1. Introduction
Oxygen supply by means of oxygen transport membranes (OTMs) can contribute to CO
2 avoidance to fulfill CCS strategies (carbon capture and storage). Demand for oxygen separated from the air exists in (raw) material production, medical applications, the chemical industry, and water treatment, in addition to the efficient combustion of fossil or renewable fuels. It is important that the oxygen enrichment or separation equipment itself can operate safely and energy efficiently at typical process temperatures of about 850 °C. Oxygen output is maximized for thin membranes, high gradients of oxygen partial pressure on both sides of the membrane, high operating temperatures, and membrane materials with high non-stoichiometry δ. As result of a high non-stoichiometry, the combined coefficient of thermal and chemical expansion increases, as in the case of the membrane material Ba
0.5Sr
0.5Co
0.8Fe
0.2O
3-δ (BSCF) [
1,
2,
3,
4].
OTM concepts therefore vary in the selected membrane materials, the geometry of the membrane component, the process gases, and their partial pressures but also the sealing concepts. Demonstrator modules often work with tubular membranes that are glued with adhesives to metal sleeves [
5,
6,
7], as schematically also shown in
Figure 1a. Due to the water cooling in the flange and the insulation to the heated pressure vessel, the temperature-sensitive adhesives and rubber sealing rings remain cool. However, an axial temperature gradient occurs along the length of the membrane.
Observed BSCF membrane fractures in a previous study suggest that this axial temperature gradient leads to a local stress maximum as a result of the different minimum temperatures for the onset of chemical expansion or creep relaxation [
8]. In tubular membranes; however, it is, compared with planar membrane stacks, relatively easy to shift the axial temperature gradient to the metallic joining partner. For this purpose, the permanent cooled adhesive joint between the membrane tube and the metal sleeve used in the membrane module must be replaced by a gas-tight and high-temperature-resistant joint; see
Figure 1b. In addition to increasing the reliability, this can increase the efficiency as a result of an increase in the effective membrane area contributing to permeation and heat losses due to water cooling.
To join fragile BSCF membrane tubes to metals in a gas-tight and high-temperature-resistant manner, only brazing methods can be considered. For permeation studies of thin membrane discs at the laboratory scale, press-fit joints with gold gaskets and spring elements are often used [
9,
10]. However, these compression joints are not suitable for small-scale joining of long, free-hanging membrane tubes due to boundary conditions. As early as 1994, Sarocco et al. identified the joining technology as a key problem for the application of OTMs as membrane reactors, which five years later was said to have received too little attention and progress [
11,
12].
Among the brazing techniques, some established technologies exist to realize ceramic–metal joints [
13]. However, this is more difficult due to the strong ionic or ionic-covalent bonds in ceramics. Moreover, the processes of vacuum brazing, active brazing, or brazing of a metallized ceramic are not suitable, because BSCF chemically decomposes under low-oxygen partial pressures or through reducing reactions with the reactive elements [
14,
15]. In addition, these braze alloys or the locally reduced ceramic oxidize during subsequent application under air, which causes volume expansion and leads to cracks and delamination [
15,
16,
17]. Glass or glass–ceramic solders, as used for joints in solid oxide fuel cells stacks [
18,
19], are currently not suitable for BSCF joints. Their low thermal expansion coefficients in relation to BSCF in combination with the brittle fracture lead to high internal stresses and cracks upon cooling [
20], which can be detected, e.g., by N
2 contents in the permeate [
21]. There are approaches to increase the coefficient of thermal expansion of glass solders by targeted crystallization of oxide phases. Approaches exist to increase the thermal expansion coefficient of glass solders by selective crystallization of oxide phases [
22]. Chemical interactions can be reduced by gold coating of the membrane [
23], but thermal cycling also changes the degree of crystallization and the thermomechanical properties [
24], which can damage the solder and ceramic membrane.
