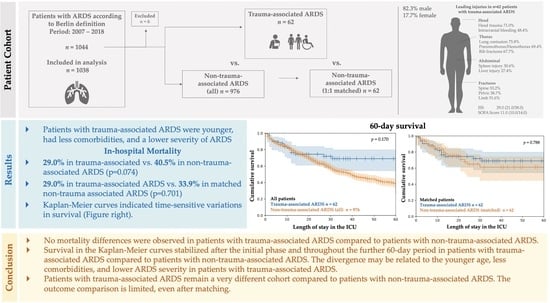
Outcome Comparison of Acute Respiratory Distress Syndrome (ARDS) in Patients with Trauma-Associated and Non-Trauma-Associated ARDS: A Retrospective 11-Year Period Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria, Setting and Data Source
2.2. Outcome Measures and Variables of Interest
2.3. Group Categorization and Matching
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Primary Outcome
3.2.1. Mortality (28-Day In-Hospital, 60-Day In-Hospital, and Overall In-Hospital)
3.2.2. Mortality (28-Day In-Hospital Mortality, 60-Day In-Hospital Mortality, and Overall In-Hospital) in Patients with Extracorporeal Lung Support (ECLS) Treatment
3.2.3. Kaplan–Meier Survival Curves (28-Day in-Hospital and 60-Day in-Hospital)
3.2.4. Kaplan–Meier Survival Curves (28-Day In-Hospital Survival and 60-Day In-Hospital Survival) in Patients with Extracorporeal Lung Support (ECLS)
3.3. Secondary Outcomes
3.3.1. Mechanical Ventilation, Pulmonary Gas Exchange, and Supportive Therapies
3.3.2. Transfusions
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case characteristics, resource use, and outcomes of 10,021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir. Med. 2020, 8, 853–862. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Kasotakis, G.; Stanfield, B.; Haines, K.; Vatsaas, C.; Alger, A.; Vaslef, S.N.; Brooks, K.; Agarwal, S. Acute Respiratory Distress Syndrome (ARDS) after trauma: Improving incidence, but increasing mortality. J. Crit. Care 2021, 64, 213–218. [Google Scholar] [CrossRef]
- Robinson, B.R.; Cotton, B.A.; Pritts, T.A.; Branson, R.; Holcomb, J.B.; Muskat, P.C.; Fox, E.E.; Wade, C.E.; del Junco, D.J.; Bulger, E.M.; et al. Application of the Berlin definition in PROMMTT patients: The impact of resuscitation on the incidence of hypoxemia. J. Trauma Acute Care Surg. 2013, 75, S61–S67. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.M.; Kornblith, L.Z.; Hendrickson, C.M.; Redick, B.J.; Conroy, A.S.; Nelson, M.F.; Callcut, R.A.; Calfee, C.S.; Cohen, M.J. Differences in degree, differences in kind: Characterizing lung injury in trauma. J. Trauma Acute Care Surg. 2015, 78, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Prinz, V.; Manekeller, L.; Menk, M.; Hecht, N.; Weber-Carstens, S.; Vajkoczy, P.; Finger, T. Clinical management and outcome of adult patients with extracorporeal life support device-associated intracerebral hemorrhage—A neurocritical perspective and grading. Neurosurg. Rev. 2021, 44, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Ferbert, A.; Deinsberger, W.; Kleffmann, J.; Kästner, S.; Godau, J.; Schüler, M.; Tryba, M.; Gehling, M. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit. Care 2014, 21, 186–191. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Nakanishi, N.; Liu, K.; Kawakami, D.; Kawai, Y.; Morisawa, T.; Nishida, T.; Sumita, H.; Unoki, T.; Hifumi, T.; Iida, Y.; et al. Post-Intensive Care Syndrome and Its New Challenges in Coronavirus Disease 2019 (COVID-19) Pandemic: A Review of Recent Advances and Perspectives. J. Clin. Med. 2021, 10, 3870. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Qin, T.; Xi, Z.; Sun, L.; Wu, H.; Li, D. Extracorporeal membrane oxygenation in trauma patients: A systematic review. World J. Emerg. Surg. 2020, 15, 51. [Google Scholar] [CrossRef]
