Synthesis of Imidazolium based PILs and Investigation of Their Blend Membranes for Gas Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PIL Synthesis
2.2.1. Synthesis of Pyridinium-Based PILs (PILPyr)
2.2.2. Synthesis of Imidazolium-Based PILs (P(VBCImY-co-AAx))
Synthesis of Precursor Copolymers P(VBC-co-AAx)
Synthesis of Imidazolium Functionalized P(VBCImCl-co-AAx)
Anion Exchange of Imidazolium Functionalized P(VBCImCl-co-AAx)
2.3. Membrane Preparation
2.4. Characterization
2.5. Gas Separation Experiments
3. Results and Discussion
3.1. Synthesis and Characterization of Imidazolium-Based PILs
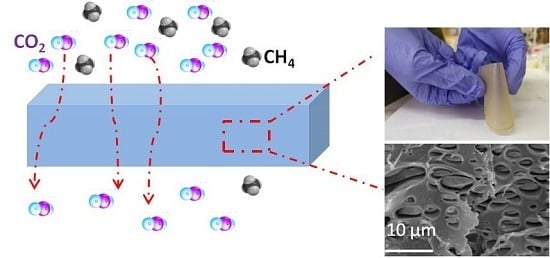
3.2. Preparation and Characterization of PIL Blend Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Tomé, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–27824. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquids): An Update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Tang, J.; Tang, H.; Sun, W.; Radosz, M.; Shen, Y. Atom transfer radical polymerization of styrenic ionic liquid monomers and carbon dioxide absorption of the polymerized ionic liquids. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5477–5489. [Google Scholar] [CrossRef]
- Bara, J.E.; Gabriel, C.J.; Hatakeyama, E.S.; Carlisle, T.K.; Lessmann, S.; Noble, R.D.; Gin, D.L. Improving CO2 selectivity in polymerized room temperature ionic liquid gas separation membranes through incorporation of polar substituents. J. Membr. Sci. 2008, 321, 3–7. [Google Scholar] [CrossRef]
- Tomé, L.C.; Isik, M.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Novel pyrrolidinium-based polymeric ionic liquids with cyano counter-anions: High performance membrane materials for post-combustion CO2 separation. J. Membr. Sci. 2015, 483, 155–165. [Google Scholar] [CrossRef]
- Zulficar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef] [Green Version]
- Vollas, A.; Chouliaras, T.; Deimede, V.; Ioannides, T.; Kallitsis, J.K. New pyridinium type Poly(ionic liquids) as membranes for CO2 separation. Polymers 2018, 10, 912. [Google Scholar] [CrossRef] [Green Version]
- Bhavasar, R.S.; Kumbharkar, S.C.; Rewar, A.S.; Kharul, U.K. Polybenzimidazole based film forming polymeric ionic liquids: Synthesis and effects of cation-anion variation on their physical properties. Polym. Chem. 2014, 5, 4083–4096. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Morozova, S.M.; Lozinskaya, E.I.; Vlasov, P.S.; Gouveia, A.S.L.; Tomé, L.C.; Marrucho, I.M.; Vygodskii, Y.S. Turning into poly(ionic liquid)s as a tool for polyimide modification: Synthesis, characterization and CO2 separation properties. Polym. Chem. 2016, 7, 580–591. [Google Scholar] [CrossRef]
- Robeson, L.M. Polymer Blends in Membrane Transport Processes. Ind. Eng. Chem. Res. 2010, 49, 11859–11865. [Google Scholar] [CrossRef]
- Mannan, H.A.; Mukhtar, H.; Murugesan, T.; Nasir, R.; Mohshim, D.F.; Mushtaq, A. Recent Applications of Polymer Blends in Gas separation Membranes. Chem. Eng. Technol. 2013, 36, 1–10. [Google Scholar] [CrossRef]
- Panapitiya, N.; Wijenayake, S.; Nguyen, D.; Karunaweera, C.; Huang, Y.; Balkus, K.; Musselman, I., Jr.; Ferraris, J. Compatibilized immiscible polymer blends for gas separations. Materials 2016, 9, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, S.S.; Teoh, M.M.; Chung, T.S. Hydrogen separation and purification in membranes of miscible polymer blends with interpenetration networks. Polymer 2008, 49, 1594–1603. [Google Scholar] [CrossRef]
