Key Parameters Impacting the Crystal Formation in Antisolvent Membrane-Assisted Crystallization
Abstract
:1. Introduction
| Crystallization System * | Solution Velocity | Antisolvent Composition | Temperature [K] | Gravity | Key Results | Ref. | |
|---|---|---|---|---|---|---|---|
| Crystallizing Solution | Antisolvent Solution | ||||||
| [A] Water and ethanol mixture at 30 C; [CS] Glycine solution in bi-distilled water at 10 C. | - | - | 40% ethanol in ethanol/water mixture | Antisolvent and crystallizing solution were set at 303.15 and 283.15 K respectively. | - | The transmembrane flux was J = 1.53 or 9.1 × 10−4 Lh−1. | [24] |
| [A] Ethanol; [CS] A solution of erythritol and ultrapure water. | 10 to 20 mL·min−1 | 60 to 120 mL·min−1 | 100% ethanol | 293.15 | From the schematic, the hollow fiber module was perhaps placed vertically. | The transmembrane flux ranged between 2 and 4 Kg·m−2·h−1; CV was 48%. | [20] |
| [A] Ethanol; [CS] A solution of NaCl and ultrapure water | 93 mL·min−1 | 10 mL·min−1 | 100% ethanol | Ambient temperature, which in China can be 303.15 | The hollow fiber module was perhaps placed vertically. | CV was 43.5% | [28] |
| [A] Deionized water; [CS] Indomethacin in ethanol. | 10–15 and 20 mL·min−1 | 25 mL·min−1 | 100% water | - | The hollow fiber module was placed horizontally. | Average velocity across membrane was 1.2–1.4 m/s Particle median diameter was D50 = 0.3 to 0.35 µm. | [21] |
| [A] Deionized water; [CS] Drug particles of Griseofulvin in acetone. | 6–12 mL·min−1 | 6–11 mL·min−1 | 100% water | - | The hollow fiber module was placed horizontally. | Median particle size was 1.6–11.7 µm | [29] |
| [A] 2-Propanol; [CS] L-asparagine in de-ionized water. | 0.175, 0.235 and 0.325 m·s−1. | 50% of CS velocity; which means 0.087, 0.115 and 0.162 m·s−1 | 100% 2-propanol | - | The hollow fiber module appears to be placed vertically on the schematic. | Cross flow velocity 100 to 500 µm·s−1 Median crystal size was c.a. 70 µm | [22] |
| [A] Ethanol; [CS] Erythritol in water. | 20 mL·min−1 | 60–120 mL·min−1 | 100% ethanol | - | The hollow fiber module was placed at angles 33, 50 and 55°. | Flux was 1.2–2.8 Kg·m−2·h−1; CV was 50.9% | [23] |
| [A] Ethanol; [CS] Glycine in water | 0.00017–0.0005 m/s | 0.00017–0.0005 m/s | 40–100 wt.% ethanol | 298.15–308.15 | The solution circulation path was either through the upper or lower membrane cell. | Flux was 0.0002–0.001 Kg/m2·s; CV was 50–60% Mean particle size 23–40 µm | This study |
2. Materials and Methods
2.1. Material
2.2. Membrane Performance for MAAC
2.2.1. Antisolvent Transmembrane Flux Transport
2.2.2. Gas Chromatography (GC) for the Quantitative Analysis of Ethanol
2.3. Crystal Quality
2.3.1. Crystal Shape
2.3.2. Crystalline Form
2.3.3. Crystal Size Distribution
2.3.4. In-Line Turbidity Measurement
3. Results and Discussion
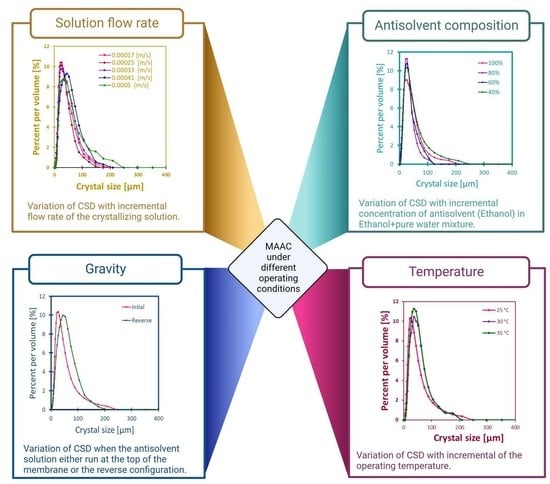
3.1. Impact of Solution Velocity
3.2. Impact of Antisolvent Composition
3.3. Impact of Temperature