- ARDS Definition Task Force. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Hunsicker, O.; Materne, L.; Bünger, V.; Krannich, A.; Balzer, F.; Spies, C.; Francis, R.C.; Weber-Carstens, S.; Menk, M.; Graw, J.A. Lower versus higher hemoglobin threshold for transfusion in ARDS patients with and without ECMO. Crit. Care 2020, 24, 697. [Google Scholar] [CrossRef] [PubMed]
- Ried, M.; Bein, T.; Philipp, A.; Müller, T.; Graf, B.; Schmid, C.; Zonies, D.; Diez, C.; Hofmann, S. Extracorporeal lung support in trauma patients with severe chest injury and acute lung failure: A 10-year institutional experience. Crit. Care 2013, 17, R110. [Google Scholar] [CrossRef] [PubMed]
- Ull, C.; Schildhauer, T.A.; Strauch, J.T.; Swol, J. Outcome measures of extracorporeal life support (ECLS) in trauma patients versus patients without trauma: A 7-year single-center retrospective cohort study. J. Artif. Organs 2017, 20, 117–124. [Google Scholar] [CrossRef]
- Amos, T.; Bannon-Murphy, H.; Yeung, M.; Gooi, J.; Marasco, S.; Udy, A.; Fitzgerald, M. ECMO (extra corporeal membrane oxygenation) in major trauma: A 10 year single centre experience. Injury 2021, 52, 2515–2521. [Google Scholar] [CrossRef]
- Swol, J.; Brodie, D.; Napolitano, L.; Park, P.K.; Thiagarajan, R.; Barbaro, R.P.; Lorusso, R.; McMullan, D.; Cavarocchi, N.; Hssain, A.A.; et al. Indications and outcomes of extracorporeal life support in trauma patients. J. Trauma Acute Care Surg. 2018, 84, 831–837. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Hooft, N.M.; Robinson, B.R.; Todd, E.; Bremner, R.M.; Petersen, S.R.; Smith, M.A. The use of extracorporeal membrane oxygenation in blunt thoracic trauma: A study of the Extracorporeal Life Support Organization database. J. Trauma Acute Care Surg. 2015, 79, 1049–1054. [Google Scholar] [CrossRef]
- Hunsicker, O.; Beck, L.; Krannich, A.; Finger, T.; Prinz, V.; Spies, C.; Weber-Carstens, S.; Wolf, S.; Graw, J.A.; Menk, M. Timing, Outcome and Risk Factors of Intracranial Hemorrhage in Acute Respiratory Distress Syndrome Patients During Venovenous Extracorporeal Membrane Oxygenation. Crit. Care Med. 2021, 49, e120–e129. [Google Scholar] [CrossRef]
- Ahmad, S.B.; Menaker, J.; Kufera, J.; O’Connor, J.; Scalea, T.M.; Stein, D.M. Extracorporeal membrane oxygenation after traumatic injury. J. Trauma Acute Care Surg. 2017, 82, 587–591. [Google Scholar] [CrossRef]
- Bonacchi, M.; Spina, R.; Torracchi, L.; Harmelin, G.; Sani, G.; Peris, A. Extracorporeal life support in patients with severe trauma: An advanced treatment strategy for refractory clinical settings. J. Thorac. Cardiovasc. Surg. 2013, 145, 1617–1626. [Google Scholar] [CrossRef]
