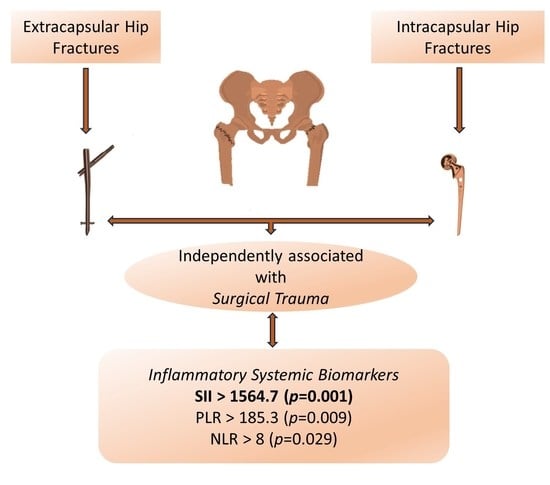
Correlation between Inflammatory Systemic Biomarkers and Surgical Trauma in Elderly Patients with Hip Fractures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection
2.3. Inflammatory Systemic Biomarkers
2.4. Surgical Technique and Postoperative Care
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schemitsch, E.H.; Miclau, T.; Karachalios, T.; Nowak, L.L.; Sancheti, P.; Poolman, R.W.; Caminis, J.; Daizadeh, N.; Dent-Acosta, R.E.; Egbuna, O.; et al. A Randomized, Placebo-Controlled Study of Romosozumab for the Treatment of Hip Fractures. J. Bone Jt. Surg. Am. 2020, 102, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Dhanwal, D.K.; Dennison, E.M.; Harvey, N.C.; Cooper, C. Epidemiology of hip fracture: Worldwide geographic variation. Indian J. Orthop. 2011, 45, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Maleitzke, T.; Eder, C.; Ahmad, S.; Stöckle, U.; Braun, K.F. Management of proximal femur fractures in the elderly: Current concepts and treatment options. Eur. J. Med. Res. 2021, 26, 86. [Google Scholar] [CrossRef]
- Trejo-Ávila, M.E.; Valenzuela-Salazar, C.; Betancourt-Ferreyra, J.; Fernández-Enríquez, E.; Romero-Loera, S.; Moreno-Portillo, M. Laparoscopic Versus Open Surgery for Abdominal Trauma: A Case-Matched Study. J. Laparoendosc. Adv. Surg. Tech. A 2017, 27, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kong, L.; Kou, N.; Wang, Y.; Yu, K.; Bai, J.; Tian, D. The comparison of limited-incision versus standard-incision in treatment of carpal tunnel syndrome: A meta-analysis of randomized controlled trials. Medicine 2019, 98, e15372. [Google Scholar] [CrossRef]
- Chang, W.; Zhang, D.; Liu, W.; Lian, X.; Jiao, Z.; Chen, W. Posterior paraspinal muscle versus post-middle approach for the treatment of thoracolumbar burst fractures: A randomized controlled trial. Medicine 2018, 97, e11193. [Google Scholar] [CrossRef]
- Foy, B.H.; Sundt, T.; Carlson, J.C.T.; Aguirre, A.D.; Higgins, J.M. White Blood Cell and Platelet Dynamics Define Human Inflammatory Recovery. Nat. Commun. 2022, 13, 4705. [Google Scholar] [CrossRef]
- Gros, A.; Ollivier, V.; Ho-Tin-Noé, B. Platelets in inflammation: Regulation of leukocyte activities and vascular repair. Front. Immunol. 2015, 5, 678. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Ismail, A.M.; Ahmed Ali, M.; Ismail, F.H. Leukocyte Count as a Predictor of Severity of Injury in Pediatric Blunt Abdominal Trauma. World J. Surg. Surg. Res. 2019, 2, 1107. [Google Scholar]
- Kovtun, A.; Messerer, D.A.C.; Scharffetter-Kochanek, K.; Huber-Lang, M.; Ignatius, A. Neutrophils in Tissue Trauma of the Skin, Bone, and Lung: Two Sides of the Same Coin. J. Immunol. Res. 2018, 2018, 8173983. [Google Scholar] [CrossRef]
