Post-Traumatic Headache: A Review of Prevalence, Clinical Features, Risk Factors, and Treatment Strategies
Abstract
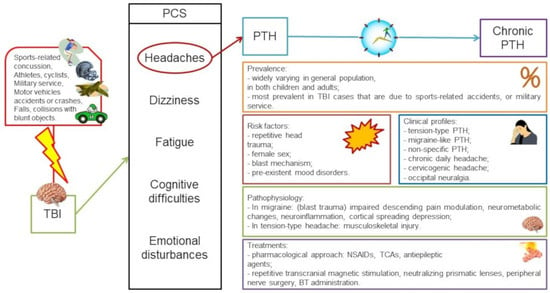
:1. Introduction
2. Prevalence of Post-Traumatic Headaches
3. Clinical Profiles of Chronic Post-Traumatic Headache
4. Diagnosis of Post-Traumatic Headache
5. Treatment
5.1. Pharmacological Treatments
5.2. Repetitive Transcranial Magnetic Stimulation
5.3. Neutralizing Prismatic Lenses
5.4. Peripheral Nerve Surgery
5.5. Botulinum Toxin
6. Prognosis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucas, S.; Blume, H.K. Sport-Related Headache. Neurol. Clin. 2017, 35, 501–521. [Google Scholar] [CrossRef] [PubMed]
- McCrory, P.R.; Ariens, T.; Berkovic, S.F. The nature and duration of acute concussive symptoms in Australian football. Clin. J. Sport Med. 2000, 10, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Zemek, R.; Barrowman, N.; Freedman, S.B.; Gravel, J.; Gagnon, I.; McGahern, C.; Aglipay, M.; Sangha, G.; Boutis, K.; Beer, D.; et al. Pediatric Emergency Research Canada (PERC) Concussion Team. Clinical Risk Score for Persistent Postconcussion Symptoms Among Children With Acute Concussion in the ED. JAMA 2016, 315, 1014–1025, Erratum in JAMA 2016, 315, 2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theeler, B.J.; Flynn, F.G.; Erickson, J.C. Chronic daily headache in U.S. soldiers after concussion. Headache 2012, 52, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Maleki, N.; Finkel, A.; Cai, G.; Ross, A.; Moore, R.D.; Feng, X.; Androulakis, X.M. Post-traumatic headache: Epidemiology and pathophysiological insights. Nat. Rev. Neurol. 2019, 15, 607. [Google Scholar]
- Ladak, A.A.; Enam, S.A.; Ibrahim, M.T. A Review of the Molecular Mechanisms of Traumatic Brain Injury. World Neurosurg. 2019, 131, 126. [Google Scholar]
- Shina, H.; Iljazi, A.; Al-Khazali, H.M.; Christensen, C.E.; Amin, F.M.; Ashina, M.; Schytz, H.W. Hypersensitivity to Calcitonin Gene-Related Peptide in Post-Traumatic Headache. Ann. Neurol. 2020, 88, 1220. [Google Scholar]
- Triplett, G.; Hill, C.; Freeman, L.; Rajan, U.; Templer, D.I. Incidence of head injury: Lasting effects among college students and working adults in the general population. Percept. Mot. Ski. 1996, 83 Pt 2, 1344–1346. [Google Scholar] [CrossRef]
- Charles, A.; Pozo-Rosich, P. Targeting calcitonin gene-related peptide: A new era in migraine therapy. Lancet 2019, 394, 1765. [Google Scholar]
- Ashina, H.; Iljazi, A.; Al-Khazali, H.M.; Eigenbrodt, A.K.; Larsen, E.L.; Andersen, A.M.; Hansen, K.J.; Bräuner, K.B.; Mørch-Jessen, T.; Chaudhry, B.; et al. Efficacy, tolerability, and safety of erenumab for the preventive treatment of persistent post-traumatic headache attributed to mild traumatic brain injury: An open-label study. J. Headache Pain 2020, 21, 62. [Google Scholar]
- Vanderploeg, R.D.; Belanger, H.G.; Horner, R.D.; Spehar, A.M.; Powell-Cope, G.; Luther, S.L.; Scott, S.G. Health outcomes associated with military deployment: Mild traumatic brain injury.; blast.; trauma.; and combat associations in the Florida National Guard. Arch. Phys. Med. Rehabil. 2012, 93, 1887–1895. [Google Scholar] [CrossRef]