- Lockey, D.J.; Coats, T.; Parr, M.J. Aspiration in severe trauma: A prospective study. Anaesthesia 1999, 54, 1097–1098. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, K.; Nemzek, J.; Napolitano, L.M.; Knight, P.R. Aspiration-Induced lung injury. Crit. Care Med. 2011, 39, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.A.; Sperry, J.L.; Rosengart, M.R.; Minei, J.P.; Harbrecht, B.G.; Moore, E.E.; Cuschieri, J.; Maier, R.V.; Billiar, T.R.; Peitzman, A.B. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J. Trauma 2009, 67, 221–227; discussion 228–230. [Google Scholar] [CrossRef] [PubMed]
- Van den Akker, T.A.; Grimes, Z.M.; Friedman, M.T. Transfusion-Associated Circulatory Overload and Transfusion-Related Acute Lung InjuryA Review of Underreported Entities with Current Updates. Am. J. Clin. Pathol. 2021, 156, 529–539. [Google Scholar] [CrossRef]
- Funk, M.B.; Guenay, S.; Lohmann, A.; Henseler, O.; Heiden, M.; Hanschmann, K.M.; Keller-Stanislawski, B. Benefit of transfusion-related acute lung injury risk-minimization measures—German haemovigilance data (2006–2010). Vox Sang. 2012, 102, 317–323. [Google Scholar] [CrossRef]
- Rhee, P.; Joseph, B.; Pandit, V.; Aziz, H.; Vercruysse, G.; Kulvatunyou, N.; Friese, R.S. Increasing Trauma Deaths in the United States. Ann. Surg. 2014, 260, 13–21. [Google Scholar] [CrossRef]




| A. Trauma- Associated ARDS n = 62 | B1. Non-Trauma- Associated ARDS (all) n = 976 | A vs. B1 p-Value | B2. Non-Trauma- Associated ARDS (matched) n = 62 | A vs. B2 p-Value | |
|---|---|---|---|---|---|
| Age (years) | 45.0 (27.0/60.0) | 53.0 (41.0/64.0) | 0.003 | 44.5 (27.0/58.0) | 0.383 |
| Sex (f/m) (n, %) | 11/51 (17.7/82.3) | 351/625 (36.0/64.0) | 0.004 | 11/51 (17.7/82.3) | 1.00 |
| SOFA at ICU admission | 11.0 (10.0/14.0) | 11.0 (8.0/14.0) | 0.778 | 11.0 (9.0/13.0) | 0.944 |
| Oxygenation Index (OI) | 11.9 (6.7/20.8) | 15.5 (9.8/23.5) | 0.009 | 12.3 (8.0/19.4) | 0.546 |
| Body mass index (kg/m2) | 26.3 (24.7/30.2) | 26.2 (22.9/31.3) | 0.449 | 26.1 (23.6/28.6) | 0.104 |
| APACHE * II | 24.0 (19.0/33.0) | 26.0 (19.0/33.0) | 0.404 | 25.5 (17.0/31.0) | 0.760 |
| TISS 28 | 51.0 (42.0/58.0) | 48.0 (41.0/56.0) | 0.077 | 47.0 (42.0/54.0) | 0.110 |
| SAPS II | 47.5 (37.0/63.0) | 55.0 (39.5/68.5) | 0.051 | 53.0 (38.0/64.0) | 0.053 |
| Glasgow Coma Scale (GCS) | 3.0 (3.0/3.0) | 3.0 (3.0/3.0) | 0.312 | 3.0 (3.0/3.0) | 1.00 |
| Septic shock (n,%) | 37 (59.7) | 430 (44.1) | 0.022 | 31 (50.0) | 0.458 |
| Injury Severity Score (ISS) | 29.0 (21.0/38.0) | ||||
| LOS ** in ICU of ARDS center (d) | 21.4 (10.2/37.3) | 17.0 (8.2/30.2) | 0.118 | 18.7 (12.1/39.3) | 0.853 |
| Charlson Comorbidity Index | 0.5 (0.0/2.0) | 3.0 (1.0/5.0) | <0.001 | 2.0 (0.0/5.0) | <0.001 |
| Cardiopulmonary resuscitation total (n,%) | 20 (32.3) | 270 (27.7) | 0.434 | 20 (32.3) | 1.00 |
| Cardiopulmonary resuscitation extern (n,%) | 11 (17.7) | 137 (14.0) | 0.418 | 9 (14.5) | 0.804 |
| A. Trauma- Associated ARDS n = 62 | B1. Non-Trauma- Associated ARDS (all) n = 976 | A vs. B1 p-Value | B2. Non-Trauma- Associated ARDS (matched) n = 62 | A vs. B2 p-Value | |
|---|---|---|---|---|---|
| a | |||||
| 28-day mortality (n,%) | 16 (25.8) | 299 (30.6) | 0.423 | 18 (29.0) | 0.845 |
| 60-day mortality (n,%) | 17 (27.4) | 373 (38.2) | 0.089 | 19 (30.6) | 0.845 |