- Baht, G.S.; Vi, L.; Alman, B.A. The Role of the Immune Cells in Fracture Healing. Curr. Osteoporos. Rep. 2018, 16, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.Z.; Zhao, X.J.; Deng, J.X.; Du, Z.; Wang, T.B.; Zhu, F.X. Early changes within the lymphocyte population are associated with the long term prognosis in severely injured patients. Beijing Da Xue Xue Bao Yi Xue Ban 2022, 54, 552–556. [Google Scholar] [PubMed]
- Wu, L.; Zou, S.; Wang, C.; Tan, X.; Yu, M. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in Chinese Han population from Chaoshan region in South China. BMC Cardiovasc. Disord. 2019, 19, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelesoglu Dincer, A.B.; Sezer, S. Systemic Immune Inflammation Index as a Reliable Disease Activity Marker in Psoriatic Arthritis. J. Coll. Physicians Surg. Pak. 2022, 32, 773–778. [Google Scholar]
- Hernandez-Ainsa, M.; Velamazan, R.; Lanas, A.; Carrera-Lasfuentes, P.; Piazuelo, E. Blood-Cell-Based Inflammatory Markers as a Useful Tool for Early Diagnosis in Colorectal Cancer. Front. Med. 2022, 9, 843074. [Google Scholar] [CrossRef]
- Kamiya, N.; Ishikawa, Y.; Kotani, K.; Hatakeyama, S.; Matsumura, M. Monocyte-to-Lymphocyte Ratio in the Diagnosis of Lymphoma in Adult Patients. Int. J. Gen. Med. 2022, 15, 4221–4226. [Google Scholar] [CrossRef] [PubMed]
- Tzikos, G.; Alexiou, I.; Tsagkaropoulos, S.; Menni, A.-E.; Chatziantoniou, G.; Doutsini, S.; Papavramidis, T.; Grosomanidis, V.; Stavrou, G.; Kotzampassi, K. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Predictive Factors for Mortality and Length of Hospital Stay after Cardiac Surgery. J. Pers. Med. 2023, 13, 473. [Google Scholar] [CrossRef] [PubMed]
- Parmana, I.M.A.; Boom, C.E.; Poernomo, H.; Gani, C.; Nugroho, B.; Cintyandy, R.; Sanjaya, L.; Hadinata, Y.; Parna, D.R.; Hanafy, D.A. Systemic Immune-Inflammation Index Predicts Prolonged Mechanical Ventilation and Intensive Care Unit Stay After off-Pump Coronary Artery Bypass Graft Surgery: A Single-Center Retrospective Study. Vasc. Health Risk Manag. 2023, 19, 353–361. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, Y.; Chen, W.; Zhang, Y. Are postoperative NLR and PLR associated with the magnitude of surgery-related trauma in young and middle-aged patients with bicondylar tibial plateau fractures? A retrospective study. BMC Musculoskelet Disord. 2021, 22, 816. [Google Scholar] [CrossRef]
- Moldovan, F.; Gligor, A.; Moldovan, L.; Bataga, T. An Investigation for Future Practice of Elective Hip and Knee Arthroplasties during COVID-19 in Romania. Medicina 2023, 59, 314. [Google Scholar] [CrossRef]
- Mikolajczyk, T.; Moldovan, L.; Chalupczak, A.; Moldovan, F. Computer aided learning process. Procedia Eng. 2017, 181, 1028–1035. [Google Scholar] [CrossRef]
- Mikolajczyk, T.; Moldovan, F.; Ciobanu, I.; Chalupczak, A.; Marin, A.G. Brain research Using Computer Test. Procedia Technol. 2016, 22, 1113–1120. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Tian, S.; Zhao, K.; Zhang, R.; Yin, Y.; Zhu, Y.; Hou, Z.; Zhang, Y. Neutrophil to lymphocyte ratio and fracture severity in young and middle-aged patients with tibial plateau fractures. Int. Orthop. 2020, 44, 2769–2777. [Google Scholar] [CrossRef]