- Andersen, A.M.; Ashina, H.; Iljazi, A.; Al-Khazali, H.M.; Chaudhry, B.; Ashina, M.; Ashina, S.; Schytz, H.W. Risk Factors for the Development of Post-Traumatic Headache Attributed to Traumatic Brain Injury: A Systematic Review. Headache 2020, 60, 1066. [Google Scholar]
- Lucas, S.; Hoffman, J.M.; Bell, K.R.; Dikmen, S. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 2014, 34, 93. [Google Scholar]
- Yilmaz, T.; Roks, G.; de Koning, M.; Scheenen, M.; van der Horn, H.; Plas, G.; Hageman, G.; Schoonman, G.; Spikman, J.; van der Naalt, J. Risk factors and outcomes associated with post-traumatic headache after mild traumatic brain injury. Emerg. Med. J. 2017, 34, 800. [Google Scholar]
- Hoffman, J.M.; Lucas, S.; Dikmen, S.; Braden, C.A.; Brown, A.W.; Brunner, R.; Diaz-Arrastia, R.; Walker, W.C.; Watanabe, T.K.; Bell, K.R. Natural history of headache after traumatic brain injury. J. Neurotrauma 2011, 28, 1719–1725. [Google Scholar] [CrossRef] [Green Version]
- Stacey, A.; Lucas, S.; Dikmen, S.; Temkin, N.; Bell, K.R.; Brown, A.; Brunner, R.; Diaz-Arrastia, R.; Watanabe, T.K.; Weintraub, A.; et al. Natural History of Headache Five Years after Traumatic Brain Injury. J. Neurotrauma 2017, 34, 1558–1564. [Google Scholar]
- Sawyer, K.; Bell, K.R.; Ehde, D.M.; Temkin, N.; Dikmen, S.; Williams, R.M.; Dillworth, T.; Hoffman, J.M. Longitudinal Study of Headache Trajectories in the Year After Mild Traumatic Brain Injury: Relation to Posttraumatic Stress Disorder Symptoms. Arch. Phys. Med. Rehabil. 2015, 96, 2000. [Google Scholar]
- Babcock, L.; Byczkowski, T.; Wade, S.L.; Ho, M.; Mookerjee, S.; Bazarian, J.J. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA Pediatr. 2013, 167, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Root, J.M.; Gai, J.; Sady, M.D.; Vaughan, C.G.; Madati, P.J. Identifying Risks for Persistent Postconcussive Symptoms in a Pediatric Emergency Department: An Examination of a Clinical Risk Score. Arch. Clin. Neuropsychol. 2022, 37, 30–39. [Google Scholar] [CrossRef]
- Guty, E.; Riegler, K.; Meyer, J.; Walter, A.E.; Slobounov, S.M.; Arnett, P. Symptom Factors and Neuropsychological Performance in Collegiate Athletes with Chronic Concussion Symptoms. Arch. Clin. Neuropsychol. 2021, 36, 746–756. [Google Scholar] [CrossRef]
- Ahman, S.; Saveman, B.I.; Styrke, J.; Björnstig, U.; Stålnacke, B.M. Long-term follow-up of patients with mild traumatic brain injury: A mixed-method study. J. Rehabil. Med. 2013, 45, 758–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.H.; Bowles, A.O.; Kennedy, J.E.; Eapen, B.C.; Cooper, D.B. Single-Item Versus Multiple-Item Headache Ratings in Service Members Seeking Treatment for Brain Injury. Mil. Med. 2020, 185, e43–e46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilk, J.E.; Thomas, J.L.; McGurk, D.M.; Riviere, L.A.; Castro, C.A.; Hoge, C.W. Mild traumatic brain injury (concussion) during combat: Lack of association of blast mechanism with persistent postconcussive symptoms. J. Head Trauma Rehabil. 2010, 25, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wilk, J.E.; Herrell, R.K.; Wynn, G.H.; Riviere, L.A.; Hoge, C.W. Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: Association with postdeployment symptoms. Psychosom. Med. 2012, 74, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ferdosi, H.; Schwab, K.A.; Metti, A.; Brenner, L.A.; Terrio, H.; Pazdan, R.M.; Cole, W.R.; Scher, A.I. Trajectory of Postconcussive Symptoms 12 Months After Deployment in Soldiers With and Without Mild Traumatic Brain Injury: Warrior Strong Study. Am. J. Epidemiol. 2019, 188, 77–86, Erratum in Am. J. Epidemiol. 2019, 188, 260. [Google Scholar] [CrossRef]