Reactive brazing in air has been shown to be suitable for joining BSCF to high-temperature-resistant metals [
25,
26,
27,
28]. In addition, other OTM materials such as LSCF [
29,
30], BCFN [
31], or dual-phase membranes [
32,
33,
34] were joined using reactive air brazing. Suitable metallic joining partners show a similar thermal expansion to the ceramic OTM. Examples include ferritic steels such as Kanthal [
35], Crofer APU or H [
36], AISI 430 [
37], AISI 446 [
38], austenitic heat-resistant steels such as AISI 310S [
33], AISI 314 [
39], and nickel-base alloys such as alloy 602 CA [
40], Haynes 214 [
35], or Inconel 600 [
33].
The BSCF membrane tubes shown in
Figure 1b are brazed to steel sleeves in this manner using Ag-3Cu (3 mol% Cu, 97 mol% Ag). A similar concept with Ag-1Cu was shown by Zhang et al. [
31,
41]. During the brazing process in air, the reactive element copper is oxidized and wets reactively both the oxide ceramic and the passivated metal surface in the silver melt. However, during membrane operation, the progressive diffusion of copper, chromium, and oxygen leads to the growth of the reaction layers, which strongly decreases the strength of the joints [
42,
43,
44]. In particular, the diffusion of chromium from the metallic joining partner leads to microstructural decomposition [
35,
42,
43] and a decrease in permeation performance [
45].
Some coatings have been tested to limit chromium diffusion in brazed joints and show stabilized microstructures. The Ni-based alloy 602 CA was coated with an Al
2O
3 top layer using the reactive air aluminizing technique [
46] and then air brazed. Strength studies after aging have not been published. In another study [
47], AISI 314 was coated with Ni-Al
2O
3 and YSZ via thermal spraying and then brazed in air using pure silver. After 1500 h of Al aging at 850 °C, no growing reaction layers were observed in cross-sections. In [
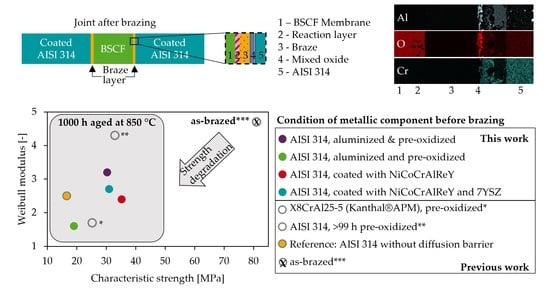
39], we demonstrated how a pre-oxidation of AISI 314 at 1050 °C can reduce the strength degradation of BSCF-Ag-3CuO-AISI 314 joints after 1000 h of isothermal aging at 850 °C. Without pre-oxidation, the characteristic joint strength decreases from 81 MPa immediately after brazing to 17 MPa after 1000 h, and chromium poisoning is present. With pre-oxidation before brazing, 33 MPa joint strength is achieved, and no chromium is detected in the BSCF.
Dense outer Al
2O
3 passivation layers could be even better diffusion barriers. They are only formed in materials with sufficient Al reservoirs that are >6 wt%. However, there are no heat-resistant austenitic steels with sufficiently high Al content [
48], since these would form intermetallic phases during cooling. The choice of a ferritic high-Al steel led to cracking due to the large differences in thermal expansion coefficients [
39]. In the present study, therefore, aluminum-containing coatings or diffusion layers were applied onto the metallic joining partner before brazing to BSCF and aging. Four-point bending tests, fracture surface analyses, and categorization as well as microstructure analyses of cross-sections enable the discussion of the efficacy of the applied coatings as a diffusion barrier for air brazed BSCF–steel joints.
2. Materials and Methods
Ba0,5Sr0,5Co0,8Fe0,2O3-δ powder (Treibacher GmbH, Althofen, Austria) with an average particle size of 3 µm was transformed into granules appropriate for pressing by spray drying (Dorst Technologies GmbH, Kochel am See, Germany, 125 µm average granule size). Cylindric bars were pressed in two stages to realize the desired high aspect ratio h/d ≈ 1.3. An amount of 2.8 mg granules was pressed in a double-acting die at 50 MPa on a manual hydraulic uniaxial press (PW40, Paul-Otto We-ber, Remshalden, Germany) to cylindrical green bodies with a diameter of 9.5 mm. After vacuuming in foil, cold isostatic pressing (EPSI at 180 MPa for 60 s) was carried out. Sintering of the rods was conducted in a chamber furnace (HT64/16, Nabertherm, Lilienthal, Germany) at 1100 °C for 5 h in air atmosphere.