- Chung, T.S.; Guo, W.F.; Liu, Y. Enhanced matrimid membranes for pervoration by homogeneous blends with polybenzimidazole (PBI). J. Membr. Sci. 2006, 271, 221–231. [Google Scholar] [CrossRef]
- Ismail, A.F.; Rahim, R.A.; Rahman, W.A. Characterization of polyethersulfone/Matrimid® 5218 miscible blend mixed matrix membranes for O2/N2 gas separation. Sep. Purif. Technol. 2008, 63, 200–206. [Google Scholar] [CrossRef]
- Pérez-Fransisco, J.M.; Santiago-Garcia, J.L.; Loria-Bastarrachea, M.I.; Aguilar-Vega, M. Evaluation of gas transport properties of highly rigid aromatic PI DPPD-IMM/PBI blends. Ind. Eng. Chem. Res. 2017, 56, 9355–9366. [Google Scholar] [CrossRef]
- Moon, J.D.; Bridge, A.T.; D’Ambra, C.; Freeman, B.D.; Paul, D.R. Gas separation properties of polybenzimidazole/thermally-rearranged polymer blends. J. Membr. Sci. 2019, 582, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Pefkianakis, E.K.; Deimede, V.; Daletou, M.K.; Gourdoupi, N.; Kallitsis, J.K. Novel Polymer Electrolyte membrane based on pyridine containing poly(ether sulfone), for application in High-Temperature Fuel Cells. Macromol. Rapid Commun. 2005, 26, 1724–1728. [Google Scholar] [CrossRef]
- Geormezi, M.; Chochos, C.; Gourdoupi, N.; Neophytides, S.G.; Kallitsis, J.K. High performance polymer electrolytes based on main and side chain pyridine aromatic polyethers for high and medium temperature proton exchange membrane fuel cells. J. Power Sour. 2011, 196, 9382–9390. [Google Scholar] [CrossRef]
- Geormezi, M.; Deimede, V.; Kallitsis, J.K.; Neophytides, S. Polymer blends based on copolymers bearing both side and main chain pyridine units as proton exchange membranes for high temperature fuel cells. J. Membr. Sci. 2012, 396, 57–66. [Google Scholar] [CrossRef]
- Voege, A.; Deimede, V.; Kallitsis, J.K. Side chain crosslinking of aromatic polyethers for high temperature polymer electrolyte membrane fuel cell applications. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 207–216. [Google Scholar] [CrossRef]
- Deimede, V.; Voege, A.; Lainioti, G.; Elmasides, C.; Kallitsis, J.K. Large-Scale Separators Based on Blends of Aromatic Polyethers with PEO for Li-Ion batteries: Improving Thermal shrinkage and wettability behavior. Energy Technol. 2014, 2, 275–283. [Google Scholar] [CrossRef]
- Kougia, E.; Tselepi, M.; Vasilopoulos, G.; Lainioti, G.; Koromilas, N.D.; Druvari, D.; Bokias, G.; Vantarakis, A.; Kallitsis, J.K. Evaluation of Antimicrobial Efficiency of New Polymers Comprised by Covalently Attached and/or Electrostatically Bound Bacteriostatic Species, Based on Quaternary Ammonium Compounds. Molecules 2015, 20, 21313–21327. [Google Scholar] [CrossRef] [PubMed]
- Druvari, D.; Koromilas, N.D.; Lainioti, G.; Bokias, G.; Vasilopoulos, G.; Vasilopoulos, G.; Baras, I.; Dourala, N.; Kallitsis, J.K. Polymeric Quaternary Ammonium-Containing Coatings with Potential Dual Contact-Based and Release-Based Antimicrobial Activity. ACS Appl. Mater. Interfaces 2016, 8, 35593–35605. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1984; pp. 127–128. [Google Scholar]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Pinnau, I.; Freeman, B.D. (Eds.) Materials Science of Membranes for Gas and Vapor Separation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 251–270. [Google Scholar]
