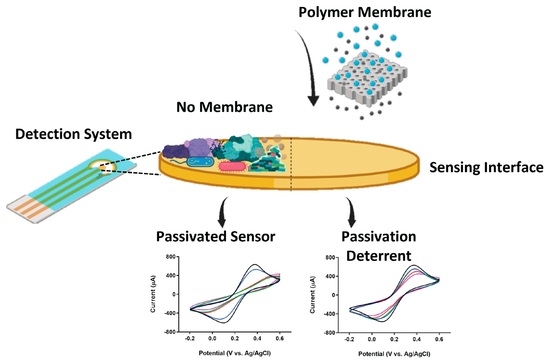
PCL/PEO Polymer Membrane Prevents Biofouling in Wearable Detection Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer Solution
2.2. Preparation of PCL/PEO Membrane
2.3. Membrane Characterization
2.3.1. Optical Imaging
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Laser Confocal Microscopy
2.3.5. Scanning Electron Microscopy (SEM) and Overall Porosity
2.3.6. Wettability
2.3.7. Water Adsorption and Swelling
2.3.8. Gravimetrical Analysis
2.4. Microfluidic Flow Cell
2.5. Permeability and Transfer Efficiency
2.6. Biofouling Experiments
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the PCL/PEO Membrane
3.2. Membrane Permeability, Transfer Efficiency and Pore Activation
3.3. Biofouling Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Smith, A.A.; Li, R.; Tse, Z.T.H. Reshaping Healthcare with Wearable Biosensors. Sci. Rep. 2023, 13, 4998. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device Integration of Electrochemical Biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-R.; Kim, H.S.; Qazi, R.; Kwon, Y.-T.; Jeong, J.-W.; Yeo, W.-H. Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater. 2020, 32, e1901924. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Kim, J.K.; Choi, J. Multifunctional Wearable System for Mapping Body Temperature and Analyzing Sweat. ACS Sens. 2023, 8, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liang, C.; Huang, Y.; Zhou, G.; Xiao, Y.; Ji, N.; Zhang, Y.-T.; Zhao, N. Emerging Sensing and Modeling Technologies for Wearable and Cuffless Blood Pressure Monitoring. NPJ Digit. Med. 2023, 6, 93. [Google Scholar] [CrossRef]
- Song, Y.; Min, J.; Yu, Y.; Wang, H.; Yang, Y.; Zhang, H.; Gao, W. Wireless Battery-Free Wearable Sweat Sensor Powered by Human Motion. Sci. Adv. 2020, 6, eaay9842. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Li, G.; Yan, D.; Liu, C.; Xu, T.; Zhang, X. Ultra-Small Wearable Flexible Biosensor for Continuous Sweat Analysis. ACS Sens. 2022, 7, 3102–3107. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeang, W.J.; Ghaffari, R.; Rogers, J.A. Wearable Sensors for Biochemical Sweat Analysis. Annu. Rev. Anal. Chem. 2019, 12, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and Flexible Electrochemical Sensors for Sweat Analysis: A Review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Tu, J.; Xu, C.; Lukas, H.; Shin, S.; Yang, Y.; Solomon, S.A.; Mukasa, D.; Gao, W. Skin-Interfaced Wearable Sweat Sensors for Precision Medicine. Chem. Rev. 2023, 123, 5049–5138. [Google Scholar] [PubMed]
- Hadid, A.; McDonald, E.G.; Cheng, M.P.; Papenburg, J.; Libman, M.; Dixon, P.C.; Jensen, D. The WE SENSE Study Protocol: A Controlled, Longitudinal Clinical Trial on the Use of Wearable Sensors for Early Detection and Tracking of Viral Respiratory Tract Infections. Contemp. Clin. Trials 2023, 128, 107103. [Google Scholar] [CrossRef] [PubMed]
