A Complex Investigation of LATP Ceramic Stability and LATP+PVDF Composite Membrane Performance: The Effect of Solvent in Tape-Casting Fabrication
Abstract
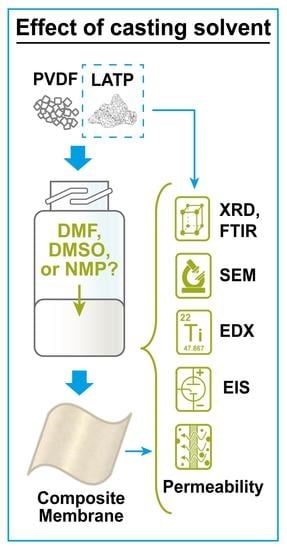
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ceramic
2.2. Fabrication of Composite Membranes
2.3. Soaking Experiments
2.4. Structure, Composition, and Morphology Analysis
2.5. Electrochemical Analysis
3. Results
3.1. Investigation of Ceramic’s Stability toward Casting Solvents
3.1.1. LATP’s Microstructure
3.1.2. Crystal Structure and IC
3.2. Structural, Morphological, and Electrochemical Properties of LATP+PVDF Composite Membranes
3.2.1. LATP’s Crystal Structure within the Composite Membrane
3.2.2. Morphological Properties, Conductivity, and Permeability of LATP+PVDF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bindra, H.; Revankar, S. Storage and Hybridization of Nuclear Energy: Techno-Economic Integration of Renewable and Nuclear Energy; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128139752. [Google Scholar]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox Flow Batteries: A Review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef] [Green Version]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox Flow Batteries for the Storage of Renewable Energy: A Review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Noack, J.; Roznyatovskaya, N.; Herr, T.; Fischer, P. The Chemistry of Redox-Flow Batteries. Angew. Chem. Int. Ed. 2015, 54, 9776–9809. [Google Scholar] [CrossRef]
- Leung, P.; Li, X.; de León, C.P.; Berlouis, L.; Low, C.T.J.; Walsh, F.C. Progress in Redox Flow Batteries, Remaining Challenges and Their Applications in Energy Storage. RSC Adv. 2012, 2, 10125–10156. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent Progress in Redox Flow Battery Research and Development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of Material Research and Development for Vanadium Redox Flow Battery Applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, M.S.; Kim, Y.J.; Kim, J.H.; Dou, S.X.; Skyllas-Kazacos, M. A Technology Review of Electrodes and Reaction Mechanisms in Vanadium Redox Flow Batteries. J. Mater. Chem. A 2015, 3, 16913–16933. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane Development for Vanadium Redox Flow Batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef]
- Hosseiny, S.S.; Wessling, M. Ion Exchange Membranes for Vanadium Redox Flow Batteries. In Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2011; pp. 413–434. ISBN 9781845699697. [Google Scholar]
- Gubler, L. Membranes and Separators for Redox Flow Batteries. Curr. Opin. Electrochem. 2019, 18, 31–36. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, L.; Chen, Y.; Peng, S.; Yu, G. A Chemistry and Microstructure Perspective on Ion-Conducting Membranes for Redox Flow Batteries. Angew. Chem. Int. Ed. 2021, 60, 24770–24798. [Google Scholar] [CrossRef]
