Kim, D.W. A comprehensive review of MXene-based water-treatment membranes and technologies: Recent progress and perspectives. Desalination 2022, 522, 115448. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staszak, K.; Wieszczycka, K. Recovery of Metals from Wastewater—State-of-the-Art Solutions with the Support of Membrane Technology. Membranes 2023, 13, 114. https://doi.org/10.3390/membranes13010114
Staszak K, Wieszczycka K. Recovery of Metals from Wastewater—State-of-the-Art Solutions with the Support of Membrane Technology. Membranes. 2023; 13(1):114. https://doi.org/10.3390/membranes13010114
Chicago/Turabian StyleStaszak, Katarzyna, and Karolina Wieszczycka. 2023. "Recovery of Metals from Wastewater—State-of-the-Art Solutions with the Support of Membrane Technology" Membranes 13, no. 1: 114. https://doi.org/10.3390/membranes13010114
APA StyleStaszak, K., & Wieszczycka, K. (2023). Recovery of Metals from Wastewater—State-of-the-Art Solutions with the Support of Membrane Technology. Membranes, 13(1), 114. https://doi.org/10.3390/membranes13010114