- Zhou, W.; Mao, Z.; Wang, Z.; Zhu, H.; Zhao, Y.; Zhang, Z.; Zeng, Y.; Li, M. Diagnostic and Predictive Value of Novel Inflammatory Markers of the Severity of Acute Traumatic Spinal Cord Injury: A Retrospective Study. World Neurosurg. 2023, 171, e349–e354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Chen, J.; Wang, J.; Wang, S.; Xia, J.; Wei, Y.; Wu, J.; Huang, G.; Chen, F.; Shi, J.; et al. Predictive values of the postoperative neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio for the diagnosis of early periprosthetic joint infections: A preliminary study. J. Orthop. Surg Res. 2020, 15, 571. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Zhang, Z.; Yao, Y.; Xu, X.; Jiang, Q.; Shi, D. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio for acute deep vein thrombosis after total joint arthroplasty: A retrospective study. J. Orthop. Surg. Res. 2018, 13, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, K.; Si, L.; Pan, X.; Sun, L.; Wang, Y.; Lu, J.; Wang, X. Preoperative Systemic Immune–Inflammation Index (SII) as a Superior Predictor of Long-Term Survival Outcome in Patients with Stage I–II Gastric Cancer After Radical Surgery. Front. Oncol. 2022, 12, 829689. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Zhu, Y.; Wu, Q.; Yao, C.; Xia, H.; Li, C. Postoperative Systemic Immune-Inflammation Index (SII): A Superior Prognostic Factor of Endometrial Cancer. Front. Surg. 2021, 8, 704235. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Z.; Pei, F.; Huang, F.; Chen, S.; Xiang, Z. A meta-analysis of the Gamma nail and dynamic hip screw in treating peritrochanteric fractures. Int. Orthop. 2010, 34, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Todor, A.; Pojar, A.; Lucaciu, D. Minimally invasive treatment of trochanteric fractures with intramedullary nails. Technique and results. Clujul Med. 2013, 86, 40–42. [Google Scholar]
- Fu, M.; Shen, J.; Ren, Z.; Lv, Y.; Wang, J.; Jiang, W. A systematic review and meta-analysis of cemented and uncemented bipolar hemiarthroplasty for the treatment of femoral neck fractures in elderly patients over 60 years old. Front. Med. 2023, 10, 1085485. [Google Scholar] [CrossRef]
- Khan, A.M.; Anwar, S.F.; Hafeez, S. Clinical outcome of cemented bipolar hemiarthoplasty versus Austin Moore hemiarthroplasty for displaced intracapsular fractures of hip in terms of anterior thigh pain in elderly. J. Pak. Med. Assoc. 2015, 65 (Suppl. 3), S49–S51. [Google Scholar]
- Mishra, A.K.; Chalise, P.K.; Shah, S.B.; Adhikari, V.; Singh, R.P. Comparative study in surgical outcome of intracapsular fracture neck of femur in active elderly patients treated with hemiarthroplasty with Austin Moore’s and bipolar prosthesis. Nepal Med. Coll. J. 2013, 15, 81–83. [Google Scholar]