- Zhang, W.; Yoon, S.; Jin, L.; Lim, H.; Jeon, M.; Jang, H.; Ahmed, F.; Kim, W. Lithium Salt Catalyzed Ring-Opening Polymerized Solid-State Electrolyte with Comparable Ionic Conductivity and Better Interface Compatibility for Li-Ion Batteries. Membranes 2022, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Falina, I.; Kononenko, N.; Timofeev, S.; Rybalko, M.; Demidenko, K. Nanocomposite Membranes Based on Fluoropolymers for Electrochemical Energy Sources. Membranes 2022, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Xu, W.; Long, J.; Huang, W.; He, Z.; Liu, S.; Zhang, Y. A Sulfonated Polyimide/Nafion Blend Membrane with High Proton Selectivity and Remarkable Stability for Vanadium Redox Flow Battery. Membranes 2021, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Pan, Z.-Z.Z.; Jin, Y.; Qiu, Q.; Zhang, C.; Zhao, Y.; Li, Y. Membranes in Non-Aqueous Redox Flow Battery: A Review. J. Power Sources 2021, 500, 229983. [Google Scholar] [CrossRef]
- Shin, S.H.; Yun, S.H.; Moon, S.H. A Review of Current Developments in Non-Aqueous Redox Flow Batteries: Characterization of Their Membranes for Design Perspective. RSC Adv. 2013, 3, 9095–9116. [Google Scholar] [CrossRef]
- Huang, Y.; Gu, S.; Yan, Y.; Li, S.F.Y. Nonaqueous redox-flow batteries: Features, challenges, and prospects. Curr. Opin. Chem. Eng. 2015, 8, 105–113. [Google Scholar] [CrossRef]
- Jia, C.; Pan, F.; Zhu, Y.G.; Huang, Q.; Lu, L.; Wang, Q. Energy Storage: High-Energy Density Nonaqueous All Redox Flow Lithium Battery Enabled with a Polymeric Membrane. Sci Adv. 2015, 1, e1500886. [Google Scholar] [CrossRef] [Green Version]
- Monchak, M.; Hupfer, T.; Senyshyn, A.; Boysen, H.; Chernyshov, D.; Hansen, T.; Schell, K.G.; Bucharsky, E.C.; Hoffmann, M.J.; Ehrenberg, H. Lithium Diffusion Pathway in Li1.3Al0.3Ti1.7(PO4)3 (LATP) Superionic Conductor. Inorg. Chem. 2016, 55, 2941–2945. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Kim, Y.; Yun, S.H.; Maurya, S.; Moon, S.H. Influence of Membrane Structure on the Operating Current Densities of Non-Aqueous Redox Flow Batteries: Organic-Inorganic Composite Membranes Based on a Semi-Interpenetrating Polymer Network. J. Power Sources 2015, 296, 245–254. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Li, B.; Song, H.; Cheng, Z.; Chen, M.; He, P.; Zhou, H. Germanium Thin Film Protected Lithium Aluminum Germanium Phosphate for Solid-State Li Batteries. Adv. Energy Mater 2018, 8, 1702374. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.; Lv, W.; He, Y.B.; Yang, Q.H. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauskopf, T.; Hartmann, H.; Zeier, W.G.; Janek, J. Toward a Fundamental Understanding of the Lithium Metal Anode in Solid-State Batteries—An Electrochemo-Mechanical Study on the Garnet-Type Solid Electrolyte Li 6.25 Al 0.25 La 3 Zr 2 O 12. ACS Appl. Mater. Interfaces 2019, 11, 14463–14477. [Google Scholar] [CrossRef]
- Fergus, J.W. Ceramic and Polymeric Solid Electrolytes for Lithium-Ion Batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y. Perovskite-type Li-ion Solid Electrolytes: A Review. J. Mater. Sci. Mater. Electron. 2021, 32, 9736–9754. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and Prospects of PVDF Based Polymer Electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO Based Solid Polymer Electrolytes for All-Solid State Lithium Ion Batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeWees, R.; Wang, H. Synthesis and Properties of NaSICON-Type LATP and LAGP Solid Electrolytes. ChemSusChem 2019, 12, 3713–3725. [Google Scholar] [CrossRef]