Metallic braze foil with an average composition of 97 mol% silver and 3 mol% copper was produced by electroplating 75 µm thick silver foils with 1.6 µm copper layers on both sides. The braze foil was punched out with a diameter of 8 mm, cleaned, and smoothed. During the brazing process, the oxidation of the reactive element copper under the formation of composition Ag-3CuO took place.
Bars made of the heat-resistant austenitic steel AISI 314 (DIN X15CrNiSi25-21, EN 1.4841) were coated with different variants. As a reference condition, we refer to results published in [
39] where blank AISI 314 was brazed to BSCF without any diffusion barrier. Aluminizing was carried out by solid-state diffusion in the pack cementation process at Chromin Maastricht BV, Maastricht, The Netherlands. Aluminized metal components were oxidized in air at 1050 °C either for 1 h or for 100 h to improve the wetting of the braze. NiCoCrAlReY layers were applied by high-velocity flame spraying (HVOF) at the Institute for Surface Technology in Aachen (series H). The same institute carried out an additional coating with 7 mol% yttria-stabilized zirconia (7YSZ) by atmospheric plasma spraying (APS) on some samples with NiCoCrAlReY coating.
Table 1 gives an overview of the abbreviations used.
The brazing of the joints was conducted after the vertical arrangement of all components in a specially constructed brazing frame; see
Figure 1a,b. Details regarding the brazing frame are given in [
39]. The programmed temperature–time regime of a brazing cycle is given in
Table 2.
After brazing, the isothermal aging was conducted at 850 °C for 1000 h (Ecotop 20, Rohde, München, Germany). To avoid stress cracks, the maximum heating and cooling rate here was limited to 150 K/h.
The aged joints were tested at room temperature in accordance with the four-point bending test of design B standardized in DIN EN 843-1 for monolithic ceramics. The specimen lay symmetrically on the support rollers with a distance of L = 40 mm and thus both joint areas within the span of l = 20 mm between the two load rollers. After applying a pre-load of 1 N, the centrally placed joints were loaded until failure in a universal testing machine (Z020, Zwick/Roell, Ulm, Germany). The fracture stress
is calculated with the measured maximum force
and the joint diameter d = 8.1 mm.
Representative cross-sections were made of specimens with a bending strength close to the average strength of the series. Overview images in the light microscope (Axioscope 7, Zeiss, Jena, Germany) gave an overview of typical defects in the joints. This was followed by higher-magnification analyses in a scanning electron microscope (FEI Helios Nanolab G3 CX DualBeam, Thermofisher, Waltham, MA, USA). The fracture surfaces of all joints were assigned to the three fracture types, “ceramic fracture”, “mixed fracture”, and “delamination”, after images were taken under the stereo microscope (KL 1500LCD, Olympus, Tokyo, Japan).
Figure 2c,d illustrate the representative crack paths of each fracture types where “x” marks the fracture origin at the tensile loaded position.
4. Discussion
In thin-walled tubular membrane joints with 920 µm wall thickness and contact to the gaseous atmosphere on both sides, distances in the order of magnitude of the wall thickness can be bridged by diffusion after only a short aging time [
59]. In addition to the strength-determining defects, special attention must be paid to the microstructural changes at the edges and center of the BSCF.
Table 5 compares these as numbered microstructural features of the braze and the BSCF to discuss possible causal relationships between individual observed microstructural changes.
In the previous work, we discussed why chromium is probably transported to BSCF via internal Ba-Cr oxides in the braze in the uncoated Series R. The diffusion barrier studies now performed seem to confirm this, as neither the Ba-Cr oxide phases nor chromium poisoning of the BSCF ⑥ were detected by EDX.
The different braze infiltration ① is clearly illustrated by the Ag mappings in
Figure 12. In joints (series R) with strong braze infiltration, no pore coarsening occurred in the BSCF ③. This is in agreement with observations in [
42], where infiltration without pore coarsening occurred in BSCF-Ag-xCuO-X15CrNiSi25-21 joints, but pore coarsening without infiltration occurred in the wetting test BSCF-Ag-xCuO. The mechanisms of pore coarsening due to locally increased grain boundary mobility have been described in [
60], but it is unclear when it occurs.