- Salma, R.G.; Al-Shammari, F.M.; Al-Garni, B.A.; Al-Qarzaee, M.A. Operative time, blood loss, hemoglobin drop, blood transfusion, and hospital stay in orthognathic surgery. Oral Maxillofac. Surg. 2017, 21, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.D.; Navo, P.; Ramsey, F.; Jallow, S.; Rehman, S. “Hidden” Preoperative Blood Loss with Extracapsular Versus Intracapsular Hip Fractures: What Is the Difference? Geriatr. Orthop. Surg. Rehabil. 2017, 8, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Menges, P.; Kessler, W.; Kloecker, C.; Feuerherd, M.; Gaubert, S.; Diedrich, S.; van der Linde, J.; Hegenbart, A.; Busemann, A.; Traeger, T.; et al. Surgical trauma and postoperative immune dysfunction. Eur. Surg. Res. 2012, 48, 180–186. [Google Scholar] [CrossRef]
- Grzelak, I.; Olszewski, W.L.; Zaleska, M.; Durlik, M.; Lagiewska, B.; Muszynski, M.; Rowinski, W. Blood cytokine levels rise even after minor surgical trauma. J. Clin. Immunol. 1996, 16, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Jaradeh, M.; Curran, B.; Poulikidis, K.; Rodrigues, A.; Jeske, W.; Abdelsattar, Z.M.; Lubawski, J.; Walenga, J.; Vigneswaran, W.T. Inflammatory cytokines in robot-assisted thoracic surgery versus video-assisted thoracic surgery. J. Thorac. Dis. 2022, 14, 2000–2010. [Google Scholar] [CrossRef]
- Reikeras, O.; Borgen, P.; Reseland, J.E.; Lyngstadaas, S.P. Changes in serum cytokines in response to musculoskeletal surgical trauma. BMC Res. Notes 2014, 7, 128. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Yang, L.; Jiang, W.; Chen, X.; Liu, Y. High platelet-to-lymphocyte ratio predicts poor survival of elderly patients with hip fracture. Int. Orthop. 2021, 45, 13–21. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Jiang, W.; Chen, X.; Yang, L.; Wang, H.; Liu, Y.-H. Systemic immune-inflammation index independently predicts poor survival of older adults with hip fracture: A prospective cohort study. BMC Geriatr. 2021, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fu, J.; Zhao, Q.; Lin, S.; Zhu, H. Effect of neutrophil-to-lymphocyte ratio on short-term prognosis of elderly patients with hip fracture. Am. J. Transl. Res. 2021, 13, 9122–9128. [Google Scholar] [PubMed]
- Downey, C.; Kelly, M.; Quinlan, J.F. Changing trends in the mortality rate at 1-year post hip fracture—A systematic review. World J. Orthop. 2019, 10, 166–175. [Google Scholar] [CrossRef] [PubMed]



| Variables | Cut-off Values | AUC | 95% CI | p-Value |
|---|---|---|---|---|
| NLR—Admission | 8.9 | 0.47 | 0.37–0.57 | 0.551 |
| PLR—Admission | 217.5 | 0.48 | 0.38–0.58 | 0.799 |
| MLR—Admission | 0.6 | 0.40 | 0.30–0.50 | 0.057 |
| SII—Admission | 1606.5 | 0.47 | 0.37–0.57 | 0.627 |
| NLR—Postoperative | 8 | 0.57 | 0.47–0.67 | 0.156 |
| PLR—Postoperative | 185.3 | 0.58 | 0.47–0.68 | 0.119 |
| MLR—Postoperative | 0.8 | 0.47 | 0.37–0.57 | 0.637 |
| SII—Postoperative | 1564.7 | 0.60 | 0.50–0.70 | 0.038 |