- Womble, M.N.; McAllister-Deitrick, J.; Marchetti, G.F.; Reynolds, E.; Collins, M.W.; Elbin, R.J.; Kontos, A.P. Risk Factors for Vestibular and Oculomotor Outcomes After Sport-Related Concussion. Clin. J. Sport Med. 2021, 31, e193–e199. [Google Scholar] [CrossRef]
- Kraemer, Y.; Mäki, K.; Marinkovic, I.; Nybo, T.; Isokuortti, H.; Huovinen, A.; Korvenoja, A.; Melkas, S.; Harno, H. Post-traumatic headache after mild traumatic brain injury in a one-year follow up study-risk factors and return to work. J. Headache Pain 2022, 23, 27. [Google Scholar] [CrossRef]
- Ingebrigtsen, T.; Waterloo, K.; Marup-Jensen, S.; Attner, E.; Romner, B. Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J. Neurol. 1998, 245, 609–612. [Google Scholar] [CrossRef]
- Aita, S.L.; Schuler, K.R.; Isaak, S.L.; Borgogna, N.C.; Moncrief, G.G.; Hollis, S.D.; Hill, B.D. Posttraumatic Stress Disorder Complicated by Traumatic Brain Injury: A Narrative Review. SN Compr. Clin. Med. 2023, 5, 1–14. [Google Scholar] [CrossRef]
- Nampiaparampil, D.E. Prevalence of chronic pain after traumatic brain injury: A systematic review. JAMA 2008, 300, 711. [Google Scholar]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar]
- Cnossen, M.C.; van der Naalt, J.; Spikman, J.M.; Nieboer, D.; Yue, J.K.; Winkler, E.A.; Manley, G.T.; von Steinbuechel, N.; Polinder, S.; Steyerberg, E.W.; et al. Prediction of Persistent Post-Concussion Symptoms after Mild Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2691–2698. [Google Scholar]
- Theeler, B.J.; Flynn, F.G.; Erickson, J.C. Headaches after concussion in US soldiers returning from Iraq or Afghanistan. Headache 2010, 50, 1262–1272. [Google Scholar] [CrossRef]
- Voormolen, D.C.; Haagsma, J.A.; Polinder, S.; Maas, A.I.; Steyerberg, E.W.; Vuleković, P.; Sewalt, C.A.; Gravesteijn, B.Y.; Covic, A.; Andelic, N.; et al. Post-Concussion Symptoms in Complicated vs. Uncomplicated Mild Traumatic Brain Injury Patients at Three and Six Months Post-Injury: Results from the CENTER-TBI Study. J. Clin. Med. 2019, 8, 1921. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Pi, H.; Ma, L.; Su, X.; Wang, J. Incidence of Headache After Traumatic Brain Injury in China: A Large Prospective Study. World Neurosurg. 2016, 88, 289–296. [Google Scholar]
- Aaseth, K.; Grande, R.; Kvárner, K.; Gulbrandsen, P.; Lundqvist, C.; Russell, M. Prevalence of Secondary Chronic Headaches in a Population-Based Sample of 30-44-Year-Old Persons. The Akershus Study of Chronic Headache. Cephalalgia 2008, 28, 705–713. [Google Scholar]
- Rasmussen, B.K.; Olesen, J. Symptomatic and nonsymptomatic headaches in a general population. Neurology 1992, 42, 1225. [Google Scholar] [CrossRef]
- Dondi, A.; Biserni, G.B.; Scarpini, S.; Fetta, A.; Moscano, F.; Corsini, I.; Borelli, G.; Cordelli, D.M.; Lanari, M. Post-Traumatic Headache in Children after Minor Head Trauma: Incidence, Phenotypes, and Risk Factors. Children 2023, 10, 534. [Google Scholar] [CrossRef]