- Kalasin, S.; Surareungchai, W. Challenges of Emerging Wearable Sensors for Remote Monitoring toward Telemedicine Healthcare. Anal. Chem. 2023, 95, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, A.; Yu, B.; Zhang, L.; Pang, W.; Zhang, H.; Niu, P. Uncovering the Sweat Biofouling Components and Distributions in Electrochemical Sensors. Anal. Chem. 2022, 94, 14402–14409. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Wang, X.; Niu, P.; He, A.; Yu, B.; Zhang, M.; Pang, W. Miniature Sono-Electrochemical Platform Enabling Effective and Gentle Electrode Biofouling Removal for Continuous Sweat Measurements. Chem. Eng. J. 2022, 431, 133354. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Wang, J.; Liu, Z. A Laser-Induced Photoelectrochemical Sensor for Natural Sweat Cu2+ Detection. Chemosensors 2022, 10, 169. [Google Scholar] [CrossRef]
- Ding, R.; Joon, N.K.; Ahamed, A.; Shafaat, A.; Guzinski, M.; Wagner, M.; Ruzgas, T.; Bobacka, J.; Lisak, G. Gold-Modified Paper as Microfluidic Substrates with Reduced Biofouling in Potentiometric Ion Sensing. Sens. Actuators B Chem. 2021, 344, 130200. [Google Scholar] [CrossRef]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef]
- Su, T.; Mi, Z.; Xia, Y.; Jin, D.; Xu, Q.; Hu, X.; Shu, Y. A Wearable Sweat Electrochemical Aptasensor Based on the Ni–Co MOF Nanosheet-Decorated CNTs/PU Film for Monitoring of Stress Biomarker. Talanta 2023, 260, 124620. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Machado, S.A.S.; Oliveira, O.N., Jr. Wearable Glove-Embedded Sensors for Therapeutic Drug Monitoring in Sweat for Personalized Medicine. Chem. Eng. J. 2022, 435, 135047. [Google Scholar] [CrossRef]
- Stefanov, C.; Cioates Negut, C.; Dinu Gugoasa, L.A.; Frederick van Staden, J. (Koos) Sensitive Voltammetric Determination of Riboflavin in Pharmaceutical and Biological Samples Using FSN-Zonyl-Nafion Modified Carbon Paste Electrode. Microchem. J. 2020, 155, 104729. [Google Scholar] [CrossRef]
- Long, X.; Deng, C.; Xiao, G.; Cheng, F.; Zhou, Y.; Zhao, L.; Cai, L.; Chen, L.; Du, J. Electrochemical Sensors with Antifouling Properties for Sensitive Detection of Isoproturon Based on Glassy Carbon Electrode Modified with Nafion Membrane. Int. J. Electrochem. Sci. 2020, 15, 4964–4977. [Google Scholar] [CrossRef]
- Jayakumar, K.; Lielpetere, A.; Domingo-Lopez, D.A.; Levey, R.E.; Duffy, G.P.; Schuhmann, W.; Leech, D. Tethering Zwitterionic Polymer Coatings to Mediated Glucose Biosensor Enzyme Electrodes Can Decrease Sensor Foreign Body Response yet Retain Sensor Sensitivity to Glucose. Biosens. Bioelectron. 2023, 219, 114815. [Google Scholar] [CrossRef] [PubMed]
- Sievers, M.; Fitridge, I.; Bui, S.; Dempster, T. To Treat or Not to Treat: A Quantitative Review of the Effect of Biofouling and Control Methods in Shellfish Aquaculture to Evaluate the Necessity of Removal. Biofouling 2017, 33, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lee, H.-J.; Wu, Y.; Ganzoury, M.A.; de Lannoy, C.-F. Integrating Biofouling Sensing with Fouling Mitigation in a Two-Electrode Electrically Conductive Membrane Filtration System. Sep. Purif. Technol. 2022, 288, 120679. [Google Scholar]
- Qi, L.; Jiang, T.; Liang, R.; Qin, W. Polymeric Membrane Ion-Selective Electrodes with Anti-Biofouling Properties by Surface Modification of Silver Nanoparticles. Sens. Actuators B Chem. 2021, 328, 129014. [Google Scholar]