- Makrygianni, M.; Lada, Z.G.; Manousou, A.; Aggelopoulos, C.A.; Deimede, V. Removal of anionic dyes from aqueous solution by novel pyrrolidinium based Polymeric Ionic Liquid (PIL) as adsorbent: Investigation of the adsorption kinetics, equilibrium isotherms and the adsorption mechanism involved. J. Environ. Chem. Eng. 2019, 7, 103163. [Google Scholar] [CrossRef]
- Gong, X.; Yan, X.; Li, T.; Wu, X.; Chen, W.; Huang, S.; Wu, Y.; Zhen, D.; He, G. Design of pendent imidazolium side chain with flexible ether-containing spacer for alkaline anion exchange membrane. J. Membr. Sci. 2017, 523, 216–224. [Google Scholar] [CrossRef]
- Lu, W.; Shao, Z.-G.; Zhang, G.; Zhao, Y.; Yi, B. Crosslinked poly(vinylbenzyl chloride) with a macromolecular crosslinker for anion exchange membrane fuel cells. J. Power Sour. 2014, 248, 905–914. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, W.; Yuan, J. Reprocessable porous poly(ionic liquid) membranes derived from main-chain polyimidazolium. Eur. Polym. J. 2018, 103, 214–219. [Google Scholar] [CrossRef]
- Suarez, P.A.Z.; Dullius, J.E.L.; Einloft, S.; De Souza, R.F.; Dupont, J. The use of new ionic liquids in two phase catalytic hydrogenation reaction by rhodium complexes. Polyhedron 1996, 15, 1217–1219. [Google Scholar] [CrossRef]
- Depuydt, D.; Van den Bossche, A.; Dehaen, W.; Binnemans, K. Halogen-free synthesis of symmetrical 1,3-dialkylimidazolium ionic liquids using non- enolisable starting materials. RSC Adv. 2016, 6, 8848–8859. [Google Scholar] [CrossRef]
- Wang, C.; Luo, X.; Luo, H.; Jiang, D.-E.; Li, H.; Dai, S. Tuning the basicity of ionic liquids of equimolar CO2 capture. Angew. Chem. 2011, 50, 4918–4922. [Google Scholar] [CrossRef]
- Hameed, N.; Guo, Q. Self-Assembled complexes of poly(acrylic acid) and poly(styrene)-block-poly(4-vinyl pyridine). J. Polym. Sci. Part B Polym. Phys. 2009, 47, 1192–1202. [Google Scholar] [CrossRef]
- Sakurai, K.; Douglas, E.P.; MacKnight, W.J. Spectroscopic study of an ionic blend made from the acid form of sulfonated polystyrene and poly[ethyl acrylate-co-(4-vinylpyridine)]. Macromolecules 1992, 25, 4506–4510. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Li, B.; Pan, Y.; Wu, G. Morphology evolution of immiscible polymer blends as directed by nanoparticle self-agglomeration. Polymer 2012, 53, 259–266. [Google Scholar] [CrossRef]
- Park, C.; Jo, W.H.; Park, H.C.; Kang, Y.S. Morphological effect of dispersed phase on gas permeation properties through heterophase polymer membrane: Theoretical and experimental approaches. Polymer 2000, 41, 1765–1771. [Google Scholar] [CrossRef]
- Lafitte, G.; Espuche, E.; Gérard, J.-F. Polyamide 11/poly(hydroxyl amino ether) blends: Influence of the blend composition and morphology on the barrier and mechanical properties. Eur. Polym. J. 2011, 47, 1994–2002. [Google Scholar] [CrossRef]
- Bara, J.E.; Lessmann, S.; Gabriel, C.J.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. Synthesis and performance of polymerizable room-temperature ionic liquids as gas separation membranes. Ind. Eng. Chem. Res. 2007, 46, 5397–5404. [Google Scholar] [CrossRef]
- Li, S.; Jiang, X.; Jang, X.; Bai, Y.; Shao, L. Nanoporous framework “reservoir” maximizing low-molecular-weight enhancer impregnation into CO2-philic membranes for highly-efficient CO2 capture. J. Membr. Sci. 2019, 570–571, 278–285. [Google Scholar] [CrossRef]