- van Ierssel, J.J.; Tang, K.; Beauchamp, M.; Bresee, N.; Cortel-LeBlanc, A.; Craig, W.; Doan, Q.; Gravel, J.; Lyons, T.; Mannix, R.; et al. Association of Posttraumatic Headache With Symptom Burden After Concussion in Children. JAMA Netw. Open 2023, 6, e231993. [Google Scholar]
- McConnell, B.; Duffield, T.; Hall, T.; Piantino, J.; Seitz, D.; Soden, D.; Williams, C. Post-traumatic Headache After Pediatric Traumatic Brain Injury: Prevalence, Risk Factors, and Association With Neurocognitive Outcomes. J. Child Neurol. 2020, 35, 63–70. [Google Scholar] [CrossRef]
- Kjeldgaard, D.; Forchhammer, H.B.; Teasdale, T.W.; Jensen, R.H. Cognitive behavioural treatment for the chronic post-traumatic headache patient: A randomized controlled trial. J. Headache Pain 2014, 15, 81. [Google Scholar] [PubMed]
- Bree, D.; Stratton, J.; Levy, D. Increased severity of closed head injury or repetitive subconcussive head impacts enhances post-traumatic headache-like behaviors in a rat model. Cephalalgia 2020, 40, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Ashina, H.; Eigenbrodt, A.K.; Seifert, T.; Sinclair, A.J.; I Scher, A.; Schytz, H.W.; Lee, M.J.; De Icco, R.; Finkel, A.G.; Ashina, M. Post-traumatic headache attributed to traumatic brain injury: Classification, clinical characteristics, and treatment. Lancet Neurol. 2021, 20, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.R.; Rinaldi, R.; Didehbani, N. Chapter 6—Assessment and Management of Sports Concussion. In Rehabilitation after Traumatic Brain Injury; Eapen, B.C., Cifu, D.X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 59–71. ISBN 9780323544566. [Google Scholar] [CrossRef]
- Guidry, A.; Crutchfield, K. Chapter 41—Athletes with neurologic disease. In Handbook of Clinical Neurology; Hainline, B., Stern, R.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 158, pp. 445–462. ISBN 9780444639547. [Google Scholar] [CrossRef]
- Lucas, L. Chapter 13—Post-Traumatic Headache. In Headache and Migraine Biology and Management; Diamond, S., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 161–174. ISBN 9780128009017. [Google Scholar] [CrossRef]
- Ashina, H.; Iljazi, A.; Al-Khazali, H.M.; Ashina, S.; Jensen, R.H.; Amin, F.M.; Ashina, M.; Schytz, H.W. Persistent post-traumatic headache attributed to mild traumatic brain injury: Deep phenotyping and treatment patterns. Cephalalgia 2020, 40, 554–564. [Google Scholar] [PubMed]
- Haas, D.C. Chronic post-traumatic headaches classified and compared with natural headaches. Cephalalgia 1996, 16, 486. [Google Scholar]
- Baandrup, L.; Jensen, R. Chronic post-traumatic headache--a clinical analysis in relation to the International Headache Classification 2nd Edition. Cephalalgia 2005, 25, 132. [Google Scholar]
- Vargas, B.B. Posttraumatic headache in combat soldiers and civilians: What factors influence the expression of tension-type versus migraine headache? Curr. Pain Headache Rep. 2009, 13, 470. [Google Scholar]
- Tad, S. Migraine with aura is the predominant phenotype among acute post-traumatic headache in sports. Neurol. Dec. 2018, 91 (Suppl. 1), S28–S29. [Google Scholar] [CrossRef] [Green Version]