- Chao, L.; Kexin, Y.; Shaogang, H.; Wulin, Y. Cathodic Biofouling Control by Microbial Separators in Air-Breathing Microbial Fuel Cells. Environ. Sci. Ecotechnol. 2023, 15, 100251. [Google Scholar]
- Sánchez, J.; Oyarce, E.; Roa, K.; Salfate, G. The Importance of Polymers in the Preparation of Biomaterials for Removal of Metal and Control of Bacterial Infections for Healthcare Applications. In Polymeric Biomaterials for Healthcare Applications; Woodhead Publishing: Cambridge, UK, 2022; pp. 235–256. [Google Scholar]
- Karimi-Maleh, H.; Orooji, Y.; Karimi, F.; Alizadeh, M.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; et al. A Critical Review on the Use of Potentiometric Based Biosensors for Biomarkers Detection. Biosens. Bioelectron. 2021, 184, 113252. [Google Scholar] [CrossRef]
- Kohlová, M.; Amorim, C.G.; da Nova Araújo, A.; Santos-Silva, A.; Solich, P.; Montenegro, M.C.B.S.M. In Vitro Assessment of Polyethylene Glycol and Polyvinylpyrrolidone as Hydrophilic Additives on Bioseparation by Polysulfone Membranes. J. Mater. Sci. 2019, 55, 1292–1307. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-Based Biomaterials for Tissue Engineering and Drug Delivery: Current Scenario and Challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Yao, Y.; Wei, H.; Wang, J.; Lu, H.; Jinsong, L.; Hui, D. Fabrication of Hybrid Membrane of Electrospun Polycaprolactone and Polyethylene Oxide with Shape Memory Property. Compos. Part B 2015, 83, 264–269. [Google Scholar] [CrossRef]
- Mu, Y.; Feng, H.; Wang, S.; Zhang, S.; Luan, J.; Zhang, M.; Yu, Z.; Wang, G. Combined Strategy of Blending and Surface Modification as an Effective Route to Prepare Antifouling Ultrafiltration Membranes. J. Colloid Interface Sci. 2021, 589, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B. Sweating Rate and Sweat Sodium Concentration in Athletes: A Review of Methodology and Intra/Interindividual Variability. Sports Med. 2017, 47, 111–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, L.B.; Ungaro, C.T.; Sopeña, B.C.; Nuccio, R.P.; Reimel, A.J.; Carter, J.M.; Stofan, J.R.; Barnes, K.A. Body Map of Regional vs. Whole Body Sweating Rate and Sweat Electrolyte Concentrations in Men and Women during Moderate Exercise-Heat Stress. J. Appl. Physiol. 2018, 124, 1304–1318. [Google Scholar] [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Srinivasarao, M.; Collings, D.; Philips, A.; Patel, S. Three-Dimensionally Ordered Array of Air Bubbles in a Polymer Film. Science 2001, 292, 79–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janarthanan, G.; Kim, I.G.; Chung, E.-J.; Noh, I. Comparative Studies on Thin Polycaprolactone-Tricalcium Phosphate Composite Scaffolds and Its Interaction with Mesenchymal Stem Cells. Biomater. Res. 2019, 23, 1. [Google Scholar] [CrossRef] [Green Version]
- Gohary, M.I.E.; Hady, B.M.A.E.; Saeed, A.A.A.; Tolba, E.; Rashedi, A.M.I.E.; Saleh, S. Electrospinning of Doxorubicin Loaded Silica/Poly(ɛ-Caprolactone) Hybrid Fiber Mats for Sustained Drug Release. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025002. [Google Scholar] [CrossRef]