3.4. Impact of Gravity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García Alvarez, M.; Sefidi, V.S.; Beguin, M.; Collet, A.; Soria, R.B.; Luis, P. Osmotic Membrane Distillation Crystallization of NaHCO3. Energies 2022, 15, 2682. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Z.; Liu, T.; Wu, Y.; Lan, C.; Jiang, W.; Zhu, L.; Wang, Y.; Yang, D.; Shao, Z. A membrane-based seawater electrolyser for hydrogen generation. Nature 2022, 612, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hartanto, Y.; Nikolaeva, D.; He, Z.; Chergaoui, S.; Luis, P. High-performance ZIF-8/biopolymer chitosan mixed-matrix pervaporation membrane for methanol/dimethyl carbonate separation. Sep. Purif. Technol. 2022, 293, 121085. [Google Scholar] [CrossRef]
- Molina-Fernández, C.; Péters, A.; Debecker, D.P.; Luis, P. Immobilization of carbonic anhydrase in a hydrophobic poly(ionic liquid): A new functional solid for CO2 capture. Biochem. Eng. J. 2022, 187, 108639. [Google Scholar] [CrossRef]
- Bakker, W.J.W.; Geertman, R.M.; Reedijk, M.F.; Baltussen, J.J.M.; Bargeman, G.; Van Lare, C.E.J. Antisolvent Solidification Process. U.S. Patent 13/099,926, 3 November 2011. [Google Scholar]
- Anisi, F.; Thomas, K.M.; Kramer, H.J.M. Membrane-assisted crystallization: Membrane characterization, modelling and experiments. Chem. Eng. Sci. 2017, 158, 277–286. [Google Scholar] [CrossRef]
- Pal, S.; Madane, K.; Kulkarni, A.A. Antisolvent based precipitation: Batch, capillary flow reactor and impinging jet reactor. Chem. Eng. J. 2019, 369, 1161–1171. [Google Scholar] [CrossRef]
- Jiang, X.; Shao, Y.; Sheng, L.; Li, P.; He, G. Membrane Crystallization for Process Intensification and Control: A Review. Engineering 2021, 7, 50–62. [Google Scholar] [CrossRef]
- Diao, Y.; Harada, T.; Myerson, A.S.; Hatton, T.A.; Trout, B.L. The role of nanopore shape in surface-induced crystallization. Nat. Mater. 2011, 10, 867–871. [Google Scholar] [CrossRef]
- Chabanon, E.; Mangin, D.; Charcosset, C. Membranes and crystallization processes: State of the art and prospects. J. Membr. Sci. 2016, 509, 57–67. [Google Scholar] [CrossRef]
- Curcio, E.; Criscuoli, A.; Drioli, E. Membrane Crystallizers. Ind. Eng. Chem. Res. 2001, 40, 2679–2684. [Google Scholar] [CrossRef]
- Testa, C.J.; Shvedova, K.; Hu, C.; Wu, W.; Born, S.C.; Takizawa, B.; Mascia, S. Heterogeneous Crystallization as a Process Intensification Technology in an Integrated Continuous Manufacturing Process for Pharmaceuticals. Org. Process Res. Dev. 2021, 25, 225–238. [Google Scholar] [CrossRef]
- Yu, Q.; Jia, W.; Pu, J.; Wang, Y.; Yang, H. Cocrystallization of urea and succinic acid in ‘‘Nano-Crystallizer”. Chem. Eng. Sci. 2021, 229, 116082. [Google Scholar] [CrossRef]
- Trampuž, M.; Teslić, D.; Likozar, B. Process analytical technology-based (PAT) model simulations of a combined cooling, seeded and antisolvent crystallization of an active pharmaceutical ingredient (API). Powder Technol. 2020, 366, 873–890. [Google Scholar] [CrossRef]
- Li, Z.-H.; Yu, T.; Lee, T.; Kim, W.-S. Cocrystallization of a Caffeine–Maleic Acid in a Batchelor Vortex Flow. Cryst. Growth Des. 2020, 20, 1618–1627. [Google Scholar] [CrossRef]