- Couch, J.R.; Bearss, C. Chronic daily headache in the post-trauma syndrome: Relation to extent of head injury. Headache 2001, 41, 559. [Google Scholar] [CrossRef]
- Formisano, R.; Angelini, A.; De Vuono, G.; Calisse, P.; Fiacco, F.; Catarci, T.; Bozzao, L.; Cerbo, R. Cluster-like headache and head injury: Case report. Neurol. Sci. 1990, 11, 303–305. [Google Scholar] [CrossRef]
- Jacob, S.; Saha, A.; Rajabally, Y. Post-traumatic short-lasting unilateral headache with cranial autonomic symptoms (SUNA). Cephalalgia 2008, 28, 991. [Google Scholar] [CrossRef]
- Matharu, M.J.; Goadsby, P.J. Post-traumatic chronic paroxysmal hemicrania (CPH) with aura. Neurology 2001, 56, 273. [Google Scholar] [CrossRef]
- Lay, C.L.; Newman, L.C. Posttraumatic hemicrania continua. Headache 1999, 39, 275. [Google Scholar]
- Lieba-Samal, D.; Platzer, P.; Seidel, S.; Klaschterka, P.; Knopf, A.; Wöber, C. Characteristics of acute posttraumatic headache following mild head injury. Cephalalgia 2011, 31, 1618–1626. [Google Scholar]
- Levy, D.; Gruener, H.; Riabinin, M.; Feingold, Y.; Schreiber, S.; Pick, C.G.; Defrin, R. Different clinical phenotypes of persistent post-traumatic headache exhibit distinct sensory profiles. Cephalalgia 2019, 40, 675–688. [Google Scholar]
- Ducic, I.; Sinkin, J.C.; Crutchfield, K.E. Interdisciplinary treatment of post-concussion and post-traumatic headaches. Microsurgery 2015, 35, 603–607. [Google Scholar] [CrossRef]
- Seifert, T. Post-Traumatic Headache Therapy in the Athlete. Curr. Pain Headache Rep. 2016, 20, 41. [Google Scholar] [CrossRef]
- Shaw, L.; Morozova, M.; Abu-Arafeh, I. Chronic post-traumatic headache in children and adolescents: Systematic review of prevalence and headache features. Pain Manag. 2018, 8, 57–64. [Google Scholar] [CrossRef]
- Anonymous. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1. [Google Scholar]
- Langer, L.K.; Bayley, M.T.; Lawrence, D.W.; Comper, P.; Kam, A.; Tam, A.; Saverino, C.; Wiseman-Hakes, C.; Ruttan, L.; Chandra, T.; et al. Revisiting the ICHD-3 criteria for headache attributed to mild traumatic injury to the head: Insights from the Toronto Concussion Study Analysis of Acute Headaches Following Concussion. Cephalalgia 2022, 42, 1172–1183. [Google Scholar] [CrossRef]
- Schwedt, T.J. Structural and Functional Brain Alterations in Post-traumatic Headache Attributed to Mild Traumatic Brain Injury: A Narrative Review. Front. Neurol. 2019, 10, 615. [Google Scholar] [PubMed] [Green Version]
- Chong, C.D.; Berisha, V.; Chiang, C.; Ross, K.; Schwedt, T.J. Less Cortical Thickness in Patients With Persistent Post-Traumatic Headache Compared With Healthy Controls: An MRI Study. Headache 2017, 58, 53–61. [Google Scholar] [PubMed]
- Dumkrieger, G.; Chong, C.D.; Ross, K.; Berisha, V.; Schwedt, T.J. Static and dynamic functional connectivity differences between migraine and persistent post-traumatic headache: A resting-state magnetic resonance imaging study. Cephalalgia 2019, 39, 1366–1381. [Google Scholar] [PubMed]
- Schwedt, T.J.; Chong, C.D.; Peplinski, J.; Ross, K.; Berisha, V. Persistent post-traumatic headache vs. migraine: An MRI study demonstrating differences in brain structure. J. Headache Pain 2017, 18, 1–8. [Google Scholar]