Braze infiltration did not directly correlate with the local Cu concentration in the mixed oxide layer ②. However, it was noticeable that braze infiltration was pronounced when either many elements of the BSCF diffused through the braze to the metal-side mixed oxide layer ⑦ or the copper was concentrated at this layer ②. For series R, O1, and O99+, a continuous Co-Cu mixed oxide was formed, and for series K, a thick layer of (Al)-Ba-Co-Cr-Fe-Sr mixed oxide was deposited on the metal-side Al
2O
3 top layer [
39]. Weak infiltration was observed in series A100, H, and Z, where a thin Al-Cu mixed oxide was observed (A100, H), and few distinct Ba-Sr-Co-(Fe) crystallites were deposited on the zirconia layer (Z). In series A1, the pure Al
2O
3 top layer was present at the interface to the braze, and no infiltration occurred at all. A possible explanation is the following:
If elements of the BSCF ⑦ or copper ② diffused from the braze to the metal-side interface, voids were created. These were filled either by silver diffusing in or by BSCF (= pore coarsening ③). This would mean that silver could enter between the BSCF grains both primarily during brazing and secondarily by solid-state diffusion during aging. Proof of this hypothesis should be clarified by subsequent studies. This could also be the reason why “infiltration structures” were observed preferentially at wetting gaps and edges, as illustrated, for example, by the overview image of the joints from series A100 in
Figure 3b. Continuous braze layers with as few wetting gaps as possible were obviously important not only for beneficial stress transfer and resulting strength but also for microstructural stability.
The occurrence of needle-shaped phases ④ correlated with the observation of decomposition features of the BSCF ⑤. By EDX in
Figure 6e, the decomposition was described as enrichment of cobalt with simultaneous depletion of strontium both in the needles and in the surrounding BSCF. The local decomposition of the BSCF was attributed to chromium and sulfur poisoning in [
1]. The results now available show that decomposition starts even in the absence of chromium ⑥ and sulfur contamination. If elemental mappings of cobalt and strontium of the same microstructural sections are compared in
Figure 12b,c, this tendency can be traced for all sample series except series A1.
The inhomogeneity in the Co and Sr mappings correlated with the formation of needle-like phases (R, H and Z series). Where only differences in the contour of defined structures between Co and Sr mapping were apparent (outlined areas in
Figure 12), needles were either weak or absent. However, it can be assumed that the concentration differences of cobalt and strontium in these joints will increase in prolonged aging, and thus, the precipitation of the acicular structures is also thermodynamically favored. Since no Co/Sr inhomogeneity occurred after 1000 h aging of the A1 series, it is possible that the formation of needles will also be absent in long-term use.
A phase analysis of the needles was difficult since only EDX measurements were available where the excitation volume exceeded the volume of the needles. The decomposition of the cubic phase was indicated by the high Goldschmidt factor of 1.07, which clearly exceeded the tolerance range of 0.8–1 for the cubic perovskite structure. This factor was calculated based on the Ba:Sr and Co:Fe cation ratios determined by EDX (
Figure 6e). Similar lamellar or plate-like structures were studied in [
61,
62,
63,
64] using TEM lamellae, XRD, and dilatometry. The authors describe Co enrichment and Sr depletion in a trigonal phase Ba
3Co
10O
17B with high iron solubility. Depending on the p
O2-T history, the lamellar trigonal phase was often surrounded by hexagonal phase. Müller observed nucleation at CoO precipitates and a volume fraction of 8% after annealing at 850 °C for 10 h [
61]. Decomposition of the BSCF into the iron-free hexagonal Ba(Sr)CoO
3-δ phase and cobalt-free cubic Sr(Ba)FeO
3-δ phase, as observed in [
65] by TEM for the hexagonal and cubic phases, was apparently not present. Moreover, the hexagonal phase showed a different morphology and occurred preferentially at grain boundaries, where it appeared bright in the BSE image [
8].