- Di Profio, G.; Grosso, V.; Caridi, A.; Caliandro, R.; Guagliardi, A.; Chita, G.; Curcio, E.; Drioli, E. Direct production of carbamazepine–saccharin cocrystals from water/ethanol solvent mixtures by membrane-based crystallization technology. CrystEngComm 2011, 13, 5670. [Google Scholar] [CrossRef]
- Bevernage, J.; Brouwers, J.; Brewster, M.E.; Augustijns, P. Evaluation of gastrointestinal drug supersaturation and precipitation: Strategies and issues. Int. J. Pharm. 2013, 453, 25–35. [Google Scholar] [CrossRef]
- Chen, D.; Singh, D.; Sirkar, K.K.; Pfeffer, R. Porous Hollow Fiber Membrane-Based Continuous Technique of Polymer Coating on Submicron and Nanoparticles via Antisolvent Crystallization. Ind. Eng. Chem. Res. 2015, 54, 5237–5245. [Google Scholar] [CrossRef]
- Drioli, E.; Di Profio, G.; Curcio, E. Membrane-Assisted Crystallization Technology; Advances in Chemical and Process Engineering; Imperial College Press: London, UK, 2015; Vol. 2. [Google Scholar] [CrossRef] [Green Version]
- Tuo, L.; Ruan, X.; Xiao, W.; Li, X.; He, G.; Jiang, X. A novel hollow fiber membrane-assisted antisolvent crystallization for enhanced mass transfer process control. AIChE J. 2019, 65, 734–744. [Google Scholar] [CrossRef]
- Fern, J.C.W.; Ohsaki, S.; Watano, S.; Pfeffer, R. Continuous synthesis of nano-drug particles by antisolvent crystallization using a porous hollow-fiber membrane module. Int. J. Pharm. 2018, 543, 139–150. [Google Scholar] [CrossRef]
- Zarkadas, D.M.; Sirkar, K.K. Antisolvent crystallization in porous hollow fiber devices. Chem. Eng. Sci. 2006, 61, 5030–5048. [Google Scholar] [CrossRef]
- Li, J.; Sheng, L.; Tuo, L.; Xiao, W.; Ruan, X.; Yan, X.; He, G.; Jiang, X. Membrane-Assisted Antisolvent Crystallization: Interfacial Mass-Transfer Simulation and Multistage Process Control. Ind. Eng. Chem. Res. 2020, 59, 10160–10171. [Google Scholar] [CrossRef]
- Di Profio, G.; Stabile, C.; Caridi, A.; Curcio, E.; Drioli, E. Antisolvent membrane crystallization of pharmaceutical compounds. J. Pharm. Sci. 2009, 98, 4902–4913. [Google Scholar] [CrossRef] [PubMed]
- Terterov, I.; Koniakhin, S.; Vyazmin, S.; Boitsov, V.; Dubina, M. Molecular dynamic simulations of glycine amino acid association with potassium and sodium ions in explicit solvent. F1000Research 2017, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Guin, P.S.; Ghosh, B.; Debnath, S.C.; Baskey, S.; Roy, S.; Mahali, K. Solubility and solvation thermodynamics of glycine, dl-alanine and l-threonine in pure aqueous and aqueous (NH4)2SO4 solution. Chem. Phys. Lett. 2022, 788, 139284. [Google Scholar] [CrossRef]
- Ramakers, L.A.I.; McGinty, J.; Beckmann, W.; Levilain, G.; Lee, M.; Wheatcroft, H.; Houson, I.; Sefcik, J. Investigation of Metastable Zones and Induction Times in Glycine Crystallization across Three Different Antisolvents. Cryst. Growth Des. 2020, 20, 4935–4944. [Google Scholar] [CrossRef]
- Sheng, L.; Li, J.; He, G.; Xiao, W.; Yan, X.; Li, X.; Ruan, X.; Jiang, X. Visual study and simulation of interfacial liquid layer mass transfer in membrane-assisted antisolvent crystallization. Chem. Eng. Sci. 2020, 228, 116003. [Google Scholar] [CrossRef]