- Chong, C.D.; Peplinski, J.; Berisha, V.; Ross, K.; Schwedt, T.J. Differences in fibertract profiles between patients with migraine and those with persistent post-traumatic headache. Cephalalgia 2019, 39, 1121–1133. [Google Scholar]
- De Kruijk, J.R.; Leffers, P.; A Menheere, P.P.C.; Meerhoff, S.; Rutten, J.; Twijnstra, A. Prediction of post-traumatic complaints after mild traumatic brain injury: Early symptoms and biochemical markers. J. Neurol. Neurosurg. Psychiatry 2002, 73, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Ashina, H.; Al-Khazali, H.M.; Iljazi, A.; Ashina, S.; Jørgensen, N.R.; Amin, F.M.; Ashina, M.; Schytz, H.W. Low plasma levels of calcitonin gene-related peptide in persistent post-traumatic headache attributed to mild traumatic brain injury. Cephalalgia 2020, 40, 1276–1282. [Google Scholar] [CrossRef]
- Domenichiello, A.F.; Jensen, J.R.; Zamora, D.; Horowitz, M.; Yuan, Z.-X.; Faurot, K.; Mann, J.D.; Mannes, A.J.; Ramsden, C.E. Identifying oxidized lipid mediators as prognostic biomarkers of chronic posttraumatic headache. Pain 2020, 161, 2775–2785. [Google Scholar]
- Friedman, D.I. Headaches Due to Low and High Intracranial Pressure. Continuum 2018, 24, 1066. [Google Scholar]
- Chen, J.C.; Levy, M.L. Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg. Clin. N. Am. 2000, 11, 399. [Google Scholar]
- Ledoux, A.; Tang, K.; Freedman, S.B.; Gravel, J.; Boutis, K.; Yeates, K.O.; Mannix, R.C.; Richer, L.R.; Bell, M.J.; Zemek, R.L. Pediatric Emergency Research Canada 5P Study Group. Early analgesic administration and headache presence 7 days post-concussion in children. Can. J. Emerg. Med. 2022, 24, 876–884. [Google Scholar] [CrossRef]
- Cushman, D.M.; Borowski, L.; Hansen, C.; Hendrick, J.; Bushman, T.; Teramoto, M. Gabapentin and Tricyclics in the Treatment of Post-Concussive Headache.; a Retrospective Cohort Study. Headache 2019, 59, 371–382. [Google Scholar] [CrossRef]
- Erickson, J.C. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: An observational study. Headache 2011, 51, 932–944. [Google Scholar] [CrossRef]
- Mollica, A.; Safavifar, F.; Fralick, M.; Giacobbe, P.; Lipsman, N.; Burke, M.J. Transcranial Magnetic Stimulation for the Treatment of Concussion: A Systematic Review. Neuromodulation 2021, 24, 803–812. [Google Scholar] [CrossRef]
- Rosner, M.S.; Feinberg, D.L.; Doble, J.E.; Rosner, A.J. Treatment of vertical heterophoria ameliorates persistent post-concussive symptoms: A retrospective analysis utilizing a multi-faceted assessment battery. Brain Inj. 2016, 30, 311–317. [Google Scholar] [CrossRef]
- Lippert-Grüner, M. Botulinum toxin in the treatment of post-traumatic headache-case study. Neurol. Neurochir. Pol. 2012, 46, 591–594. [Google Scholar] [CrossRef] [Green Version]
- Yerry, J.A.; Kuehn, D.; Finkel, A.G. Onabotulinum toxin a for the treatment of headache in service members with a history of mild traumatic brain injury: A cohort study. Headache 2015, 55, 395–406. [Google Scholar] [CrossRef]
- Klein, S.K.; Brown, C.B.; Ostrowski-Delahanty, S.; Bruckman, D.; Victorio, M.C. Identifying Migraine Phenotype Post Traumatic Headache (MPTH) to Guide Overall Recovery From Traumatic Brain Injury. J. Child Neurol. 2022.