- Candadai, A.A.; Nadler, E.J.; Burke, J.S.; Weibel, J.A.; Marconnet, A.M. Thermal and Mechanical Characterization of High Performance Polymer Fabrics for Applications in Wearable Devices. Sci. Rep. 2021, 11, 8705. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Wang, Z.; Horseman, T.; Livingston, J.L.; Lin, S. Interpreting Contact Angles of Surfactant Solutions on Microporous Hydrophobic Membranes. J. Membr. Sci. Lett. 2022, 2, 100015. [Google Scholar] [CrossRef]
- Shay, T.; Saha, T.; Dickey, M.D.; Velev, O.D. Principles of Long-Term Fluids Handling in Paper-Based Wearables with Capillary-Evaporative Transport. Biomicrofluidics 2020, 14, 034112. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.; Mastronardi, V.M.; Guido, F.; Algieri, L.; Qualtieri, A.; Fiammengo, R.; Rizzi, F.; De Vittorio, M. Wearable Piezoelectric Mass Sensor Based on PH Sensitive Hydrogels for Sweat PH Monitoring. Sci. Rep. 2020, 10, 6840. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rivera, G.; Suleiman, D. High-performance Blended Membranes Based on Poly(Arylene Ether Sulfone) and Sulfonated Poly(Styrene-isobutylene-styrene) for Direct Methanol Fuel Cell Applications. J. Appl. Polym. Sci. 2022, 139, 52027. [Google Scholar] [CrossRef]
- Xu, J.H.; Luo, G.S.; Chen, G.G.; Tan, B. Mass Transfer Performance and Two-Phase Flow Characteristic in Membrane Dispersion Mini-Extractor. J. Memb. Sci. 2005, 249, 75–81. [Google Scholar] [CrossRef]
- Dourandish, Z.; Tajik, S.; Beitollahi, H.; Jahani, P.M.; Nejad, F.G.; Sheikhshoaie, I.; Di Bartolomeo, A. A Comprehensive Review of Metal–Organic Framework: Synthesis, Characterization, and Investigation of Their Application in Electrochemical Biosensors for Biomedical Analysis. Sensors 2022, 22, 2238. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.; Orozco, J. Wearable Electrochemical Biosensors to Measure Biomarkers with Complex Blood-to-Sweat Partition Such as Proteins and Hormones. Microchim. Acta 2022, 189, 127. [Google Scholar] [CrossRef]
- López, L.; Hernández, N.; Reyes Morales, J.; Cruz, J.; Flores, K.; González-Amoretti, J.; Rivera, V.; Cunci, L. Measurement of Neuropeptide Y Using Aptamer-Modified Microelectrodes by Electrochemical Impedance Spectroscopy. Anal. Chem. 2021, 93, 973–980. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Rivera, R.; García-Rodríguez, W.; López, L.; Cunci, L.; Resto, P.J.; Domenech, M. PCL/PEO Polymer Membrane Prevents Biofouling in Wearable Detection Sensors. Membranes 2023, 13, 728. https://doi.org/10.3390/membranes13080728
Delgado-Rivera R, García-Rodríguez W, López L, Cunci L, Resto PJ, Domenech M. PCL/PEO Polymer Membrane Prevents Biofouling in Wearable Detection Sensors. Membranes. 2023; 13(8):728. https://doi.org/10.3390/membranes13080728
Chicago/Turabian StyleDelgado-Rivera, Roberto, William García-Rodríguez, Luis López, Lisandro Cunci, Pedro J. Resto, and Maribella Domenech. 2023. "PCL/PEO Polymer Membrane Prevents Biofouling in Wearable Detection Sensors" Membranes 13, no. 8: 728. https://doi.org/10.3390/membranes13080728
APA StyleDelgado-Rivera, R., García-Rodríguez, W., López, L., Cunci, L., Resto, P. J., & Domenech, M. (2023). PCL/PEO Polymer Membrane Prevents Biofouling in Wearable Detection Sensors. Membranes, 13(8), 728. https://doi.org/10.3390/membranes13080728