| In-hospital mortality (n,%) | 18 (29.0) | 395 (40.5) | 0.074 | 21 (33.9) | 0.701 |
| b | |||||
| A. Trauma- Associated ARDS and ECLS n = 24 | B1. Non-Trauma- Associated ARDS and ECLS (all) n = 558 | A vs. B1 p-Value | B2. Non-Trauma- Associated ARDS and ECLS (matched) n= 29 | A vs. B2 p-Value | |
| 28-day mortality in ECLS patients (n,%) | 11 (45.8) | 205 (36.7) | 0.366 | 8 (27.6) | 0.124 |
| 60-day mortality in ECLS patients (n,%) | 11 (45.8) | 267 (47.8) | 0.846 | 8 (27.6) | 0.124 |
| In-hospital mortality in ECLS patients (n,%) | 12 (50.0) | 282 (50.5) | 0.959 | 9 (31.0) | 0.206 |
| A. Trauma- Associated ARDS n = 62 | B1. Non-Trauma- Associated ARDS (all) n = 976 | A vs. B1 p-Value | B2. Non-Trauma-Associated ARDS (matched) n = 62 | A vs. B2 p-Value | |
|---|---|---|---|---|---|
| Mild: PaO2/FiO2 201–300 mmHg, PEEP ≥5 cmH2O (n,%) | 8 (12.9) | 28 (2.9) | <0.001 | 2 (3.2) | 0.149 |
| Moderate: PaO2/FiO2 101–200 mmHg, PEEP ≥5 cmH2O (n,%) | 13 (21.0) | 139 (14.2) | 16 (25.8) | ||
| Severe: PaO2/FiO2 ≤100 mmHg, PEEP ≥5 cmH2O (n,%) or patient with ECLS | 41 (66.1) | 809 (82.9) | 44 (71.0) | ||
| Mechanical ventilation parameters in the first 6h after ICU admission | |||||
| Pmax (cm H2O) | 31.0 (25.5/36.0) | 34.0 (30.5/37.9) | 0.005 | 33.3 (30.6/37.0) | 0.864 |
| Pmean (cm H2O) | 20.5 (16.5/25.5) | 23.0 (20.0/26.0) | 0.026 | 22.0 (19.0/28.0) | 0.743 |
| PEEP * (cm H2O) | 14.7 (10.0/18.0) | 16.3 (13.6/19.0) | 0.019 | 16.4 (13.0/19.0) | 0.218 |
| Respiratory rate (n/min) | 19.5 (16.5/22.0) | 21.0 (18.0/24.0) | 0.007 | 21.0 (18.0/23.0) | 0.078 |
| Respiratory volume (L/min) | 9.2 (7.4/11.1) | 8.3 (6.3/10.4) | 0.019 | 8.8 (6.8/10.5) | 0.217 |
| FiO2 (%) | 74.0 (60.5/90.0) | 82.0 (70.0/94.0) | 0.017 | 77.0 (68.0/91.0) | 0.215 |
| Pulmonary compliance (mL/cm H2O) | 36.4 (30.0/52.3) | 30.7 (20.8/43.1) | <0.001 | 34.3 (24.8/44.9) | 0.172 |
| Pulmonary gas exchange in the first 6 h after ICU admission | |||||
| PaO2 (mmHg) | 114.3 (91.1/147.2) | 112.3 (83.3/152.2) | 0.454 | 122.7 (90.9/176.6) | 0.544 |
| PaCO2 (mmHg) | 44.0 (38.9/54.2) | 51.6 (42.7/62.4) | <0.001 | 49.2 (39.7/61.2) | 0.046 |
| pH value | 7.38 (7.28/7.44) | 7.32 (7.24/7.39) | 0.002 | 7.33 (7.26/7.41) | 0.131 |
| Mechanical ventilation, ECLS and supportive therapies | |||||
| ECLS patients total (n,%) | 24 (38.7) | 558 (57.2) | 0.005 | 29 (46.8) | 0.424 |
| ECMO (n,%) | 15 (24.2) | 402 (41.2) | 0.108 | 20 (32.3) | 0.271 |
| ECLA (n,%) | 8 (12.9) | 97 (9.9) | 6 (9.7) | ||
| ECMO and ECLA | 1 (1.6) | 59 (6.0) | 3 (4.8) | ||
| NO inhalation (n,%) | 36 (58.1) | 683 (70.0) | 0.049 | 38 (61.3) | 0.839 |
| Prone positioning (n,%) | 34 (54.8) | 661 (67.7) | 0.036 | 45 (72.6) | 0.052 |
| Prone positions per patient | 2.5 (2.0/7.0) | 3.0 (2.0/5.0) | 0.718 | 3.0 (2.0/6.0) | 0.473 |
| Side positioning 135° (n,%) | 15 (24.2) | 313 (32.1) | 0.196 | 21 (33.9) | 0.286 |
| Side positions 135° per patient | 1.0 (1.0/5.0) | 2.0 (1.0/5.0) | 0.288 | 2.0 (1.0/10.0) | 0.043 |
| Tracheotomy (n,%) | 50 (80.6) | 748 (76.6) | 0.468 | 48 (77.4) | 0.815 |
| Spontaneous breathing achieved (n, %) | 48 (77.4) | 683 (70.0) | 0.213 | 47 (75.8) | 1.00 |