- Chen, D.; Singh, D.; Sirkar, K.K.; Pfeffer, R. Continuous Synthesis of Polymer-Coated Drug Particles by Porous Hollow Fiber Membrane-Based Antisolvent Crystallization. Langmuir 2015, 31, 432–441. [Google Scholar] [CrossRef]
- Kanda, H.; Katsube, T.; Hoshino, R.; Kishino, M.; Wahyudiono; Goto, M. Ethanol-free antisolvent crystallization of glycine by liquefied dimethyl ether. Heliyon 2020, 6, e05258. [Google Scholar] [CrossRef]
- Pudasaini, N.; Upadhyay, P.P.; Parker, C.R.; Hagen, S.U.; Bond, A.D.; Rantanen, J. Downstream Processability of Crystal Habit-Modified Active Pharmaceutical Ingredient. Org. Process Res. Dev. 2017, 21, 571–577. [Google Scholar] [CrossRef]
- Kaerger, J.S.; Edge, S.; Price, R. Influence of particle size and shape on flowability and compactibility of binary mixtures of paracetamol and microcrystalline cellulose. Eur. J. Pharm. Sci. 2004, 22, 173–179. [Google Scholar] [CrossRef]
- Swaminathan, V.; Kildsig, D.O. The Effect of Particle Morphology on the Physical Stability of Pharmaceutical Powder Mixtures: The Effect of Surface Roughness of the Carrier on the Stability of Ordered Mixtures. Drug Dev. Ind. Pharm. 2000, 26, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Vecino, X.; Gibert, O.; Valderrama, C.; Cortina, J.L. Study of the operational parameters in the hollow fibre liquid-liquid membrane contactors process for ammonia valorisation as liquid fertiliser. Sep. Purif. Technol. 2021, 255, 117768. [Google Scholar] [CrossRef]
- Chuang, W. Swelling behavior of hydrophobic polymers in water/ethanol mixtures. Polymer 2000, 41, 8339–8347. [Google Scholar] [CrossRef]
- Park, S.; Yeo, S. Antisolvent Crystallization of Sulfa Drugs and the Effect of Process Parameters. Sep. Sci. Technol. 2007, 42, 2645–2660. [Google Scholar] [CrossRef]










| Parameter | Variable |
|---|---|
| Solution velocity, either the crystallizing solution or the antisolvent. | 0.00017, 0.00025, 0.00033, 0.00041 and 0.0005 m/s |
| Antisolvent composition | 40, 60, 80 and 100 wt.% |
| Temperature | 25, 30 and 35 °C |
| Gravity | Antisolvent circulating in the upper or the lower membrane cell. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chergaoui, S.; Debecker, D.P.; Leyssens, T.; Luis, P. Key Parameters Impacting the Crystal Formation in Antisolvent Membrane-Assisted Crystallization. Membranes 2023, 13, 140. https://doi.org/10.3390/membranes13020140
Chergaoui S, Debecker DP, Leyssens T, Luis P. Key Parameters Impacting the Crystal Formation in Antisolvent Membrane-Assisted Crystallization. Membranes. 2023; 13(2):140. https://doi.org/10.3390/membranes13020140
Chicago/Turabian StyleChergaoui, Sara, Damien P. Debecker, Tom Leyssens, and Patricia Luis. 2023. "Key Parameters Impacting the Crystal Formation in Antisolvent Membrane-Assisted Crystallization" Membranes 13, no. 2: 140. https://doi.org/10.3390/membranes13020140
APA StyleChergaoui, S., Debecker, D. P., Leyssens, T., & Luis, P. (2023). Key Parameters Impacting the Crystal Formation in Antisolvent Membrane-Assisted Crystallization. Membranes, 13(2), 140. https://doi.org/10.3390/membranes13020140