- Carroll, L.; Cassidy, J.D.; Peloso, P.; Borg, J.; von Holst, H.; Holm, L.; Paniak, C.; Pépin, M. Prognosis for mild traumatic brain injury: Results of the who collaborating centre task force on mild traumatic brain injury. J. Rehabil. Med. 2004, 36, 84–105. [Google Scholar] [CrossRef] [Green Version]
- Kashluba, S.; Paniak, C.; Blake, T.; Reynolds, S.; Toller-Lobe, G.; Nagy, J. A longitudinal, controlled study of patient complaints following treated mild traumatic brain injury. Arch. Clin. Neuropsychol. 2004, 19, 805–816. [Google Scholar] [CrossRef] [Green Version]
- Lane, R.; Davies, P. Post traumatic headache (PTH) in a cohort of UK compensation claimants. Cephalalgia 2019, 39, 641. [Google Scholar] [CrossRef] [PubMed]
- Ruff, R.L.; Blake, K. Pathophysiological links between traumatic brain injury and post-traumatic headaches. F1000Research 2016, 5. [Google Scholar] [CrossRef] [Green Version]
| Pathophysiology Features | Description |
|---|---|
| Impaired descending pain modulation [5] | Abnormalities in the central nervous system’s pain control system leading to an increase in pain sensitivity. |
| Neurometabolic changes [5] | Changes in the brain’s metabolic activity, including an increase in lactate and a decrease in glucose metabolism, leading to a cascade of neuroinflammatory events. |
| Neuroinflammation [5] | A response to the injury that leads to an increase in pro-inflammatory cytokines and chemokines and activation of microglia and astrocytes. |
| Cortical spreading depression [5] | A wave of depolarization that spreads across the cortex leading to the release of inflammatory molecules and a decrease in cerebral blood flow. |
| Release of calcitonin gene-related peptide (CGRP) [5] | A neuropeptide that may mediate trigeminovascular pain transmission and trigger a migraine attack. |
| Trigeminal system activation [5] | Activation of the trigeminal nerve, which is implicated in migraine and other primary headache disorders. |
| Secondary cascade of metabolic and cellular excitotoxic and inflammatory changes [6] | TBI can lead to a secondary cascade of events that can promote the development of PTH. |
| Hypersensitivity to CGRP [9,11] | Patients with PTH who have no prior history of migraine exhibit hypersensitivity to CGRP. |
| Erenumab treatment [10] | A CGRP receptor antagonist that has been shown to reduce the number of days with moderate or severe headache in patients with PTH. |
| Risk Factor | Description |
|---|---|
| Headache at injury | Early post-injury headache or a previous history of headache are positively associated with prolonged PCS and persistent PTHs [18,19,20]. |
| Female sex | Female sex is a risk factor for PTHs [21,22]. |
| Pre-existing conditions | Pre-existing conditions, such as migraines, are risk factors for PTHs [22]. |
| Blast mechanism | Loss of consciousness due to a blast mechanism of concussion is associated with an increased risk of headaches [23]. |
| Multiple mTBIs | Multiple mTBIs with loss of consciousness are associated with an increased risk of headaches [24,25]. |
| PTSD and depression | There is a link between PTSD, depression, and PTHs [8,11,23,24]. |
| Post-traumatic migraine symptoms | Early symptoms indicative of post-traumatic migraines are associated with PTHs [26]. |
| Insomnia | Insomnia was reported as a risk factor for PTHs [27]. |
| Vertigo | Vertigo was reported as a risk factor for PTHs [27]. |
| Older age | Older age was reported as a risk factor for PTHs [27]. |
| Somatic pain | Somatic pain was reported as a risk factor for PTHs [27]. |
| Clinical Features of Post-Traumatic Headache (PTH) |
|---|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavroudis, I.; Ciobica, A.; Luca, A.C.; Balmus, I.-M. Post-Traumatic Headache: A Review of Prevalence, Clinical Features, Risk Factors, and Treatment Strategies. J. Clin. Med. 2023, 12, 4233. https://doi.org/10.3390/jcm12134233
Mavroudis I, Ciobica A, Luca AC, Balmus I-M. Post-Traumatic Headache: A Review of Prevalence, Clinical Features, Risk Factors, and Treatment Strategies. Journal of Clinical Medicine. 2023; 12(13):4233. https://doi.org/10.3390/jcm12134233
Chicago/Turabian StyleMavroudis, Ioannis, Alin Ciobica, Alina Costina Luca, and Ioana-Miruna Balmus. 2023. "Post-Traumatic Headache: A Review of Prevalence, Clinical Features, Risk Factors, and Treatment Strategies" Journal of Clinical Medicine 12, no. 13: 4233. https://doi.org/10.3390/jcm12134233
APA StyleMavroudis, I., Ciobica, A., Luca, A. C., & Balmus, I. -M. (2023). Post-Traumatic Headache: A Review of Prevalence, Clinical Features, Risk Factors, and Treatment Strategies. Journal of Clinical Medicine, 12(13), 4233. https://doi.org/10.3390/jcm12134233