| A. Trauma- Associated ARDS n = 62 | B1. Non-Trauma- Associated ARDS (all) n = 976 | A vs. B1 p-Value | B2. Non-Trauma-Associated ARDS (matched) n = 62 | A vs. B2 p-Value | |
|---|---|---|---|---|---|
| Number of patients who received transfusions | |||||
| Packed red blood cells transfused (n,%) | 52 (83.9) | 849 (87.0) | 0.482 | 55 (88.7) | 0.607 |
| FFP transfused (n,%) | 49 (79.0) | 722 (74.0) | 0.377 | 47 (75.8) | 0.824 |
| Platelet concentrates transfused (n,%) | 33 (53.2) | 426 (43.6) | 0.141 | 30 (48.4) | 0.720 |
| Number of units of transfusions in patients who received transfusions | |||||
| Packed red blood cells until day 7 per patient (n per patient) | 7.0 (4.0/13.0) | 6.0 (3.0/10.0) | 0.194 | 5.0 (2.0/9.0) | 0.005 |
| Packed red blood cells until day 14 per patient (n per patient) | 8.0 (6.0/19.5) | 8.0 (4.0/15.0) | 0.326 | 6.0 (3.0/11.0) | 0.014 |
| Packed red blood cells until day 28 per patient (n per patient) | 11.0 (6.0/27.0) | 10.0 (5.0/20.0) | 0.407 | 9.0 (4.0/18.0) | 0.040 |
| Packed red blood cells during ICU stay (n per patient) | 12.5 (6.0/28.0) | 11.0 (5.0/25.0) | 0.357 | 10.0 (4.5/21.5) | 0.099 |
| FFP during ICU stay (n per patient) | 28.0 (10.0/61.0) | 21.0 (8.0/46.0) | 0.170 | 19.0 (6.0/48.0) | 0.015 |
| Platelet concentrates (n per patient) | 6.0 (3.0/13.0) | 6.0 (2.0/13.0) | 0.962 | 4.0 (2.0/14.0) | 0.346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engelhardt, L.J.; Olbricht, C.; Niemann, M.; Graw, J.A.; Hunsicker, O.; Weiss, B.; Bünger, V.; Weber-Carstens, S.; Boie, S.D.; Piper, S.K.; et al. Outcome Comparison of Acute Respiratory Distress Syndrome (ARDS) in Patients with Trauma-Associated and Non-Trauma-Associated ARDS: A Retrospective 11-Year Period Analysis. J. Clin. Med. 2022, 11, 5734. https://doi.org/10.3390/jcm11195734
Engelhardt LJ, Olbricht C, Niemann M, Graw JA, Hunsicker O, Weiss B, Bünger V, Weber-Carstens S, Boie SD, Piper SK, et al. Outcome Comparison of Acute Respiratory Distress Syndrome (ARDS) in Patients with Trauma-Associated and Non-Trauma-Associated ARDS: A Retrospective 11-Year Period Analysis. Journal of Clinical Medicine. 2022; 11(19):5734. https://doi.org/10.3390/jcm11195734
Chicago/Turabian StyleEngelhardt, Lilian Jo, Claudio Olbricht, Marcel Niemann, Jan Adriaan Graw, Oliver Hunsicker, Björn Weiss, Victoria Bünger, Steffen Weber-Carstens, Sebastian Daniel Boie, Sophie K. Piper, and et al. 2022. "Outcome Comparison of Acute Respiratory Distress Syndrome (ARDS) in Patients with Trauma-Associated and Non-Trauma-Associated ARDS: A Retrospective 11-Year Period Analysis" Journal of Clinical Medicine 11, no. 19: 5734. https://doi.org/10.3390/jcm11195734
APA StyleEngelhardt, L. J., Olbricht, C., Niemann, M., Graw, J. A., Hunsicker, O., Weiss, B., Bünger, V., Weber-Carstens, S., Boie, S. D., Piper, S. K., Balzer, F., & Menk, M. (2022). Outcome Comparison of Acute Respiratory Distress Syndrome (ARDS) in Patients with Trauma-Associated and Non-Trauma-Associated ARDS: A Retrospective 11-Year Period Analysis. Journal of Clinical Medicine, 11(19), 5734. https://doi.org/10.3390/jcm11195734