- Park, J.Y.; Paul, D.R. Correlating and prediction of gas permeability in glassy polymer membrane materials via a modified free volume based group contribution method. J. Membr. Sci. 1997, 125, 23–39. [Google Scholar] [CrossRef]
- Nagy, E. Basic Equation of Mass Transport through a Membrane Layer, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Paul, D.R.; Bucknall, C.B. Polymer Blends; John Wiley & Sons: New York, NY, USA, 2000; Volumes 1–2. [Google Scholar]
- Paul, D.R.; Yampolskii, Y.P. Polymeric Gas Separation Membranes; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Hosseini, S.S.; Chung, T.S. Carbon membranes from blends of PBI and Polyimides for N2/CH4 and CO2/CH4 separation and hydrogen purification. J. Membr. Sci. 2009, 328, 174–185. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.Y.; Xiao, Y.C.; Li, P.; Pramoda, K.P.; Tong, Y.W.; Chung, T.S. Molecular engineering of PIM-1/Matrimid blend membranes for gas separation. J. Membr. Sci. 2012, 407–408, 47–57. [Google Scholar] [CrossRef]










| Blend Composition PILPyr-X/P(VBCImY-co-AAx) | PILPyr-X | P(VBCImY-co-AAx) | |
|---|---|---|---|
| - | X-Counter Anion | Y-Counter Anion | x Molar Fraction of AA Units |
| PILPyr-BF4/ P(VBCImBF4-co-AA10) | |||
| 100/0 | BF4 | - | - |
| 80/20 | BF4 | BF4 | 10 |
| 70/30 | BF4 | BF4 | 10 |
| PILPyr-TFSI/ P(VBCImTFSI-co-AA10) | |||
| 100/0 | TFSI | - | - |
| 80/20 | TFSI | TFSI | 10 |
| 70/30 | TFSI | TFSI | 10 |
| PILPyr-TFSI/ P(VBCImTFSI-co-AA20) | |||
| 80/20 | TFSI | TFSI | 20 |
| 70/30 | TFSI | TFSI | 20 |
| 60/40 | TFSI | TFSI | 20 |
| Polymer Blends | Composition | PCO2 (Barrer) | PCH4 (Barrer) | αCO2/CH4 |
|---|---|---|---|---|
| PILPyr-BF4 /(VBCImBF4-co-AA10) | 100/0 | 2.0 ± 0.20 a | 0.040 ± 0.01 a | 50 ± 5 |
| PILPyr-BF4/P(VBCImBF4-co-AA10) | 80/20 | 1.97 ± 0.25 | 0.041 ± 0.0002 | 77 ± 2 |
| PILPyr-BF4/P(VBCImBF4-co-AA10) | 70/30 | 2.78 ± 0.15 | 0.018 ± 0.0002 | 154 ± 4 |
| PILPyr-TFSI/P(VBCImBF4-co-AA10) | 100/0 | 4.1 ± 0.10 a | 0.100 ± 0.01 a | 41 ± 1 |
| PILPyr-TFSI/P(VBCImTFSI-co-AA10) | 80/20 | 6.47 ± 0.04 | 0.094 ± 0,019 | 69 ± 5 |
| PILPyr-TFSI/P(VBCImTFSI-co-AA10) | 70/30 | 7.37 ± 0.03 | 0.200 ± 0.014 | 37 ± 2 |
| PILPyr-TFSI/P(VBCImTFSI-co-AA20) | 80/20 | 7.00 ± 0.12 | 0.068 ± 0.039 | 103 ± 25 |
| PILPyr-TFSI/P(VBCImTFSI-co-AA20) | 70/30 | 8.94 ± 0.59 | 1.43 ± 0.154 | 6 ± 1 |
| PILPyr-TFSI/P(VBCImTFSI-co-AA20) | 60/40 | 17.84 ± 0.08 | 8.22 ± 0.260 | 2 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouliaras, T.; Vollas, A.; Ioannides, T.; Deimede, V.; Kallitsis, J. Synthesis of Imidazolium based PILs and Investigation of Their Blend Membranes for Gas Separation. Membranes 2019, 9, 164. https://doi.org/10.3390/membranes9120164
Chouliaras T, Vollas A, Ioannides T, Deimede V, Kallitsis J. Synthesis of Imidazolium based PILs and Investigation of Their Blend Membranes for Gas Separation. Membranes. 2019; 9(12):164. https://doi.org/10.3390/membranes9120164
Chicago/Turabian StyleChouliaras, Thanasis, Aristofanis Vollas, Theophilos Ioannides, Valadoula Deimede, and Joannis Kallitsis. 2019. "Synthesis of Imidazolium based PILs and Investigation of Their Blend Membranes for Gas Separation" Membranes 9, no. 12: 164. https://doi.org/10.3390/membranes9120164
APA StyleChouliaras, T., Vollas, A., Ioannides, T., Deimede, V., & Kallitsis, J. (2019). Synthesis of Imidazolium based PILs and Investigation of Their Blend Membranes for Gas Separation. Membranes, 9(12), 164. https://doi.org/10.3390/membranes9120164