- Liang, X.; Han, D.; Wang, Y.; Lan, L.; Mao, J. Preparation and Performance Study of a PVDF-LATP Ceramic Composite Polymer Electrolyte Membrane for Solid-State Batteries. RSC Adv. 2018, 8, 40498–40504. [Google Scholar] [CrossRef] [Green Version]
- Pogosova, M.; Krasnikova, I.; Sergeev, A.; Zhugayevych, A.; Stevenson, K. Correlating Structure and Transport Properties in Pristine and Environmentally-Aged Superionic Conductors Based on Li1.3Al0.3Ti1.7(PO4)3 Ceramics. J. Power Sources 2020, 448, 227367. [Google Scholar] [CrossRef]
- Krasnikova, I.V.; Pogosova, M.A.; Sanin, A.O.; Stevenson, K.J. Toward Standardization of Electrochemical Impedance Spectroscopy Studies of Li-Ion Conductive Ceramics. Chem. Mater. 2020, 32, 2232–2241. [Google Scholar] [CrossRef]
- Pogosova, M.A.; Krasnikova, I.V.; Sanin, A.O.; Lipovskikh, S.A.; Eliseev, A.A.; Sergeev, A.V.; Stevenson, K.J. Complex Investigation of Water Impact on Li-Ion Conductivity of Li1.3Al0.3Ti1.7(PO4)3—Electrochemical, Chemical, Structural, and Morphological Aspects. Chem. Mater. 2020, 32, 3723–3732. [Google Scholar] [CrossRef]
- Ashraf Gandomi, Y.; Krasnikova, I.V.; Akhmetov, N.O.; Ovsyannikov, N.A.; Pogosova, M.A.; Matteucci, N.J.; Mallia, C.T.; Neyhouse, B.J.; Fenton, A.M.; Brushett, F.R.; et al. Synthesis and Characterization of Lithium-Conducting Composite Polymer-Ceramic Membranes for Use in Nonaqueous Redox Flow Batteries. ACS Appl. Mater. Interfaces 2021, 13, 53746–53757. [Google Scholar] [CrossRef] [PubMed]
- Akhmetov, N.; Ovsyannikov, N.; Gvozdik, N.; Pogosova, M.; Ryazantsev, S.; Lipovskikh, S.; Krasnikova, I.; Stevenson, K. Composite Lithium-Conductive LATP+PVdF Membranes: Development, Optimization, and Applicability for Li-TEMPO Hybrid Redox Flow Batteries. J. Membr. Sci. 2022, 643, 120002. [Google Scholar] [CrossRef]
- Ovsyannikov, N.A.; Romadina, E.I.; Akhmetov, N.O.; Gvozdik, N.A.; Akkuratov, A.V.; Pogosova, M.A.; Stevenson, K.J. All-Organic Non-Aqueous Redox Flow Batteries with Advanced Composite Polymer-Ceramic Li-Conductive Membrane. J. Energy Storage 2022, 46, 103810. [Google Scholar] [CrossRef]
- Li, M.; Katsouras, I.; Piliego, C.; Glasser, G.; Lieberwirth, I.; Blom, P.W.M.; de Leeuw, D.M. Controlling the Microstructure of Poly(Vinylidene-Fluoride) (PVDF) Thin Films for Microelectronics. J. Mater. Chem C 2013, 1, 7695–7702. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef]
- Huang, X. Cellular Porous Polyvinylidene Fluoride Composite Membranes for Lithium-Ion Batteries. J. Solid State Electrochem. 2013, 17, 591–597. [Google Scholar] [CrossRef]
- Solvent Physical Properties. Available online: https://people.chem.umass.edu/xray/solvent.html (accessed on 13 January 2023).
- Parker, A.J. The Effects of Solvation on the Properties of Anions in Dipolar Aprotic Solvents. Q. Rev. Chem. Soc. 1962, 16, 163–187. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M. The Crystallographic Computing System. 2000. Available online: www-xray.fzu.cz (accessed on 30 November 2022).