| Preoperative days | 2.5 | 0.59 | 0.49–0.69 | 0.067 |
| Duration of surgery (minutes) | 60.5 | 0.91 | 0.86–0.96 | <0.0001 |
| Length of hospital stay (days) | 7.5 | 0.66 | 0.56–0.75 | 0.001 |
| Variable | Total Patients (n = 129) | Extracapsular Fracture Group (n = 67) | Intracapsular Fracture Group (n = 62) | p-Value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years), median (IQR) | 81 (9) | 81 (8) | 80.50 (12) | 0.470 |
| Sex, n (%) | 0.844 | |||
| Male | 35 (27.1) | 19 (28.4) | 16 (25.8) | |
| Female | 94 (72.9) | 48 (71.6) | 46 (74.2) | |
| Alcohol (yes), n (%) | 34 (26.4) | 23 (34.3) | 11 (17.7) | 0.045 |
| Tobacco (yes), n (%) | 33 (25.6) | 20 (29.9) | 13 (21) | 0.313 |
| Obesity (yes), n (%) | 64 (49.6) | 32 (47.8) | 32 (51.6) | 0.726 |
| Living area, n (%) | 0.157 | |||
| Rural | 59 (45.7) | 35 (52.2) | 24 (38.7) | |
| Urban | 70 (54.3) | 32 (47.8) | 38 (61.3) | |
| HBP (yes), n (%) | 106 (82.2) | 57 (85.1) | 49 (79) | 0.491 |
| COPD (yes), n (%) | 37 (28.7) | 18 (26.9) | 19 (30.6) | 0.699 |
| CVI (yes), n (%) | 40 (31.0) | 24 (35.8) | 16 (25.8) | 0.256 |
| CHF (yes), n (%) | 71 (55.0) | 39 (58.2) | 32 (51.6) | 0.483 |
| CKD (yes), n (%) | 21 (16.3) | 9 (13.4) | 12 (19.4) | 0.475 |
| DM (yes), n (%) | 22 (17.1) | 9 (13.4) | 13 (21) | 0.349 |
| Surgery-related data | ||||
| ASA score, n (%) | 0.028 | |||
| I–II | 34 (26.4) | 12 (17.9) | 22 (35.5) | |
| ≥III | 95 (73.6) | 55 (82.1) | 40 (64.5) | |
| Type of anesthesia, n (%) | 0.818 | |||
| Intraspinal | 106 (82.2) | 56 (83.6) | 50 (80.6) | |
| General | 23 (17.8) | 11 (16.4) | 12 (19.4) | |
| Preoperative days, | 0.022 | |||
| 0–2.5 cut-off | 72 (55.8) | 44 (65.7) | 28 (45.7) | |
| >2.5 | 57 (44.2) | 23 (34.3) | 34 (54.8) | |
| LHS (days), | 0.008 | |||
| 0–7.5 cut-off | 62 (48.1) | 40 (59.7) | 22 (35.5) | |
| >7.5 | 67 (51.9) | 27 (40.3) | 40 (64.5) | |
| Duration of surgery (min), | <0.0001 | |||
| 0–60.5 cut-off | 65 (50.4) | 57 (85.1) | 8 (12.9) | |
| >60.5 | 64 (49.6) | 10 (14.9) | 54 (87.1) | |
| Admission laboratory data | ||||
| Neutrophil count (×103/µL), median (IQR) | 8.35 (4.28) | 8.35 (3.67) | 8.09 (4.91) | 0.925 |
| Lymphocyte count (×103/µL), median (IQR) | 1.21 (0.66) | 1.17 (0.57) | 1.21 (0.77) | 0.578 |
| Monocyte count (×103/µL), median (IQR) | 0.72 (0.36) | 0.76 (0.31) | 0.69 (0.41) | 0.038 |
| PLT count (×103/µL), median (IQR) | 220 (88) | 225 (114) | 217.5 (82) | 0.891 |
| AST/ALT (>1, reference), median (IQR) | 1.23 (0.56) | 1.21 (0.57) | 1.26 (0.56) | 0.934 |
| WBC (×103/µL), median (IQR) | 10.05 (4.52) | 10.2 (3.94) | 9.47 (5.02) | 0.810 |
| RBC (×106/µL), mean ± SD | 4.02 ± 0.67 | 3.76 ± 0.61 | 4.3 ± 0.62 | <0.0001 |
| HGB (g/dL), mean ± SD | 12.26 ± 1.84 | 11.61 ± 1.87 | 12.97 ± 1.52 | <0.0001 |
| NLR (>8.9, cut-off), n (%) | 44 (43.1) | 21 (31.3) | 23 (37.1) | 0.578 |
| PLR (>217.5, cut-off), n (%) | 45 (34.9) | 21 (31.3) | 24 (38.7) | 0.460 |
| MLR (>0.6, cut-off), n (%) | 69 (53.5) | 39 (58.2) | 30 (48.4) | 0.293 |