The morphology of the needles in the reactive air brazed BSCF, as well as their protrusion from the cross-section plane due to a locally higher hardness, agreed very well with the descriptions of the trigonal phase. According to EDX analyses in
Figure 6e, an ion ratio A:B:O of 10:30:60 was present. This was between the 12.5:25:62.5 [
64] and 10:33:56 [
62] ion ratios reported in the literature. Instead of nucleation at CoO precipitates, as observed by Müller, the phase boundaries to the braze or the joint surface could favor nucleation. Since the formation of the needles did not occur in the joints with aluminized AISI314 (the same in Series K in [
39]), the formation of the trigonal phase in brazed joints cannot depend purely on the p
O2-T history. The inhomogeneity in the Co-Sr mapping can probably be considered as an early-warning indicator to the formation of visible needles. The lack of braze infiltration ① and resulting low and flat interface to the BSCF with few nucleation sites could be a reason why the A1 series was also free of any decomposition features in the Co and Sr mapping ⑤.
For the geometry of the tested brazed joints, the largest defects in the micrograph and fracture types with the lowest strength from the fracture surface analysis were additional important criteria for discussion. In addition, the percentage of specimens that failed below a 10 MPa proof test limit that was reasonable for the application was relevant to the fabrication route.
Table 6 compares these characteristics.
In the R, O1, and O99+ series of our previous study [
39], local delamination or elongated pores between the mixed oxide layer and the steel were the largest defects in the micrograph and also the critical defects that initiated delamination. In addition, the observed cracks in series Z triggered the ceramic fracture or a mixed fracture initiated in the ceramic. However, in the series A1, A100, and H, the defects in the micrograph did not determine the fracture type with the lowest strength. The common approach in the literature of correlating joint strength with microstructural defects can lead to erroneous conclusions.
Table 7 summarizes the fracture types with the characteristic fracture patterns, defects, and average strength.
While in most series, the average strength of ceramic fractures was above that of mixed fracture and delamination fracture, this order was changed in series H. BSCF was the weakest link in these joints, and the adhesion of steel and mixed oxide layer was advantageous. The strength of the mixed fracture, which started in the mixed oxide layer but passed through the braze in the stress neutral center, was still higher probably due to the crack deflection and enlarged fracture work. An unused strength potential lay obviously in the ceramic component. Since no severe defects were observed in the BSCF and the crack progression was atypical for a flexural test, residual stresses seemed to be responsible for the ceramic fracture type.
These residual stresses were also responsible for the pre-damage caused by microcracks parallel to the braze layer in series Z. In these joints, crack propagation was influenced both by the residual stresses in the coated metal substrate and by the thermally induced stresses during the cooling of the joint. It was therefore surprising that in series H and Z, the ceramic fractures were similar. The series differed in the additional 7YSZ top layer applied in series Z. The 8YSZ layer had a comparatively low coefficient of thermal expansion in the range 12∙10
−6 K
−1, similar to that of ferritic steels used in series K [
39]. Nevertheless, the pre-damage due to axial cracking in series K and lateral cracking in series Z was completely different. The reasons for this again lie in the residual stress states, which have not yet been investigated and which, in addition to the coefficients of thermal expansion, are strongly influenced by the layer thickness or length of the components.
For the application of the tubular air brazed membrane in a membrane module outlined in
Figure 1b, the strengths achieved are theoretically sufficient. In addition to the thermally induced residual stresses, the joint will be loaded with compressive stresses in operation equal to the feed pressure or, in the depressurized state before/after operation, stresses of about 30 kPa due to the dead weight of the membrane itself. Important for the application is the significant increase in the observed minimum strength to avoid failure of the brazed joint during operation. The minimum strength was increased from 0 MPa in joints with uncoated AISI 314 to 7 and 11 MPa by applying the NiCoCrAlReY coating and NiCoCrAlReY layer with 7YSZ top layer, respectively.























 BSCF;
BSCF;  Reaction Layer;
Reaction Layer;  Braze;
Braze;  Mixed oxide;
Mixed oxide;  AISI 314.
AISI 314.