- Ferlita, R.R.; Phipps, D.; Safarik, J.; Yeh, D.H. Cryo-Snap: A Simple Modified Freeze-Fracture Method for SEM Imaging of Membrane Cross-Sections. Environ. Prog. 2008, 27, 204–209. [Google Scholar] [CrossRef]
- Dashjav, E.; Ma, Q.; Xu, Q.; Tsai, C.L.; Giarola, M.; Mariotto, G.; Tietz, F. The Influence of Water on the Electrical Conductivity of Aluminum-Substituted Lithium Titanium Phosphates. Solid State Ionics 2018, 321, 83–90. [Google Scholar] [CrossRef]
- Alberti, G.; Pica, M.; Tarpanelli, T. Presursor Organic of Tetravalent Metal Phosphates and Pyrophosphates and Their Use for Electrode Modification and for the Preparation of Composite Membrane For Fuel Cells Working at Temperatures >90 °C and/or at Low Relative Humidity. U.S. Patent 20070224483A1, 27 September 2007. [Google Scholar]
- Cotton, F.A.; Francis, R. Sulfoxides as Ligands. I. A Preliminary Survey of Methyl Sulfoxide Complexes. J. Am. Chem. Soc. 1960, 82, 2986–2991. [Google Scholar] [CrossRef]
- Wang, E.; Zhao, E.W.; Grey, C.P. New Magnetic Resonance and Computational Methods to Study Crossover Reactions in Li-Air and Redox Flow Batteries Using TEMPO. J. Phys. Chem. C 2021, 125, 27520–27533. [Google Scholar] [CrossRef]
- Wang, X.; Lashgari, A.; Chai, J.; Jiang, J. A Membrane-Free, Aqueous/Nonaqueous Hybrid Redox Flow Battery. Energy Storage Mater. 2022, 45, 1100–1108. [Google Scholar] [CrossRef]
- Hatakeyama-Sato, K.; Nagano, T.; Noguchi, S.; Sugai, Y.; Du, J.; Nishide, H.; Oyaizu, K. Hydrophilic Organic Redox-Active Polymer Nanoparticles for Higher Energy Density Flow Batteries. ACS Appl Polym Mater. 2019, 1, 188–196. [Google Scholar] [CrossRef]
- Sagane, F.; Abe, T.; Ogumi, Z. LI+-Ion Transfer through the Interface between Li +-Ion Conductive Ceramic Electrolyte and LI+-Ion- Concentrated Propylene Carbonate Solution. J. Phys. Chem. C 2009, 113, 20135–20138. [Google Scholar] [CrossRef]
- Chen, H.P.; Fergus, J.W.; Jang, B.Z. The Effect of Ethylene Carbonate and Salt Concentration on the Conductivity of Propylene Carbonate∣Lithium Perchlorate Electrolytes. J. Electrochem. Soc. 2000, 147, 399. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waris, Z.; Akhmetov, N.O.; Pogosova, M.A.; Lipovskikh, S.A.; Ryazantsev, S.V.; Stevenson, K.J. A Complex Investigation of LATP Ceramic Stability and LATP+PVDF Composite Membrane Performance: The Effect of Solvent in Tape-Casting Fabrication. Membranes 2023, 13, 155. https://doi.org/10.3390/membranes13020155
Waris Z, Akhmetov NO, Pogosova MA, Lipovskikh SA, Ryazantsev SV, Stevenson KJ. A Complex Investigation of LATP Ceramic Stability and LATP+PVDF Composite Membrane Performance: The Effect of Solvent in Tape-Casting Fabrication. Membranes. 2023; 13(2):155. https://doi.org/10.3390/membranes13020155
Chicago/Turabian StyleWaris, Zainab, Nikita O. Akhmetov, Mariam A. Pogosova, Svetlana A. Lipovskikh, Sergey V. Ryazantsev, and Keith J. Stevenson. 2023. "A Complex Investigation of LATP Ceramic Stability and LATP+PVDF Composite Membrane Performance: The Effect of Solvent in Tape-Casting Fabrication" Membranes 13, no. 2: 155. https://doi.org/10.3390/membranes13020155
APA StyleWaris, Z., Akhmetov, N. O., Pogosova, M. A., Lipovskikh, S. A., Ryazantsev, S. V., & Stevenson, K. J. (2023). A Complex Investigation of LATP Ceramic Stability and LATP+PVDF Composite Membrane Performance: The Effect of Solvent in Tape-Casting Fabrication. Membranes, 13(2), 155. https://doi.org/10.3390/membranes13020155