| SII (>1606.5, cut-off), n (%) | 58 (45) | 29 (43.3) | 29 (46.8) | 0.726 |
| Preoperative laboratory data | ||||
| Neutrophil count (×103/µL), median (IQR) | 6.79 (3.69) | 3.37 (6.49) | 4.38 (7.79) | 0.047 |
| Lymphocyte count (×103/µL), median (IQR) | 1.13 (0.71) | 0.79 (1.21) | 0.69 (1.08) | 0.546 |
| Monocyte count (×103/µL), median (IQR) | 0.82 (0.47) | 0.40 (0.85) | 0.47 (0.73) | 0.051 |
| PLT count (×103/µL) mean ± SD | 224.12 ± 70.32 | 217.21 ± 71.90 | 231.60 ± 68.32 | 0.247 |
| WBC (×103/µL), median (IQR) | 9.17 (4.31) | 8.67 (3.49) | 9.53 (4.53) | 0.172 |
| RBC (×106/µL), mean ± SD | 3.36 ± 0.65 | 3.11 ± 0.59 | 3.63 ± 0.61 | <0.0001 |
| HGB (g/dL) mean ± SD | 10.24 ± 1.85 | 9.53 ± 1.66 | 11 ± 1.75 | <0.0001 |
| NLR (>8, cut-off), n (%) | 44 (34.1) | 17 (25.4) | 27 (43.5) | 0.041 |
| PLR (>185.3, cut-off), n (%) | 64 (49.6) | 26 (38.8) | 38 (61.3) | 0.014 |
| MLR (>0.8, cut-off), n (%) | 54 (41.9) | 27 (40.3) | 27 (43.5) | 0.725 |
| SII (>1572.7, cut-off), n (%) | 59 (45.7) | 18 (26.8) | 41 (66.1) | 0.012 |
| Variable | Surgery-Related Trauma | p-Value | |
|---|---|---|---|
| OR | 95% CI | ||
| Postoperative NLR | 1.79 | 0.88–3.64 | 0.029 |
| Postoperative PLR | 2.26 | 1.07–4.77 | 0.009 |
| Postoperative MLR | 1.14 | 1.22–5.070 | 0.063 |
| Postoperative SII | 2.49 | 1.22–5.07 | 0.001 |
| Duration of surgery | 38.47 | 14.13–104.73 | <0.0001 |
| Type of anesthesia | 1.22 | 0.49–3.01 | 0.424 |
| Alcohol | 0.41 | 0.18–0.94 | 0.199 |
| Tobacco | 0.62 | 0.27–1.39 | 0.371 |
| Obesity | 1.16 | 0.58–2.32 | 0.390 |
| HBP | 0.66 | 0.26–1.64 | 0.123 |
| Asthma and COPD | 1.20 | 0.56–2.58 | 0.081 |
| CVI | 0.62 | 0.29–1.32 | 0.886 |
| CHF | 0.76 | 0.38–1.53 | 0.556 |
| CKD | 1.54 | 0.60–3.97 | 0.236 |
| DM | 1.71 | 0.67–4.33 | 0.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, F.; Ivanescu, A.D.; Fodor, P.; Moldovan, L.; Bataga, T. Correlation between Inflammatory Systemic Biomarkers and Surgical Trauma in Elderly Patients with Hip Fractures. J. Clin. Med. 2023, 12, 5147. https://doi.org/10.3390/jcm12155147
Moldovan F, Ivanescu AD, Fodor P, Moldovan L, Bataga T. Correlation between Inflammatory Systemic Biomarkers and Surgical Trauma in Elderly Patients with Hip Fractures. Journal of Clinical Medicine. 2023; 12(15):5147. https://doi.org/10.3390/jcm12155147
Chicago/Turabian StyleMoldovan, Flaviu, Adrian Dumitru Ivanescu, Pal Fodor, Liviu Moldovan, and Tiberiu Bataga. 2023. "Correlation between Inflammatory Systemic Biomarkers and Surgical Trauma in Elderly Patients with Hip Fractures" Journal of Clinical Medicine 12, no. 15: 5147. https://doi.org/10.3390/jcm12155147
APA StyleMoldovan, F., Ivanescu, A. D., Fodor, P., Moldovan, L., & Bataga, T. (2023). Correlation between Inflammatory Systemic Biomarkers and Surgical Trauma in Elderly Patients with Hip Fractures. Journal of Clinical Medicine, 12(15), 5147. https://doi.org/10.3390/jcm12155147